Back to Journals » Cancer Management and Research » Volume 10
Hematologic toxicity is rare in relapsed patients treated with belinostat: a systematic review of belinostat toxicity and safety in peripheral T-cell lymphomas
Authors Allen PB, Lechowicz MJ
Received 31 May 2018
Accepted for publication 1 September 2018
Published 6 December 2018 Volume 2018:10 Pages 6731—6742
DOI https://doi.org/10.2147/CMAR.S149241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Video abstract presented by Pamela B Allen.
Views: 383
Pamela B Allen, Mary Jo Lechowicz
Department of Hematology and Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, USA
Abstract: Peripheral T-cell lymphomas (PTCLs) are an aggressive and diverse group of lymphomas with a T-cell origin. Most patients progress following initial treatment and require salvage therapy. The burden of symptoms is high due to its extra-nodal presentation, high rate of advanced disease, and associated cytopenias combined with its predilection for an elderly population. The disease is generally incurable at relapse in the absence of transplantation and treatment is aimed at prolonging life and reducing disease-related symptoms. Belinostat is a histone deacetylate inhibitor that was granted accelerated approval by the US Food and Drug Administration on July 3, 2014, for the treatment of relapsed PTCL. Here, a systemic review was conducted to assess the safety and efficacy of belinostat. A safety analysis involved 512 patients with relapsed malignancies, and an efficacy analysis focused on patients with relapsed PTCL and included a total of 144 patients. Common adverse events were noted including fatigue (35%), nausea (42.8%), and vomiting (28.5%), but comparatively low rates of grade 3/4 hematologic toxicity overall (6.4%). Efficacy analysis demonstrated an overall response rate of 25.7% and complete responses of 10.4% with the majority of discontinuations occurring for lack of efficacy. Ultimately, these results demonstrate that belinostat has comparable efficacy to other agents used in this setting and is well tolerated in regard to hematologic events, but there is limited data on patient-reported outcomes, reduction in disease-related symptoms, or quality of life.
Keywords: peripheral T-cell lymphoma, belinostat, histone deacetylase inhibitor, hematologic toxicity, anaplastic large cell lymphoma, angioimmunoblastic T-cell lymphoma, NK/T-cell lymphoma, cytopenia
Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of aggressive lymphomas. They represent ~10% of non-Hodgkin lymphomas and consist of 23 distinct entities. Most patients present with advanced stage, which is avid by 18-fluoro-2-deoxyglucose-positron emission tomography.1 Extranodal involvement is common, as are immune-related symptoms and cytopenias. The diagnosis of PTCL and categorization into a specific subtype is generally based on a combination of pathologic and clinical criteria combining histology, immunophenotype, molecular studies, and clonality as detected on T-cell receptor gene rearrangement studies. The common PTCL subtypes include angioimmunoblastic T-cell lymphoma (AITL), anaplastic large-cell lymphoma (ALCL), and PTCL, not otherwise specified (PTCL, nos). Common types constitute ~60% of all cases. Rare subtypes are often extranodal in presentation and include extranodal NK/T-cell lymphoma, nasal type, HTLV-1-associated acute T-cell leukemia/lymphoma (ATLL), enteropathy-associated T-cell lymphoma (EATL), and monomorphic epitheliotropic intestinal T-cell lymphoma, among others.
