Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 13
Hematological Abnormalities in Culture Positive Neonatal Sepsis
Authors Adane T , Worku M , Tigabu A , Aynalem M
Received 22 February 2022
Accepted for publication 30 May 2022
Published 7 June 2022 Volume 2022:13 Pages 217—225
DOI https://doi.org/10.2147/PHMT.S361188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Tiruneh Adane,1 Minichil Worku,2 Abiye Tigabu,2 Melak Aynalem1
1Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 2Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Tiruneh Adane, Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, PO Box 196, Gondar, Ethiopia, Tel +251 949914917, Email [email protected]
Background: In neonatal sepsis, anemia, leukocytosis, thrombocytopenia, and a shortened coagulation time are the most common hematologic abnormalities. However, there is inadequate information regarding the hematological abnormalities in neonatal sepsis. Thus, we aimed to determine the magnitude of hematological abnormalities in neonatal sepsis.
Methods: This is a cross-sectional study that included 143 neonates with culture proven sepsis aged 1– 28 days from September 2020 to November 2021 at the University of Gondar Specialized Referral Hospital. The sociodemographic data was collected using a pre-tested structured questionnaire, and the clinical and laboratory data was collected using a data collection sheet. A total of 2 mL of venous blood was taken using a vacutainer collection device for the complete blood count (CBC) and blood culture analysis. A univariate and multivariate logistic regression model was used to investigate factors associated with hematological abnormalities in neonatal sepsis. Statistical significance was declared when a p-value was less than 0.05.
Results: The prevalence of anemia, thrombocytopenia, and leucopenia in neonatal sepsis was 49% (95% CI: 40.89– 57.06), 44.7% (95% CI: 36.8– 52.9), and 26.6% (95% CI: 22.01– 29.40), respectively. On the other hand, leukocytosis and thrombocytosis were found in 7.7% (95% CI: 4.35– 13.25) and 11.9% (95% CI: 7.56– 18.21), respectively. Being female (AOR: 3.3; 95% CI: 1.20– 3.82) and being aged less than 7 days (AOR: 2.44; 95% CI: 1.6– 6.9) were found to be significant predictors of anemia.
Conclusion: The magnitude of anemia, leucopenia, and thrombocytopenia is high in neonatal sepsis. Furthermore, being female and being younger than 7 days were risk factors for anemia. Thus, the diagnosis and treatment of anemia, leucopenia, and thrombocytopenia prevents further complications in neonatal sepsis.
Keywords: anemia, hematological abnormalities, neonatal sepsis, Gondar, Ethiopia
Background
Neonatal sepsis is a systemic illness caused by bacteria, viruses, or fungal infections. Furthermore, it is linked to hemodynamic alterations and clinical findings, as well as bringing substantial morbidity and mortality.1 Neonatal sepsis is a clinical syndrome caused by systemic bacterial infection documented by a positive blood culture in the first 4 weeks of life.2 It can be defined by positive blood and/or cerebrospinal fluid (CSF) culture.3 Sepsis occurring in the first 72 hours of life is defined as early-onset sepsis (EOS) and that occurring beyond 72 hours as late-onset sepsis (LOS).1,4,5
According to the global burden of disease study 2016/2017, there are 1.3 million yearly incident cases of neonatal sepsis worldwide, resulting in 203,000 sepsis-attributable deaths.6,7 In 2014, roughly 5.3–8.7 million disability-adjusted life-years were lost in Sub-Saharan Africa due to neonatal sepsis and subsequent long-term morbidity.8 A systematic review and meta-analysis conducted in Ethiopia confirmed that the prevalence of neonatal sepsis was found to be 45%.9 The Amhara region had the highest prevalence of neonatal sepsis (64%).9 Hematological parameters are straightforward variables that may be derived from a patient’s hemogram.10 The ability to count the various subsets of leukocytes in a patient’s peripheral blood is a helpful tool for detecting a variety of disorders and diseases. Neutrophils play a critical part in the immune response during infection by producing cytokines that attract macrophages and phagocytizing cellular debris. An elevated absolute neutrophil count (ANC) is a common observation in an infectious or inflammatory illness.11 WBC counts alter significantly during a systemic inflammatory response, resulting in neutrophilia and/or relative lymphocytopenia. Thrombocytopenia is common complication in neonatal sepsis. Sepsis-induced thrombocytopenia is caused by a decrease in platelet synthesis in the bone marrow, which can be caused by antibiotics, the inhibitory impact of pathogenic toxins, inflammatory mediators in hematopoiesis, or hemophagocytosis.12 Pre-existing conditions that contribute to chronic anemia before admission to the intensive care unit are frequently present in sepsis, such as low nutritional conditions, co-morbidities such as renal failure, or rigorous cancer therapy.13
In our study area, there is inadequate information regarding the hematological abnormalities and associated factors in neonatal sepsis. Thus, the present study aimed to determine the magnitude and associated factors of hematological abnormalities in neonates with sepsis. The findings could serve as a basis for further research in this field.
