Back to Journals » Journal of Pain Research » Volume 16
Hemorphin-Based Analgesia: A Mechanism of Cupping Technique?
Authors Song C , Wang Q, Song N
Received 17 March 2023
Accepted for publication 24 May 2023
Published 29 May 2023 Volume 2023:16 Pages 1751—1754
DOI https://doi.org/10.2147/JPR.S413021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Changzheng Song,1 Qingwei Wang,2 Nianci Song3
1Erythrocrine Research Initiative of Translational Medicine, Shandong Academy of Medical Sciences, Jinan, People’s Republic of China; 2Cancer Therapy Center, Qilu Hospital of Shandong University, Jinan, People’s Republic of China; 3Biodesign Lab, Geriatricare Biorobotics Studio, Baltimore, MD, USA
Correspondence: Changzheng Song, Erythrocrine Research Initiative of Translational Medicine, Shandong Academy of Medical Sciences, N-18877 Jingshi Road, Jinan, 250062, People’s Republic of China, Email [email protected]
Background and Objective: Cupping is a time-honoured traditional healing modality for pain management and remains favoured by professionals and lay people across several cultures today. However, the analgesic mechanism of cupping is still poorly understood. In addition, clinical guidelines for standardized applications of cupping are currently lacking. The awareness of cupping marks has provoked curiosity about the connection between skin color changes and their benefit for local pain relief. Computer simulation is a promising approach for numerical modeling the cupping-evoked erythrocyte emigration. Quantitative proteomic profiling of cupping-induced blister fluid exhibited a significant decrease in the abundance of haemoglobin β subunit. This finding provides a critical clue to paint a novel picture of the mechanism behind cupping. The hemorphins are a set of non-classical opioid peptides derived from the proteolysis of haemoglobin β subunit. In the present study, a probable mechanism of hemorphin-based cupping analgesia is proposed. The hemorphin could also act as a potential biomarker for objective and timely quantitative clinical assessment of cupping in the management of pain conditions. A seminal theory may open a new avenue for future translational research on promoting the efficacy and safety of cupping analgesia.
Conclusion: The local analgesic effect of cupping is probable in the context of haemoglobin degradation that bestows the appearance of hemorphins along with engaging opioid receptor signalling. Exploring the potential novel mechanism of cupping analgesia facilitates seeking non-pharmacologic pain interventions.
Keywords: cupping analgesia, erythrocyte, hemorphin, cupping mark, numerical simulation
The oldest written mention of cupping dates back to circa 1550 BC as documented in the Egyptian Ebers Papyrus.1 Unlike other ancient techniques of healing, cupping has been employed by many epochs of civilization and is still widely practised in diverse cultures around the world. According to the World Health Organization (WHO), cupping is a traditional modality involving the local suction effect produced inside a cup or jar on the skin over the target area.2 The mechanical principle of cupping only relies on the negative pressure suction which is generated either by an aspirator or cooling heated air inside the cup. While performing cupping, the applied negative pressure anchors the cup onto the intact skin, induces dynamic skin distortion, and reshapes the cutaneous tissues within the interior of the cup. Moreover, cupping usually results in visible discoloration of the treated skin (so-called cupping mark) which varies from light pink to dark red. In recent years, cupping experienced a noticeable surge in interest in pain control in the field of sports medicine.3 The color of the cupping mark as a predictive indicator of the effectiveness of cupping could enable the therapist to make evaluations and adjustments accordingly.1 Nevertheless, some receivers of cupping consider the cupping marks harmful. The appearance of unpleasant visual marks on the skin following cupping may lead to negative emotional responses to cupping healing.4 The awareness of cupping marks has provoked curiosity about a valid explanation for the connection between skin color changes and local analgesic action from a modern scientific perspective.