The common varieties are treated similarly with comparable responses to treatment in the frontline setting.2 CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)-like regimens remain the standard of care for initial therapy among the common variants. The International T-Cell Project series demonstrated a 5-year failure-free survival of 60% in ALK (+) ALCL; 20% in ALK (–) ALCL, 18% in PTCL, nos, and 36% in AITL.3 Similarly, the British Columbia Cancer Agency series reported 5-year progression-free survival ranging from 13% to 28% for the common PTCL subtypes. Autologous transplant in first remission is generally recommended for all patients except ALK (+) ALCL, due to its relatively favorable prognosis, in spite of a lack of randomized data.4–6 However, few patients are able to receive an autologous transplant in this setting due to disease progression and lack of eligibility due to age, comorbidities, or poor performance status.6,7 Patients with rare subtypes generally fare worse and may be recommended for initial consolidation with allogeneic transplantation in the case of ATLL and hepatosplenic T-cell lymphoma due to poor outcomes with standard and salvage therapies.8 The majority of patients will relapse and require salvage therapy regardless of initial therapy. Young, fit patients are generally recommended to receive high-dose chemotherapy as a bridge to allogeneic or autologous transplantation. However, those unable to receive or relapse after salvage therapy have a dismal prognosis. One study demonstrated a median overall survival (OS) and progression-free survival after relapse of 5.5 and 3.1 months, respectively, in those unable to receive salvage chemotherapy and transplantation.9
The high degree of patient- and disease-related morbidities in relapsed PTCL, combined with the poor outcomes with standard therapy, necessitates the development of targeted therapies to fill this unmet need. Candidate drugs are beginning to be identified rationally by characterizing recurrent genetic and epigenetic alterations through gene expression profiles and next-generation sequencing and linking to specific phenotypes or T-cell subtypes.10–13 Recently, four targeted agents have been approved by the US Food and Drug Administration (FDA) for use as a single agent in relapsed/refractory PTCL on the basis of early-phase, single-arm studies: the histone deacetylase inhibitors (HDACis) belinostat and romidepsin, an anti-folate agent, pralatrexate, and the CD30 drug–antibody conjugate, brentuximab vedotin. However, studies linking genetic/genomic profiles to targeted agents are largely lacking.
The HDACis are a class of medications which result in epigenetic modifications. Emerging data have demonstrated frequent epigenetic modifications across PTCL subtypes, making this a rational approach for treatment.14 Recurrent epigenetic mutations include DNMT3A, TET2, and deregulation of histone-modifying enzymes such as histone methyltransferases and acetyltransferases. Unique patterns specific to particular T-cell subtypes are also identified including SETD2 methyltransferase in hepatosplenic TCL,10 enrichment of MLL methyltransferase family (particularly KMT2B and KMT2C) in Sezary syndrome,15 and BCOR mutations in NK/T-cell lymphoma and PTCL, nos.16 Modifications affect histones and DNA, altering transcription patterns, and predict for increased responsiveness to epigenetic class of drugs. Recent systemic analysis of the epigenomic landscape in CTCL suggests that certain epigenetic alterations that increase DNA accessibility and genes associated with immune activation are associated with improved clinical responses to HDACis.17 Unfortunately, the impact of HDACi on the transcriptome and predictors of response is lacking in general for PTCL.
There are two HDACis approved in the US for PTCL, romidepsin and belinostat, while chidamide is approved in China. Romidepsin was approved based on a pivotal single-arm, Phase II study of patients with relapsed or refractory PTCL, wherein it demonstrated an overall response rate (ORR) of 38% and a complete response (CR) rate of 18%.18 However, the median duration of response (DOR) was limited at 8.9 months. Belinostat is a hydroxamic acid-derived pan-HDAC inhibitor acting on all zinc-dependent HDAC enzymes. It is indicated for the treatment of relapsed PTCL following at least one line of therapy. Phase I studies in belinostat in solid tumors and hematologic malignancies identified the most common adverse events (AEs) to be nausea, vomiting, lethargy, fatigue, constipation, flushing, and diarrhea. There were no hematological or grade 4 AEs and the maximum tolerated dose (MTD) was found to be 1,000 mg/m2.19,20
Two Phase II trials have assessed the efficacy and safety of belinostat in PTCL at the recommended Phase I dose of 1,000 mg/m2 on days 1–5 every 3 weeks. Treatment in these trials was continued until death or unacceptable toxicity. Foss et al21 assessed belinostat in a Phase II study which included 24 patients with PTCL and 29 patients with CTCL. The median number of cycles was 2 (range 1–9). One patient in the PTCL cohort underwent one dose reduction (25%) for abdominal pain considered by the investigator to be related to belinostat. AE profile was similar in PTCL patients as previously reported, but additional toxicities including grade 3 AEs of paralytic ileus, pneumonitis, rash, and a grade 4 thrombocytopenia. A total of 20 patients discontinued therapy. Reasons for discontinuation included lack of efficacy (25%), physician decision (29.2%) or treatment-emergent adverse events (TEAEs) (12.5%), and death (12.5%).
