Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Heart Rate Variability in Psychiatric Disorders: A Systematic Review
Authors Ramesh A, Nayak T , Beestrum M, Quer G, Pandit JA
Received 18 July 2023
Accepted for publication 11 October 2023
Published 20 October 2023 Volume 2023:19 Pages 2217—2239
DOI https://doi.org/10.2147/NDT.S429592
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Ashvita Ramesh,1,* Tanvi Nayak,2,* Molly Beestrum,2 Giorgio Quer,3 Jay A Pandit3
1Department of Medicine, Massachusetts General Hospital, Boston, MA, USA; 2Northwestern University Feinberg School of Medicine, Chicago, IL, USA; 3Scripps Research Translational Institute, La Jolla, CA, USA
*These authors contributed equally to this work
Correspondence: Tanvi Nayak, Tel +1 602-791-3338, Email [email protected]
Introduction: Heart rate variability (HRV) is a measure of the fluctuation in time interval between consecutive heart beats. Decreased heart rate variability has been shown to have associations with autonomic dysfunction in psychiatric conditions such as depression, substance abuse, anxiety, and schizophrenia, although its use as a prognostic tool remains highly debated. This study aims to review the current literature on heart rate variability as a diagnostic and prognostic tool in psychiatric populations.
Methods: A literature search was conducted using the MEDLINE, EMBASE, Cochrane, and PsycINFO libraries to identify full-text studies involving adult psychiatric populations that reported HRV measurements. From 1647 originally identified, 31 studies were narrowed down through an abstract and full-text screen. Studies were excluded if they enrolled adolescents or children, used animal models, enrolled patients with another primary diagnosis other than psychiatric as outlined by the diagnostic and statistical manual of mental disorders (DSM) V, or if they assessed HRV in the context of treatment rather than diagnosis. Study quality assessment was conducted using a modified Downs and Blacks quality assessment tool for observational rather than interventional studies. Data were reported in four tables: 1) summarizing study characteristics, 2) methods of HRV detection, 3) key findings and statistics, and 4) quality assessment.
Results: There is significant variability between studies in their methodology of recording as well as reporting HRV, which makes it difficult to meaningfully interpret data that is clinically applicable due to the presence of significant bias in existing studies. The presence of an association between HRV and the severity of various psychiatric disorders, however, remains promising.
Conclusion: Future studies should be done to further explore how HRV parameters may be used to enhance the diagnosis and prognosis of several psychiatric disorders.
Keywords: heart rate variability, psychiatry, digital health
Introduction
Psychiatric disorders affect 21% of adults and 49.5% of adolescents in the United States (US), growing in prevalence in recent years.1 While some disorders gradually develop over time, others can present with acute crises requiring immediate intervention, which can make the diagnosis of these conditions challenging for physicians. In addition, patients with psychiatric disorders and similar comorbidities can have vastly different clinical courses and prognoses, sometimes for reasons unknown.2
Psychiatric disorders are associated with a range of impairments including executive functioning, learning, and memory. Recent studies have shown that similar impairments can also be seen in autonomic function or involuntary regulation of processes including heart rate, blood pressure, and respiration, and are correlated with severity of the disease process. According to European Society of Cardiology in North America, heart rate variability (HRV) is one of the most promising markers of autonomic function.3 Heart rate variability (HRV) is a measure of the fluctuation in time interval between consecutive heartbeats (Figure 1). HRV has been thought to provide insight into the tightly regulated signaling pathways of the autonomic nervous system and may serve as a surrogate measure of balance between the brain and the cardiovascular system. Decreased heart rate variability has been associated with autonomic dysfunction in a variety of conditions, likely as a result of an imbalance between parasympathetic and sympathetic input on heart rate and rhythm.