Cupping has been used for chronic painful conditions since antiquity.5 Nevertheless, the mechanism of cupping analgesia remains unclear, and several theories have been proposed. Cupping induces skin deformation leading to activate mechanosensitive afferent Aδ- and C-fibers, thereby decreasing nociceptive input to the dorsal horn.6 The stimulation of cutaneous baroreceptors with cupping vacuum could cause the release of endorphins, and hence, induce analgesic effect.7 The placebo mechanism also exists that explains clinical trials on cupping as eliciting analgesic placebo effects.8
Different shades of redness, erythema and ecchymosis are the immediate skin changes following cupping.9 Furthermore, a longer duration with high negative pressure during cupping suction contributes to the blister formation.10 A characteristic ecchymosis at the cupping site is likely to be associated with severe dilatation of skin vessels and the escape of erythrocytes into extravascular spaces.11 Negative pressure is known to induce intact sub-epidermal blisters. The histological change of suction blisters includes dermo-epidermal separation between the epidermal basement membrane and the basal cell membrane.12 Quantitative proteomic analysis of cupping-induced blister fluids demonstrates that the abundance of haemoglobin β subunit was significantly decreased.13 It is logical to assume that the degradation of haemoglobin β subunit might relate to the biological actions of cupping. Insight into the local haemoglobin catabolysis may illuminate certain mediating processes that influenced the pain perception at the cupping site.
The skin covers the body’s external surface and is home to its primary functions of protection, thermal regulation, and sensation. In adults, the skin receives up to one-third of the circulating blood volume.14 The drawing force in cupping may bring about the alteration in blood flow dynamics along with the variation in dermal vascular arrangement. The pattern of the tortuous vessel network and the array of vertically oriented capillary loops might facilitate skin mobility perpendicular to the surface without the risk of injury to the dermal vessels. Junctional gaps between adjacent endothelial cell borders are predominantly encountered in the venules of the papillary dermis.15 They are functional rather than permanent, ie, they are closed except when the actual transit of blood cells occurs. The aperture calibre undergoes dynamic change according to the imposed shear stress profile. The interendothelial gaps are necessary prerequisites for efficient diapedesis of blood cells. Mature erythrocytes are typically anucleate biconcave discs full of haemoglobin, the iron-carrying protein, which imparts the characteristic red color to blood. The deformability of erythrocytes depends critically on cellular morphology, intracellular haemoglobin viscosity, and rheological properties of the membrane.
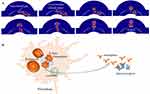
During the cupping process, negative pressure engenders a large tensile stress within the bulging tissue inside the cup. Finite element modeling of soft tissue is a convenient approach for investigating the effects of cupping on the biomechanical properties of the skin.16 Various computational methods are employed for the simulation of erythrocyte mechanics, rheology, and dynamics. A simulation model is well developed to describe a normal erythrocyte squeezing through a tiny splenic slit without damage.17,18 An elevated transmural venous pressure favours erythrocytes adhering to the gap sites and transmigrating through the venular wall into the surrounding tissues. The visible reddish marks left by cupping on the skin are probably due to varying degrees of erythrocyte extravasation. The scenario for erythrocyte crossing an interendothelial gap of submicron size can be numerically simulated (Figure 1A). Erythrocyte membrane displays liquid-like behavior and could flow as a continuum.19 Thus, the evolution of erythrocyte diapedesis may be regarded as haemoglobin flows through the micro-opening under negative transmural pressure. The numerical simulations coincide with prior evidence obtained by electronic microscopy.20
Haemoglobin performs its primary function of gas exchange and transport. It also serves as a versatile precursor for various bioactive molecules. Hemorphins are a set of atypical endogenous opioid peptides originating from proteolysis of the haemoglobin β-chain. Over the past decade, an emerging erythrocrine concept has engendered new understandings of the role of erythrocytes in analgesia.21 Physiologically, erythrocytes harbor a substantial amount of hemorphins, which originated from the proteasomal degradation of haemoglobin.22 Applying sufficiently tensile stresses to erythrocytes could trigger the release of hemorphins. Long-lasting exercises like a marathon can increase the level of hemorphin in the plasma of runners.23 Erythrocyte mechanical trauma also brings about an increased level of hemorphin in patients undergoing hemodialysis or open-heart surgery.24,25 When cupping is administered, erythrocytes gain access to the extravascular space in the skin. The extravascular erythrocytes can be captured and subsequently engulfed by dermal resident macrophages where haemoglobin is proteolytically degraded.26 As illustrated in Figure 1B, the lysosomal enzymes in macrophages are implicated in the generation of hemorphins from haemoglobin.27 The skin is the largest sensory organ of the body and is richly innervated by nociceptive afferent fibers. So far, the opioid receptors (δ-, μ-, κ-receptor) have been identified on nerve endings of the epidermis and dermis.28 Their activation by hemorphins may elicit the initiation of the hemorphin signaling cascades to exert a local analgesic effect around the cupping area.