The pivotal Phase II BELIEF trial22 was a non-randomized, open-label study of single-agent belinostat in 129 patients with relapsed or refractory PTCL. Unlike many PTCL studies, patients with low baseline platelet counts (≥50/μL) or more aggressive PTCL subtypes were eligible. Patients with adult or precursor adult TCL, prolymphocytic leukemia, T-cell Large granular lymphocytic leukemia, or primary cutaneous lymphomas were excluded. The ORR was 25.8%, with 18% CR. Among patients achieving a CR, the median DOR was not reached but exceeded 29 months. There was a difference by subtype with AITL achieving a superior ORR of 45.5%. However, only one of the patients with extranodal T-cell variants achieved a response. Importantly, belinostat monotherapy enabled 12 patients to undergo hematopoetic stem cell transplant, 10 of whom remained alive at data cutoff (OS range, 9.4–22.9 months). There were 22 deaths, and 12 of them were related to disease progression. Other AEs associated with death within 30 days included multiorgan failure (n=3), cardiac failure (n=2), lung infection (n=1), gastrointestinal (GI) hemorrhage (n=1), hepatic failure (n=1), and shock (n=1). Treatment withdrawal for TEAEs occurred in 19.4% of patients and was considered treatment related in 10.9%. TEAEs leading to withdrawal for more than one patient included multiple organ failure, fatigue, anemia, and febrile neutropenia (n=2). Overall, therapy was well tolerated. The most common AEs observed within the study were nausea, fatigue, pyrexia, anemia, and thrombocytopenia. Progressive disease in 64% of patients was the major cause for drug discontinuation. Other causes included death (11%), request by patient (8%), AEs (7%), and other (4%).
The goal of this review was to analyze the available literature to evaluate the impact of belinostat on patient-reported outcomes (PROs) in relapsed or refractory PTCL.
Methods
We performed separate analyses of safety and efficacy for belinostat monotherapy. Efficacy analyses included patients with relapsed/refractory PTCLs treated with belinostat. The National Library of Medicine’s OVID database was systematically searched for the terms: “belinostat” or “PXD 101” and “PTCL”. Results were limited to “clinical trials, all”, “case reports”, or “clinical studies” human subjects, English language, and published within the last 10 years. In order to limit publication bias, abstracts presented at the Annual Society of Hematology from the past 10 years were searched as well. The keywords “belinostat” and “peripheral T-cell lymphoma” were included.
For safety analyses, any patient with an oncologic diagnosis who was treated with belinostat monotherapy was included. To obtain relevant articles, the National Library of Medicine’s OVID database was systematically searched for the terms: “belinostat” or “PXD 101” AND “treatment” AND “cancer” in the form of abstracts, “clinical trials, all” limited to human subjects, English language, and published within the last 10 years.
The full text of potentially relevant articles was reviewed following initial title and abstract screening. Articles included in the efficacy analysis were included based on the following criteria: patients must have PTCL relapsed following at least one line of therapy and have been treated with single-agent belinostat. Only unique original cases which included efficacy results were included in the efficacy analysis. For the safely analysis, the same criteria applied, but we also allowed the addition of patients with other malignant diagnoses. For articles that reported both treatment-emergent and preexisting AEs, only the treatment-emergent events were included in the safety analysis. Data were then tabulated and summarized in a descriptive analysis.
Results
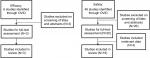
The efficacy analysis search returned a total of six articles. A total of three articles were included in the efficacy analysis. Three articles were excluded as they did not include data from patients with PTCL or patients did not receive treatment with belinostat (Figure 1).