Methods and Materials
Study Design, Setting and Populations
This study was conducted prospectively on a total of 143 neonates aged 1–28 days admitted to the University of Gondar Specialized Referral Hospital from September 2020 to November 2021. The source population included all neonates who developed sepsis and were admitted to the hospital’s neonatal intensive care unit (NICU). The study population consisted of neonates who developed sepsis that was confirmed by blood culture. Participants with preterm, induced abortion delivery, congenital anomalies, inborn error of metabolism, severe jaundice and respiratory distress syndrome, surfactant deficiency, extreme low birth weight newborns, mothers with pregnancy-induced hypertension, and neonates with asphyxia were excluded from the study.
Study Variables
Anemia, thrombocytopenia, thrombocytosis, leukopenia, and leukocytosis were considered as a dependent variable. The independent variables were sociodemographic (age, sex, and place of residence) and clinical characteristic (presence of fever, hypothermia, respiratory rate, coinfection (pneumonia, meningitis)).
Operational Definitions
Proven sepsis: There will be clinical and laboratory findings, as well as evidence of the pathogenic bacterium in sterile field cultures.14
Anemia: is considered when the hemoglobin (Hgb) value is less than 13.5 g/dl.15
Leukopenia and leukocytosis: were considered when the WBC count is less than 7.64 x109/l and greater than 22.16 x109/l, respectively.15
Thrombocytopenia and thrombocytosis: were considered when the platelet counts were less than 132.8 x109/l and 413.4 x109/l, respectively.15
Data Collection Procedures
A pre-tested structured questionnaire was used to collect sociodemographic data, and a data collection sheet was used to obtain clinical and laboratory data. Prior to the data collection procedure, the data collectors (nurses and medical laboratory personnel) received training. To ensure that the data is of high quality, the principal investigator inspects the entire data collection procedure.
Blood Sample Collection
A laboratory technologist collected venous blood samples after obtaining approval from the study participants’ parents. A total of 2 mL venous blood with K2EDTA anticoagulant was collected by using a vacutainer collection system for CBC tests and blood culture analysis. The CBC parameters were determined using a Sysmex KX21N (Sysmex Corporation, Japan) hematology analyzer. A commercially prepared known blood samples (normal, abnormal low, and abnormal high), background checks, and machine maintenance was performed to assure the quality of CBC analyzer.16
Statistical Analysis
Epidata version 4.6.02 and statistical package for social sciences (SPSS) version 25 software were used data entry and analysis, respectively. Shapiro–Wilk test was used to assert the normality of the data. Continuous quantitative data was presented using mean and standard deviation (SD), whilst qualitative data were expressed in number of cases and percentage (%). A univariate and multivariate logistic regression model was used to determine factors associated with hematological abnormalities in neonatal sepsis. Adjusted odds ratios (AOR) and crude odds ratio (COR) were estimated along with the 95% confidence intervals. The fitness of the model was checked by using Hosmer-Lemeshow goodness-of-fit test.