Cupping could positively affect erythrocyte diapedesis from superficial dermal venules. The extravasated erythrocyte may play a mediating role in the proteolytic degradation cascade of haemoglobin as depicted in Figure 1. The haemoglobin-derived hemorphins engage opioid receptor signalling and induce the local analgesic effect of cupping. Hemorphin might be used as a relevant biomarker for future testing hypothesis in clinical trials and translational research studies. A deeper knowledge of the potential mechanism will aid in deciphering the meaning of the telltale red cupping marks and shed new light on the molecular basis of cupping for pain alleviation. Rediscovering the value of cupping facilitates seeking non-pharmacologic pain interventions.
Data Sharing Statement
Data are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Not applicable for this manuscript as this research does not involve human participants or laboratory animals.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure
No competing interests exist.
References
1. Dalton EL, Velasquez BJ. Cupping therapy: an alternative method of treating pain. Public Health Open J. 2017;2(2):59–63. doi:10.17140/PHOJ-2-122
2. World Health Organization. WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region. Geneva: World Health Organization; 2007.
3. Jenkinson H, DiCicco B. Sporty spots. JAMA Dermatol. 2018;154(1):66. doi:10.1001/jamadermatol.2017.4694
4. Hong M, Lee IS, Choi DH, Chae Y. Attentional bias toward cupping therapy marks: an eye-tracking study. J Pain Res. 2020;13:1041–1047. doi:10.2147/JPR.S252675
5. Cramer H, Klose P, Teut M, et al. Cupping for patients with chronic pain: a systematic review and meta-analysis. J Pain. 2020;21(9–10):943–956. doi:10.1016/j.jpain.2020.01.002
6. Emerich M, Braeunig M, Clement HW, Lüdtke R, Huber R. Mode of action of cupping–local metabolism and pain thresholds in neck pain patients and healthy subjects. Complement Ther Med. 2014;22(1):148–158. doi:10.1016/j.ctim.2013.12.013
7. Urooj S, Jahangir U, Khan AA, Zaman F. Analgesic effect of cupping therapy in osteoarthritis on timeline: an open comparative clinical study. Int J Pharmacogn. 2016;3(7):314–318. doi:10.13040/IJPSR.0975-8232.IJP.3(7).314-318
8. Almeida Silva HJ, Barbosa GM, Scattone Silva R, et al. Dry cupping therapy is not superior to sham cupping to improve clinical outcomes in people with non-specific chronic low back pain: a randomised trial. J Physiother. 2021;67(2):132–139. doi:10.1016/j.jphys.2021.02.013
9. Lin CW, Wang JT, Choy CS, Tung HH. Iatrogenic bullae following cupping therapy. J Altern Complement Med. 2009;15(11):1243–1245. doi:10.1089/acm.2009.0282
10. Alam M, Abbas K. The role of Cupping Therapy (CT) in pain tackling, an insight into mechanism therapeutic effects and its relevance in current medical scenario. Int J Curr Sci Res Rev. 2021;4(07):732–739. doi:10.47191/ijcsrr/v4-i7-16
11. Lowe DT. Cupping therapy: an analysis of the effects of suction on skin and the possible influence on human health. Complement Ther Clin Pract. 2017;29:162–168. doi:10.1016/j.ctcp.2017.09.008