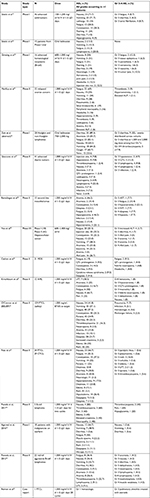
For the safety analysis, a total of 46 results returned and 15 articles, as well as one abstract, were analyzed. Overall, 31 articles were excluded for the following reasons: consisted of reviews or did not contain unique patients (n=22); involved combinations with chemotherapy (n=4); did not contain safety data (n=3); or were preclinical studies (n=2) (Figure 1, Table 1).
  | Table 1 Summary of contributing articles Abbreviation: PTCL, peripheral T-cell lymphoma. |
Additionally, the American Society of Hematology website was searched for abstracts published in Blood (supplements) for the past 10 years. A total of 32 abstracts were reviewed; 31 were excluded due to preclinical status (n=11); lack of treatment with belinostat (n=9); lack of patients with PTCL (n=1), included frontline therapy (n=2); or included subset data subsequently published in its full format (n=3). The remainder were excluded as they were not clinical studies (n=5). One abstract assessing belinostat treatment among patients with lymphoma was included in the safety analysis, but not included in efficacy because there were no details regarding lymphoma subtypes (Table 1).
This review is limited by the small number of studies involved, as well as the methods of reporting patient outcomes. For several of the safety outcomes, articles only reported symptoms that were present in more than one patient, and many did not differentiate between TEAEs and preexisting AEs. Additionally, some articles had incomplete data on all AEs and reported only the most common symptoms or serious AEs.
Safety
Belinostat is an HDACi that has been studied in a variety of malignancies including solid tumors and hematologic neoplasms with the aim of improving survival and reducing disease-related symptoms. All the cases included in the safety analysis were treated with belinostat therapy after progressing through a prior line of therapy. Studies combining belinostat with other systemic chemotherapeutic agents were excluded due to confounding effects on patient symptoms. Belinostat was evaluated in a total of 512 patients in 16 studies (Table 1). Study types included were largely Phase II (n=6) or Phase II (n=10) clinical trials, and one case report. The most common toxicities associated with belinostat were nausea (43%), vomiting (29%), fatigue (35%), constipation (16%), diarrhea (17.6%), anorexia (11.9%), fever (14.5%), dyspnea (7.8%), and hypersensitivity or injection site reactions (7.6%). Cardiac abnormalities largely consisted of QTc prolongations and were present in 4.3% of cases. Side effects occurring in <5% of patients included lethargy, flushing, dizziness, electrolyte imbalances, infections, pneumonitis, neurologic symptoms, pain, rash, and insomnia. Two patients had grade 2 cytokine release syndrome during belinostat infusion, which was managed with administration of steroids and reduction of the infusion rate. A total of 190 grade 3/4 toxicities were reported among 512 patients. Aside from hematologic AEs, the most common events were diarrhea (4.7%), fatigue (4.3%), neurologic (2.7%), and hypersensitivities (2.5%). Neurologic AEs included apraxia, status epilepticus, neuropathy, and headaches. Grade 3/4 infection occurred in five patients (1%). Other notable AEs included thrombosis in five patients (1%), pneumonitis in three patients, and tumor lysis syndrome in three patients (two were myeloma).
Hematologic AEs of any grade occurred in 26.6% of patients, and were largely characterized by thrombocytopenia (7.0%), anemia (12.7%), and lymphopenia (3.9%). Neutropenia was rated at 2.5% overall. Among the PTCL cases, the majority had underlying cytopenias that preceded treatment. The Phase I and II analyses in PTCL differentiated between treatment-emergent and preexisting cytopenias, and the data for TEAEs were reported. Grade 3/4 hematologic events occurred in 6.4% of patients. About 2.3% had a grade 3/4 thrombocytopenia, 1.2% anemia, and 1.4% neutropenia.