Ethical Declarations
The study was conducted after approval of the protocol by the Ethical Review Committee (ERC) of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, the University of Gondar, with a reference number of SBMLS/2453/12. The study followed the declaration of Helsinki. A letter of authorization was also issued by the chief clinical director of the hospital. To secure confidentiality, information obtained from participants has been encrypted. Information that could reveal the identity of the participants was not recorded. The computerized data was authorized by the principal investigator only.
Result
Participant’s Demographic and Clinical Characteristics
Of the 143 neonates with sepsis, 91 (63.6%) were male and 52 (36.4%) were females. Participant’s ages ranged from 1–40 days with a mean of 8.58 ± (6.98) days. Fever was detected in 138 (96.5) of the participants while 66 (46.2) take antibiotic therapy. Gram Positive bacteria were isolated in 73 (51%) of neonates with sepsis (Table 1).
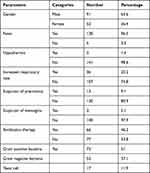 |
Table 1 Sociodemographic and Clinical Characteristics of Neonates with Culture-Proven Sepsis |
Bacterial Isolates in Neonatal Sepsis
The common bacteria isolates involved in neonatal sepsis were Coagulase-Negative Staphylococci (CoNS), Klebsiella Pneumoniae and Staph. Aureus (Figure 1).
 |
Figure 1 Common bacterial isolates in neonatal sepsis. |
Hematological Parameters
The mean and SD value of red blood cell (RBC), WBC, and platelets were 5.5±14.82, 11.8±6.88, and 203.3±153.12, respectively (Table 2).
 |
Table 2 Hematological Parameters in Neonates with Culture-Proven Sepsis |
Magnitudes of Hematological Abnormalities in Neonatal Sepsis
Anemia was determined by using the Hgb cutoff values (13.5 g/dl) after adjusting for altitude. The Hgb value was adjusted by subtracting 0.8 g/dl since the study area has an altitude of 2133 m. The prevalence of anemia, thrombocytopenia, and leucopenia in neonatal sepsis were 70/143 (49% (95% CI: 40.89–57.06)), 64/143 (44.7% (95% CI: 36.8–52.9)), and 38/143 (26.6% (95% CI: 22.01–29.40)), respectively. On the other hand, leukocytosis and thrombocytosis were found in 11/143 (7.7% (95% CI: 4.35–13.25)) and 17/143 (11.9% (95% CI: 7.56–18.21)), respectively (Figure 2).
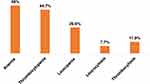 |
Figure 2 Magnitude of hematological abnormalities in neonatal sepsis. |
Anemia was more common in female neonates (67.3%) than male neonate (38.5%) (Figure 3). Besides, anemia is more common in EOS (85.7%) than LOS (14.3%). However, significant differences are not found in the magnitude of thrombocytopenia and leucopenia between EOS and LOS (Table 3).
 |
Table 3 Comparison of Hematological Abnormalities Between EOS and LOS in Neonatal Sepsis |
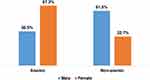 |
Figure 3 Distribution of anemia by gender in neonatal sepsis. |
Determinant Factors of Anemia in Neonatal Sepsis
In order to determine the associated factors, variables like age, gender, fever, increased respiratory rate, and suspicion of pneumonia were analyzed. Of these variables in bivariate logistic regression analysis, age and gender showed an association with anemia status in neonatal sepsis study participants. Then, those variables with a p-value less than 0.2 were conformed to multivariate logistic regression analysis. Accordingly, after adjusting for potential confounding variables, multivariate analysis affirmed that being female (AOR: 3.3; 95% CI: 1.20–3.82) and age less than 7 days (AOR: 2.44; 95% CI: 1.6–6.9) were found to be significant predictors of anemia in neonates with sepsis (Table 4).