12. Lowe LB
13. Liu Z, Chen C, Li X, et al. Is cupping blister harmful?-A proteomical analysis of blister fluid induced by cupping therapy and scald. Complement Ther Med. 2018;36:25–29. doi:10.1016/j.ctim.2017.11.002
14. Wysocki AB. Skin anatomy, physiology, and pathophysiology. Nurs Clin North Am. 1999;34(4):777–797. doi:10.1016/S0029-6465(22)02423-9
15. Braverman IM, Yen A. Ultrastructure of the human dermal microcirculation. II. The capillary loops of the dermal papillae. J Invest Dermatol. 1977;68(1):44–52. doi:10.1111/1523-1747.ep12485165
16. Tham LM, Lee HP, Lu C. Cupping: from a biomechanical perspective. J Biomech. 2006;39(12):2183–2193. doi:10.1016/j.jbiomech.2005.06.027
17. Freund JB. The flow of red blood cells through a narrow spleen-like slit. Phys Fluids. 2013;25(11):110807. doi:10.1063/1.4819341
18. Qi X, Wang S, Ma S, Han K, Xl L. Quantitative prediction of flow dynamics and mechanical retention of surface-altered red blood cells through a splenic slit. Phys Fluids. 2021;33(5):051902. doi:10.1063/5.0050747
19. Hochmuth RM. Solid and liquid behavior of red cell membrane. Annu Rev Biophys Bioeng. 1982;11(1):43–55. doi:10.1146/annurev.bb.11.060182.000355
20. Skalak R, Brånemark PI, Ekholm R. Erythrocyte adherence and diapedesis. Some aspects of a possible mechanism based on vital and electron microscopic observations. Angiology. 1970;21(4):224–239. doi:10.1177/000331977002100402
21. Song CZ, Wang QW, Song CC. Erythrocyte-based analgesic peptides. Regul Pept. 2013;180:58–61. doi:10.1016/j.regpep.2012.11.003
22. Song CZ, Wang QW, Liu H, Song CC. Inhibition of intraerythrocytic proteasome retards the generation of hemorphins. Peptides. 2012;33(1):170–173. doi:10.1016/j.peptides.2011.11.021
23. Glämsta EL, Mørkrid L, Lantz I, Nyberg F. Concomitant increase in blood plasma levels of immunoreactive hemorphin-7 and beta-endorphin following long distance running. Regul Pept. 1993;49(1):9–18. doi:10.1016/0167-0115(93)90378-l
24. Nyberg F, Sanderson K, Glämsta EL. The hemorphins: a new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers. 1997;43(2):147–156. doi:10.1002/(SICI)1097-0282(1997)43:2<147::AID-BIP8>3.0.CO;2-V
25. Nyberg G, Sanderson K, Andren P, et al. Isolation of haemorphin-related peptides from filter membranes collected in connection with haemofiltration of human subjects. J Chromatogr A. 1996;723(1):43–49. doi:10.1016/0021-9673(95)00811-X
26. Feinstein RJ, Halprin KM, Penneys NS, Taylor JR, Schenkman J. Senile purpura. Arch Dermatol. 1973;108(2):229–232. doi:10.1001/archderm.1973.01620230029011
27. Fruitier I, Garreau I, Lacroix A, Cupo A, Piot JM. Proteolytic degradation of hemoglobin by endogenous lysosomal proteases gives rise to bioactive peptides: hemorphins. FEBS Lett. 1999;447(1):81–86. doi:10.1016/s0014-5793(99)00271-9
28. Bigliardi PL, Dancik Y, Neumann C, Bigliardi-Qi M. Opioids and skin homeostasis, regeneration and ageing—what’s the evidence? Exp Dermatol. 2016;25(8):586–591. doi:10.1111/exd.13021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.