The largest safety analysis of belinostat in PTCL was included in the BELIEF trial.22 Given the high propensity for baseline cytopenias among patients with PTCL, including thrombocytopenia, patients were admitted on the trial if they had a baseline platelet count >50/μL (compared to the typical cutoff of 100/μL). Among 129 patients, only 2 had a serious adverse event related to thrombocytopenia that was considered to be related to the therapy. The authors concluded that belinostat was well tolerated, even in patients with platelet counts below 100,000/μL.22
Several dosing strategies were assessed over these 16 studies with an MTD identified at 1,000 mg/m2 IV over 5 days. Additional efforts to minimize toxicity included assessment of genetic polymorphisms that altered belinostat clearance. Plasma concentrations and incidence of hematologic toxicities were increased among patients receiving belinostat who carried the UGT1A1*28 and *60 variant alleles. Using a population pharmacokinetic analysis, a reduced dose of 400 mg/m2/24 hours (instead of 600 mg/m2/24 hours) was proposed for hepatocellular carcinoma patients homozygous for UGT1A1*28 or patients heterozygous or homozygous for UGT1A1*60.35 This genotype-based dosing recommendation is currently prospectively investigated at the NCI. Additionally, oral formulations of belinostat were assessed in two studies.24,26 No additional toxicities were noted with the oral vs IV formulation; however, there was an increased incidence of GI toxicities including diarrhea (25 patients), anorexia/decreased appetite (24 patients), nausea, and vomiting. In the Phase I study by Zain et al,26 diarrhea contributed to dose-limiting toxicities at the higher doses of belinostat of 1,500 and 2,000 mg/m2 oral. See Table 2 for a summary of studies included in the safety analysis.
Efficacy
PTCL patients from two Phase II trials were included in the efficacy analysis and one patient from a case report. A total of 145 patients were included, but data were calculated based on 144 patients (see Table 3 for details). Clinical characteristics are summarized in Table 4. About 55.2% were male and 22.8% female. The distribution of patients by race was as follows: 84.1% were white, 8.3% black, 6.9% Asian, and 3.4% other. The median age was 64 years. Performance status was only included for patients on the BELIEF trial, and the majority were performance status 0–1. By subtype, 62.8% were PTCL, nos, 17.4% AITL, 12.4% ALCL, and 6.8% other rare subtypes (EATL, extranodal T-cell lymphoma, hepatosplenic T-cell lymphoma [HSTCL], subcutaneous panniculitits-like T-cell lymphoma, PTCL, skin). About 15.1% presented as stage I–II, 33.1% as stage III, and 49.7% as stage IV.
The ORR was 25.7%, with 10.4% complete remissions and 15.3% partial responses. The DOR ranged from weeks to 39 months, with a maximum duration of treatment of 28 cycles.
Discussion
PTCL is an aggressive malignancy with high rates of relapse and a high symptom burden. Relapsed disease is rarely curable without allogeneic stem cell transplantation. However, the high median age at diagnosis and disease- and patient-related comorbidities render many patients ineligible for aggressive or curative approaches. The goals of treatment at relapse in this setting are to prolong disease-free survival and limit symptom burden. Therefore, treatments that are well tolerated and can be given continuously are often favored. Belinostat is FDA approved for relapsed PTCL based on two pivotal Phase II trials demonstrating efficacy and safety. Our review of the available data demonstrates that belinostat was well tolerated across malignancies (n=512) and effective in a subpopulation of patients with relapsed/refractory PTCLs (n=144). The ORR of ~25% and CR rate of 8%–23% seen in belinostat, are comparable to response rates of 25–38% and CR rates of 15–18% with romidepsin.18,36 The response rate is also similar to other FDA-approved agents for this indication including pralatrexate37 and brentuximab vedotin38 with the exception of brentuximab for the treatment of relapsed ALCL, which demonstrated an impressive response rate exceeding 80%.39
We report the largest systematic review of the toxicity of belinostat in relapsed malignancies. Our findings suggest the side-effect profile of belinostat is similar to other HDAC inhibitors with some critical differences: The rate of more than three hematologic AEs was lower in patients receiving belinostat, with only two reporting ≥3 grade febrile neutropenia across all belinostat studies and <10% rates of ≥3 grade hematologic toxicity, compared to data from the pivotal Phase II romidepsin trial which demonstrated higher rates of severe hematologic toxicity including 19% infections, 24% thrombocytopenia, and 20% neutropenia. The lower rate of hematologic toxicity has clinical implications, allowing for prolonged continuous treatment in patients retaining benefit, reduced need for transfusions, and reduced hospitalizations. This has additional implications for combination therapy, particularly when combined with myelotoxic chemotherapy regimens. In the Phase I/II trial of romidepsin combined with CHOP for frontline treatment of PTCL, there were hematologic dose limiting toxicities with combination therapy which included 89% of patients with neutropenia and 78% with thrombocytopenia, necessitating several dose reductions for romidepsin.40 The comparatively smaller belinostat plus CHOP, phase 1 combination study demonstrated a lower rate of hematologic toxicity with anemia (22%), neutropenia (17%), and thrombocytopenia was also less common.41 The most recently reported data demonstrate an ORR of 89% for the belinostat combination vs 68% for the romidepsin combination. We make note that the belinostat study is an ongoing Phase I trial and final results may report higher toxicity. The shortcomings of inter-trial comparisons notwithstanding, belinostat appears to limit hematologic toxicities either as a single agent or in combination therapy.