 |
Table 4 Determinant Factors of Anemia in Neonatal Sepsis |
Discussion
Neonatal sepsis results in high rates of morbidity and mortality.17 Early and precise detection of sepsis will improve clinical outcomes and reduce antibiotic usage.18 Anemia, leukocytosis, thrombocytopenia, and activation of the hemostatic system are the most common abnormalities of the hematologic system in patients with sepsis. Hematologic organ system dysfunction is a common early indicator of severe sepsis, and it can be found in almost all patients.19
The prevalence of anemia in newborn sepsis was 49% (95% CI: 40.89–57.06) in the current study. This showed that half of the infants develop anemia in neonatal sepsis. The current study confirmed that anemia is a major public health concern in newborn sepsis, according to the WHO guideline for the diagnosis and assessment of the severity of anemia.20 Changes in the mechanical characteristics of RBCs in sepsis patients may limit microcirculatory blood flow, resulting in decreased tissue oxygen supply. A low RBC count is a sign of sepsis, which can be caused by a number of mechanisms involving RBC formation or survival.21 A low RBC count is a sign of sepsis, which can be caused by a number of mechanisms involving RBC formation or survival.22 Functional iron depletion, diminished erythropoietin production, infection, and inflammation could all cause RBC production to be suppressed. In addition, RBC loss during sepsis may occur as a result of pre-existing clinical conditions, including cancer, liver disease, or renal impairment, as well as new-onset multi-organ dysfunction, especially of the liver and kidney. Disseminated intravascular coagulation (DIC), pathogen-associated hemolysis, hypoadrenalism, and dietary insufficiency are all contributory causes. Furthermore, volume resuscitation-induced hemodilution is linked to a lower RBC count. Furthermore, blood loss might occur as a result of repetitive phlebotomy, through the gastrointestinal tract, or surgical procedures. Blood withdrawal is predicted to result in a daily loss of 24 to 41 mL of blood.23 Anemia can develop in people with severe sepsis as a result of hemorrhage. In the vast majority of cases, the etiology and source of blood loss are obvious. In patients who get septic after significant trauma, such as bleeding into the soft tissues of the thigh or the retroperitoneum, the source of anemia may be less obvious. Repeated phlebotomies, which are required to take blood in order to identify and monitor patients with urgent conditions such as severe sepsis, can result in significant blood loss.19
In this study, anemia is more common in EOS than LOS patients. A study by Ogundare et al showed that the mean hematocrit and mean platelet count were all significantly lower among the babies with EOS compared with LOS.2 Studies have shown that sepsis is often complicated by anemia. Therefore, the occurrence of sepsis is often accompanied by varying degrees of a reduction in Hgb concentration. A decrease in Hgb concentration may cause ischemic and hypoxic damage to the body, causing redistribution of blood and microcirculation disorders.24
Leucopenia was seen in 26.6% of infants with sepsis in this study. Lymphocytopenia, neutrophilia, eosinopenia, and an increased neutrophil to lymphocyte ratio are all common results on a CBC of a patient with sepsis. An increase in the total WBC count is frequently associated with inflammation and infection. It can, however, be altered in a variety of clinical situations, including hemopathy and inflammatory non-infectious diseases such as rheumatoid arthritis, lupus, and septic malignancy.25 In comparison to leukocytosis, leukopenia has been demonstrated to be a better predictor of neonatal sepsis and is more informative in infections caused by gram negative bacteria. On the other hand, an increase in total leukocyte count (TLC) in severe neonatal infections, possibly as a result of the secretion of growth factors and cytokines that accelerate bone marrow production. Virus-infected neonates usually have a normal TLC or a slightly reduced WBC count.26
Thrombocytopenia commonly happens in neonates with sepsis.27 Even if the pathophysiology of neonatal sepsis and platelet count is not fully implicit, the most probable cause of platelet count reduction is mostly associated with damage that happens to the endothelial cells, which causes activation of reticuloendothelial cells and leads to platelet removal. This commonly happens at the late stage of the infection, and it indicates there is a low disease prognosis.28 Furthermore, thrombocytopenia in neonatal sepsis can be caused by reduced megakaryocyte count, high sequestration and destruction of platelets due to infection, direct cytotoxicity from bacterial endotoxins, hemophagocytic lymphohistiocytosis, DIC, and drug-induced thrombocytopenia, and is commonly related to neonatal sepsis.26,29,30 In this study, thrombocytopenia was found in 44.7% of neonates with sepsis. This magnitude is in line with a study conducted by Bhat et al in which thrombocytopenia was confirmed in 41.7% of culture proven sepsis.31 Similarly, the current study result can be considered similar to a study done in the Netherlands reporting that thrombocytopenia was found in 49% of neonates with sepsis.28 In contrast, the observed thrombocytopenia can be considered a lower finding than a report in Pakistan with 61% and India with 83.5%.32,33 However, it is higher than a finding in Nepal, where the magnitude of thrombocytopenia was detected among 24.9% of culture positive cases.34 The discrepancy might be related to changes in environmental and genetic factors.
In the current study, the dominant types of bacteria isolated were CoNS, followed by Klebsiella Pneumoniae, and Staphylococcus aureus. A study conducted by Ghotaslou et al in Iran also confirmed that the most common organism was CoNS.35 The reason for the dominancy is mostly related to the fact that these bacteria are commonly found on skin and they may be introduced to the neonate during medical procedures or the bacteria may be introduced from the environment to the neonate during handling. The CoNS are also commonly found on NICU wards.36,37 These bacteria can cause mild to moderate disease and may aggravate adverse neurodevelopmental outcomes as well as chronic lung disease.38 Also, CoNS infections can cause increased hospital costs and prolonged hospital stays.36 Klebsiella infection is frequently linked to nosocomial infection. However, if the neonates are admitted to the hospital for any reason, Klebsiella bacteria can be introduced to the neonate during medical procedures, handling, or contamination from the environment.39 It may be due to a strong correlation between the colonization of neonates at sites such as the respiratory and gastrointestinal tracts and the subsequent development of infection.40,41 As a result, this bacteria can cause prolonged hospitalization, high service costs, and high mortality rates in neonatal patients.42
In this study, multivariate analysis affirmed that being female and having less than 7 days of age were significantly associated with anemia in neonates with sepsis. However, we failed to find a published literature to justify these factors. This indicates the lack of adequate investigations into the hematological abnormalities and factors associated with them in neonates with sepsis. Consequently, we recommended researchers to investigate the independent predictors of hematological abnormalities in neonatal sepsis.
This study had its own limitation. The clinical outcomes and markers of severity of illness were not evaluated in this study. We failed to investigate inflammatory markers such as c-reactive proteins and erythrocyte sedimentation rate and also a new diagnostic markers such as neutrophil to lymphocyte ratio, mean platelet volume, immature to total neutrophil ratio in the current study.
Conclusion
The magnitude of anemia and thrombocytopenia is high in neonatal sepsis. Besides, being female and aged group less than 7 days were determinant factors of anemia. Thus, the diagnosis and treatment of anemia, leucopenia, and thrombocytopenia prevents further complications in neonatal sepsis.
Abbreviations
CBC, complete blood count; CoNS, coagulase-negative staphylococci; CSF, cerebrospinal fluid; EOS, early-onset sepsis; Hgb, hemoglobin; LOS, late-onset sepsis; NICU, neonatal intensive care unit; NLF-GNR, non-lactose fermenter gram negative road; RBC, red blood cells; TLC, total leukocyte count; WBC, white blood cells.
Data Sharing Statement
All data generated and/or analyzed in this study are available within the manuscript.
Ethics Approval
The study was conducted after approval of the protocol by the Ethical Review Committee (ERC) of School of Biomedical and Laboratory Sciences, the University of Gondar.
Funding
The authors received no specific funding for this work.
Disclosure
The authors declare no conflicts of interest in relation to this work and that there is no conflict of interests regarding the publication of this manuscript.