Cardiac toxicity including arrhythmias and QTc prolongation was rare with 4.3% of patients experiencing a related cardiac event. Eleven of 512 patients experienced a grade 3 or 4 cardiac AE. Five of these were due to QTc prolongation in a cohort of thymic cancer patients,27 suggesting an interaction between mediastinal disease and the increased incidence of cardiac events; 4 of the 11 cases were due to atrial fibrillation noted in a cohort of solid tumors.19 No grade 3/4 cardiac events were reported in studies of patients with PTCL. In the BELIEF study,22 independent central review of electrocardiogram data determined belinostat had no effect on cardiac repolarization. Additionally, pharmacokinetic and pharmacodynamic analyses showed no correlation between the concentration of belinostat and QTc changes. Overall, the cardiac abnormalities and QTc prolongation in these studies compare similarly to what was reported in the Phase II study of romidepsin in relapsed PTCL, where 6% of patients demonstrated cardiac abnormalities including 3% of patients with QTc prolongation.36
Similar to other classes of HDAC inhibitors, fatigue was a common toxicity in Phase I and II trials. One Phase I study assessed potential mechanisms for HDAC-associated fatigue. The investigators noted that patients had associated symptoms of flushing, fever, and chills, and theorized this could be related to cytokine release.19 Serum samples taken pretreatment and posttreatment demonstrated elevated IL-6 levels in two patients treated at the MTD who were identified as having clear drug-related fatigue, compared to those without fatigue. IL-6 has subsequently been evaluated as a potential biomarker of toxicity.
Our review was limited by the small number of publications in relapsed PTCL. Only two clinical trials for patients treated with belinostat were included. The safety analysis was limited by the inconsistent reporting of AEs across the trials. Certain AEs seemed related to diagnosis, as abdominal pain was strikingly increased among ovarian and hepatocellular carcinoma patients,25,29 and tumor lysis syndrome was reported only in the myeloma patients.20 Additionally, there was no data on PROs in any trials included in our safety or efficacy analyses. Clinical trials rely on standard reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) to capture symptoms. However, novel methods which incorporate PROs have recently been proposed.42 Incorporating PROs into clinical trials could more accurately describe relevant treatment-related toxicities. Furthermore, prospectively administered questionnaires including PRO-CTCAE items demonstrated favorable validity, reliability, and responsiveness in a large NCI sponsored feasibility study of patients undergoing cancer treatment.43 However, when instituted across a multicenter national trial, there was poor agreement between the investigator-reported and patient-reported symptoms, with investigators reporting fewer AEs than patients across symptoms, further highlighting the importance of the patient perspective.44
Additional concerns with the standard methods of reporting AEs are that each symptom is weighted equally regardless of its duration or impact on activities of daily living. An innovative method of incorporating quality-of-life measures into symptom assessments has been proposed. The quality-adjusted time without symptoms or toxicity (Q-TWiST) has been used to evaluate the clinical benefits and risks of oncology treatments by incorporating time into the analysis to more appropriately address quality of life during treatment.45 Q-TWiST incorporates the balance between toxicity and survival in order to evaluate the impact on quality and quantity of life. It is a multifaceted tool that delineates the impact of therapy onto three states: 1) time with grade 3 or 4 toxicity; 2) time without symptoms of disease or grade 3 or 4 toxicity of treatment (TWiST); and 3) time after tumor progression or relapse. The time spent in each state is then weighted to account for the patient’s quality of life and the final sum results in the Q-TWiST score.