References
1. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi:10.1016/S0140-6736(17)31002-4
2. Ogundare E, Akintayo A, Aladekomo T, Adeyemi L, Ogunlesi T, Oyelami O. Presentation and outcomes of early and late onset neonatal sepsis in a Nigerian Hospital. Afr Health Sci. 2019;19(3):2390–2399. doi:10.4314/ahs.v19i3.12
3. Gomella TL, Cunningham MD, Eyal FG, Tuttle DJ. Neonatology: Management, Procedures, on-Call Problems, Diseases, and Drugs. New York: McGraw-Hill Education Medical; 2013.
4. Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–826. doi:10.1542/peds.2010-2217
5. Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68:S24–S32. doi:10.1016/j.jinf.2013.09.011
6. Collaborators G. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017; 2018.
7. Harikrishnan S, Jeemon P, Mini G, Thankappan K, Sylaja P. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017; 2018.
8. Ranjeva SL, Warf BC, Schiff SJ. Economic burden of neonatal sepsis in sub-Saharan Africa. BMJ Glob Health. 2018;3(1):e000347. doi:10.1136/bmjgh-2017-000347
9. Assemie MA, Alene M, Yismaw L, et al. Prevalence of neonatal sepsis in Ethiopia: a systematic review and meta-analysis. Int J Pediatr. 2020;2020. doi:10.1155/2020/6468492
10. Gozdas HT, Gel KT, Yasayacak A, Kesgin MT, Akdeniz H. The role of hematological parameters in estimating nosocomial sepsis. Age. 2019;69:
11. Al-Gwaiz LA, Babay HH. The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract. 2007;16(5):344–347. doi:10.1159/000104806
12. Al Saleh K, AlQahtani RM. Platelet count patterns and patient outcomes in sepsis at a tertiary care center: beyond the APACHE score. Medicine. 2021;100(18):18. doi:10.1097/MD.0000000000025013
13. Straat M, van Bruggen R, de Korte D, Juffermans NP. Red blood cell clearance in inflammation. Transfus Med Hemother. 2012;39(5):353–360. doi:10.1159/000342229
14. Satar M, Arısoy AE, Çelik İH. Turkish Neonatal Society guideline on neonatal infections-diagnosis and treatment. Turk Pediatri Ars. 2018;53(Suppl 1):S88. doi:10.5152/TurkPediatriArs.2018.01809
15. Tiruneh T, Kiros T, Getu S. Hematological reference intervals among full-term newborns in Ethiopia: a cross-sectional study. BMC Pediatr. 2020;20(1):1–6. doi:10.1186/s12887-020-02320-5
16. Chandler WL, La Spada AR, Estergreen MT. Handbook of Diagnostic Hemostasis and Thrombosis Tests. labweb; 2015:03.
17. Kim F, Polin RA, Hooven TA. Neonatal sepsis. BMJ. 2020;371:1770–1780.
18. Celik IH, Hanna M, Canpolat FE, Pammi M. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. 2021;91:1–14.
19. Goyette RE, Key NS, Ely EW, editors. Hematologic changes in sepsis and their therapeutic implications. In: Seminars in Respiratory and Critical Care Medicine. 333 Seventh Avenue, New York: Copyright© 2004 by Thieme Medical Publishers, Inc.; 2004.
20. Organization WH. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. World Health Organization; 2011.
21. Bateman RM, Sharpe MD, Singer M, Ellis CG. The effect of sepsis on the erythrocyte. Int J Mol Sci. 2017;18(9):1932. doi:10.3390/ijms18091932
22. Effenberger-Neidnicht K, Hartmann M. Mechanisms of hemolysis during sepsis. Inflammation. 2018;41(5):1569–1581. doi:10.1007/s10753-018-0810-y
23. Vincent JL, Baron J-F, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–1507. doi:10.1001/jama.288.12.1499
24. Cai N, Fan W, Tao M, Liao W. A significant decrease in hemoglobin concentrations may predict occurrence of necrotizing enterocolitis in preterm infants with late-onset sepsis. J Int Med Res. 2020;48(9):0300060520952275. doi:10.1177/0300060520952275
25. Agnello L, Giglio RV, Bivona G, et al. The value of a Complete Blood Count (CBC) for sepsis diagnosis and prognosis. Diagnostics. 2021;11(10):1881. doi:10.3390/diagnostics11101881
26. Ognean ML, Boicean A, Șular F-L, Cucerea M. Complete blood count and differential in diagnosis of early onset neonatal sepsis. Rev Rom Med Lab. 2017;25(1):1–9.