Conclusion
PTCLs are an aggressive group of malignancies with limited but increasing options for therapy in the relapsed setting. Genetic analyses have demonstrated several recurrent mutations in epigenetic regulators and chromatin-modifying genes. HDACis are epigenetic modifiers that have demonstrated single-agent activity in the relapsed setting of PTCL, and are FDA approved for this designation. However, there is a lack of information on the interplay between the known genetic mutations involving epigenetic-modifying proteins and the efficacy and safety of drugs working on this pathway. Belinostat is an HDACi that has demonstrated safety and efficacy in broad studies of PTCL. Its safety profile demonstrates comparable rates of fatigue and GI toxicity but relatively few hematologic or cardiac events compared to romidepsin. Efficacy is similar to other agents approved in this setting with response rates approaching 30% overall. Small subset analyses have demonstrated increased rates of response among patients with AITL, and decreased responses in patients with rare and extra-nodal PTCL subtypes. Additional studies addressing the efficacy of belinostat in specific PTCL subtypes, particularly those with recurrent alterations in histone-modifying proteins such as AITL and HSTCL, would be provocative. Reporting of AEs in Phase I/II belinostat trials was generally according to the CTCAE standards; however, incorporation of PROs using tools such as the Q-TWiST and PRO-CTCAE would allow a well-rounded and comprehensive assessment of toxicity in future studies. This systematic review demonstrates that belinostat is well tolerated and efficacious in patients with relapsed PTCL; however, we were unable to assess its impact on patient symptoms due to the lack of reported data.
Disclosure
PBA is on the advisory board (with honorarium) of Bayer. MJL was a consultant for Allos Pharmaceuticals, Eisai Pharmaceuticals, Enzon Pharmaceuticals, and Gloucester Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15(10):1467–1475. | ||
Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123(17):2636–2644. | ||
Vose J, Armitage J, Weisenburger D, Project IT-CL, International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. . | ||
Fossard G, Broussais F, Coelho I, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol. 2018;29(3):715–723. | ||
D’Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093–3099. | ||
Wilhelm M, Smetak M, Reimer P, et al. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016;6(7):e452. | ||
Mercadal S, Briones J, Xicoy B, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol. 2008;19(5):958–963. | ||
Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23(11):1826–1838. | ||
Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970–1976. | ||
Mckinney M, Moffitt AB, Gaulard P, et al. The Genetic Basis of Hepatosplenic T-cell Lymphoma. Cancer Discov. 2017;7(4):369–379. | ||
Yoo HY, Sung MK, Lee SH, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(4):371–375. | ||
Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood. 2014;123(9):1293–1296. | ||
Crescenzo R, Abate F, Lasorsa E, et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015;27(4):516–532. | ||
van Arnam JS, Lim MS, Elenitoba-Johnson KSJ. Novel insights into the pathogenesis of T-cell lymphomas. Blood. 2018;131(21):2320–2330. | ||
da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47(12):1465–1470. . | ||
Dobashi A, Tsuyama N, Asaka R, et al. Frequent BCOR aberrations in extranodal NK/T-cell lymphoma, nasal type. Genes Chromosomes Cancer. 2016;55(5):460–471. | ||
Qu K, Zaba LC, Satpathy AT, et al. Chromatin Accessibility Landscape of Cutaneous T Cell Lymphoma and Dynamic Response to HDAC Inhibitors. Cancer Cell. 2017;32(1):e24:27–41. | ||
Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117(22):5827–5834. | ||
Steele NL, Plumb JA, Vidal L, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14(3):804–810. | ||
Gimsing P, Hansen M, Knudsen LM, et al. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol. 2008;81(3):170–176. | ||