27. Levi M, editor. Platelets in Critical Illness. Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers; 2016.
28. Ree IM, Fustolo-Gunnink SF, Bekker V, Fijnvandraat KJ, Steggerda SJ, Lopriore E. Thrombocytopenia in neonatal sepsis: incidence, severity and risk factors. PLoS One. 2017;12(10):e0185581. doi:10.1371/journal.pone.0185581
29. Vardon-Bounes F, Gratacap M-P, Groyer S, et al. Kinetics of mean platelet volume predicts mortality in patients with septic shock. PLoS One. 2019;14(10):e0223553. doi:10.1371/journal.pone.0223553
30. Vardon-Bounes F, Ruiz S, Gratacap M-P, Garcia C, Payrastre B, Minville V. Platelets are critical key players in sepsis. Int J Mol Sci. 2019;20(14):3494. doi:10.3390/ijms20143494
31. Bhat YR, Kousika P, Lewis L, Purkayastha J. Prevalence and severity of thrombocytopenia in blood culture proven neonatal sepsis: a prospective study. Arch Pediatr Infect Dis. 2018;6(2). doi:10.5812/pedinfect.12471
32. Shirazi H, Riaz S, Tahir R. Role of the hematological profile in early diagnosis of neonatal sepsis. Ann Pak Inst Med Sci. 2010;6(3):152–156.
33. Arif S, Ahmad I, Ali S, Khan H. Thrombocytopenia and bacterial sepsis in neonates. J Hematol Blood Transfus. 2012;28(3):147–151. doi:10.1007/s12288-011-0118-7
34. Gupta B, Gupta B, Shrivastava A, Chetri P. A study of neonatal sepsis and its relation to thrombocytopenia in Neonates of Tertiary Care Hospital of Western Nepal. J Preg Child Health. 2019;6(421):2.
35. Ghotaslou R, Ghorashi Z, Nahaei M. Klebsiella pneumoniae in neonatal sepsis: a 3-year-study in the pediatric hospital of Tabriz Iran. Jpn J Infect Dis. 2007;60(2/3):126.
36. Jean-Baptiste N, Benjamin DK, Cohen-Wolkowiez M, et al. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2011;32(7):679–686. doi:10.1086/660361
37. Huang Y-C, Wang YH, Chou Y-H, Lien R-I. Significance of coagulase-negative staphylococci isolated from a single blood culture from neonates in intensive care. Ann Trop Paediatr. 2006;26(4):311–318. doi:10.1179/146532806X152836
38. Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi:10.1001/jama.292.19.2357
39. Kim Y-K, Pai H, Lee H-J, et al. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother. 2002;46(5):1481–1491. doi:10.1128/AAC.46.5.1481-1491.2002
40. Mukherjee S, Mitra S, Dutta S, Basu S. Neonatal sepsis: the impact of carbapenem-resistant and hypervirulent Klebsiella pneumoniae. Front Med. 2021;8. doi:10.3389/fmed.2021.634349
41. Hill HR, Hunt CE, Matsen JM. Nosocomial colonization with Klebsiella, type 26, in a neonatal intensive-care unit associated with an outbreak of sepsis, meningitis, and necrotizing enterocolitis. J Pediatr. 1974;85(3):415–419. doi:10.1016/S0022-3476(74)80133-2
42. Hassuna NA, AbdelAziz RA, Zakaria A, Abdelhakeem M. Extensively-drug resistant Klebsiella pneumoniae recovered from neonatal sepsis cases from a major NICU in Egypt. Front Microbiol. 2020;11:1375. doi:10.3389/fmicb.2020.01375
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