Foss F, Advani R, Duvic M, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168(6):811–819. | ||
O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492–2499. | ||
Reimer P, Chawla S. Long-term complete remission with belinostat in a patient with chemotherapy refractory peripheral T-cell lymphoma. J Hematol Oncol. 2013;6:69. | ||
Steele NL, Plumb JA, Vidal L, et al. Pharmacokinetic and pharmacodynamic properties of an oral formulation of the histone deacetylase inhibitor Belinostat (PXD101). Cancer Chemother Pharmacol. 2011;67(6):1273–1279. | ||
Mackay HJ, Hirte H, Colgan T, et al. Phase II trial of the histone deacetylase inhibitor belinostat in women with platinum resistant epithelial ovarian cancer and micropapillary (LMP) ovarian tumours. Eur J Cancer. 2010;46(9):1573–1579. | ||
Zain JM, Foss FM, Diefenbach CS. Preliminary Results of An Ongoing Phase I Trial of Oral Belinostat a Novel Histone Deacetylase Inhibitor in Patients with Lymphoid Malignancies. Blood. 2011;118(21):3710. | ||
Giaccone G, Rajan A, Berman A, et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol. 2011;29(15):2052–2059. | ||
Ramalingam SS, Belani CP, Ruel C, et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol. 2009;4(1):97–101. | ||
Yeo W, Chung HC, Chan SL, et al. Epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma: a multicenter phase I/II study with biomarker and pharmacokinetic analysis of tumors from patients in the Mayo Phase II Consortium and the Cancer Therapeutics Research Group. J Clin Oncol. 2012;30(27):3361–3367. | ||
Cashen A, Juckett M, Jumonville A, et al. Phase II study of the histone deacetylase inhibitor belinostat (PXD101) for the treatment of myelodysplastic syndrome (MDS). Ann Hematol. 2012;91(1):33–38. | ||
Kirschbaum MH, Foon KA, Frankel P, et al. A phase 2 study of belinostat (PXD101) in patients with relapsed or refractory acute myeloid leukemia or patients over the age of 60 with newly diagnosed acute myeloid leukemia: a California Cancer Consortium Study. Leuk Lymphoma. 2014;55(10):2301–2304. | ||
Puvvada SD, Guillén-Rodríguez JM, Rivera XI, et al. A Phase II Exploratory Study of PXD-101 (Belinostat) Followed by Zevalin in Patients with Relapsed Aggressive High-Risk Lymphoma. Oncology. 2017;93(6):401–405. | ||
Agarwal N, Mcpherson JP, Bailey H, et al. A phase I clinical trial of the effect of belinostat on the pharmacokinetics and pharmacodynamics of warfarin. Cancer Chemother Pharmacol. 2016;77(2):299–308. | ||
Puvvada SD, Li H, Rimsza LM, et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520. Leuk Lymphoma. 2016;57(10):2359–2369. | ||
Goey AK, Figg WD. UGT genotyping in belinostat dosing. Pharmacol Res. 2016;105:22–27. | ||
Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631–636. | ||
O’Connor OA, Horwitz S, Hamlin P, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol. 2009;27(26):4357–4364. | ||
Horwitz SM, Advani RH, Bartlett NL, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014;123(20):3095–3100. | ||
Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. | ||
Dupuis J, Morschhauser F, Ghesquières H, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. Lancet Haematol. 2015;2(4):e160–e165. | ||
Johnston PB, Cashen AF, Nikolinakos PG. Safe and Effective Treatment of Patients with Peripheral T-Cell Lymphoma (PTCL) with the Novel HDAC Inhibitor, Belinostat, in Combination with CHOP: Results of the Bel-CHOP Phase 1 Trial. Blood. 2015;126(23):253. | ||
Bruner DW, Movsas B, Basch E. Capturing the patient perspective: patient-reported outcomes as clinical trial endpoints. Am Soc Clin Oncol Educ Book. 2012:139–144. | ||
Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051–1059. | ||
Basch E, Dueck AC, Rogak LJ, et al. Feasibility Assessment of Patient Reporting of Symptomatic Adverse Events in Multicenter Cancer Clinical Trials. JAMA Oncol. 2017;3(8):1043–1050. | ||
Husson O, Jones RL. Q-TWiST: what really matters to the cancer patient? Cancer. 2017;123(12):2200–2202. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.