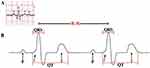 |
Figure 1 Heart rate variability beat-to-beat interval as derived from an ECG reading: (a) Example of ECG segment (b) Expansion of ECG reading with R-R interval labeled. Reprinted with permission from Dong J. The role of heart rate variability in sports physiology. Exp Ther Med, 2016;11(5):1531–1536.4 |
Prior studies have suggested that dysregulation of autonomic signaling contributes to the pathophysiology of psychiatric conditions such as depression, substance abuse, panic disorder, anxiety, and schizophrenia.5,6 This can be seen with somatic manifestations of psychiatric conditions such as panic disorder and anxiety, in which parameters such as heart rate and respiratory rate are increased during episodes with decreased vagal tone responsiveness.7 Additionally, the degree of autonomic dysfunction in psychiatric conditions may correlate to increased symptom severity and worse overall outcomes, as shown in previous studies of patients with schizophrenia and panic disorder.8
HRV remains highly debated in its use as a diagnostic tool due to its variation based on the individual person, as there are differences within patient populations based on sex, age, and BMI; however, with the increasing number of wearable devices with HRV measuring capability, it is imperative to better understand the potential for HRV to provide diagnostic and prognostic information. Current limitations in the field include the ability to diagnose and detect psychiatric conditions prior to an acute mental health crisis or to predict the prognosis of a psychiatric disorder in its initial stages.9 Utilizing markers such as HRV variation in mental health disorders early-on may allow providers to better understand the development of psychiatric conditions and provide intervention proactively.10 Furthermore, HRV might serve not only as a diagnostic tool but also one that helps understand variability in disease trajectory and response to treatment.
Previous studies have analyzed HRV via time domain and frequency components, with time domain calculations often being simpler to perform.5 Time domain analyses are further broken down into root mean square of RR intervals (RMSSD) or standard deviation of NN intervals (SDNN) with artifact often removed by the individual program.5 Similarly, HRV frequency domain consists of a power spectral analysis with low frequency (LF-HRV) defined as between 0.04 and 0.15 Hz and high frequency (HF-HRV) between 0.15 and 0.4 Hz, suggested to represent autonomic input divided based on sympathetic vs parasympathetic signals, although this distinction remains a topic of debate, as there still remains an overlap between sympathetic and parasympathetic contributions to heart rate regulation and a clear cutoff may not truly exist. The ratio of LF to HF (LF/HF), representing sympatho-vagal balance under controlled conditions, may indicate imbalances in autonomic function in certain disorders.11,12
In this review, we aim to systematically analyze studies investigating HRV in populations of psychiatric disorders, evaluating the efficacy of HRV as a diagnostic and prognostic tool in psychiatric conditions as outlined by the diagnostic and statistical manual of mental disorders (DSM) V.
Methods
Identification of Studies
A literature review was performed based on the Preferred Reporting Items for systematic reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews. A summary of the search strategy employed is shown in Figure 2.
 |
Figure 2 Flow diagram outlining identification of studies via databases and registers. |
We created a search strategy focused on mental health, mental disorders, anxiety or mood disorders, autonomic nervous systems, biomarkers, heart rate variability, prognosis, and predictive value. The search strategies were performed in MEDLINE (Ovid 1946-), EMBASE (Elsevier 1947-), Cochrane Library (Wiley), and PsycINFO (EBSCOHost). Search strategies for Embase, Cochrane Library, and PsycINFO databases were searched from inception with no date limits. Inclusion criteria were full-text studies written in English with patients above 18 years of age and reporting traditional HRV parameters in patients diagnosed with a DSM V psychiatric disorder.
Articles were excluded based on the following criteria: study type, incorrect target population (<18 years old), medical field other than psychiatry, not original research, topic not within scope or HRV parameters not reported. Studies were excluded if they enrolled pediatric patients <18 years old, focused on treatment efficacy rather than disease diagnosis or natural course, or focused on HRV as a predictor of a non-psych condition. For example, studies that evaluated a psychiatric medication and its impacts on HRV were excluded. Additionally, studies that focused on patients with another major diagnosis (eg, heart failure) who presented with psychiatric symptoms during their course were solely excluded, as these studies did not focus on a psychiatric condition or its relationship with HRV. Searches were completed on March 31, 2022.
From the initial search, 2098 sources were identified across various databases, of which 451 duplicates were removed prior to screening. Of the 1647 studies that were screened via a title/abstract screen, 1284 studies were excluded due to not meeting inclusion criteria of study type, target population, or topic not within the scope of HRV. 182 studies were selected for a full-text screen, of which 32 reports were not able to be retrieved for a full manuscript, leaving 150 manuscripts to be reviewed in depth. Of the 150 full-text sources, reports were excluded if they focused on a non-psychiatric primary diagnosis, if they were mainly evaluating a medication or treatment strategy, if they did not have original data, or if they enrolled pediatric or adolescent patients <18 years old. The final selection of studies yielded 31 eligible studies focusing on patients with psychiatric diagnoses and their respective HRV analyses.
Data Extraction
Data were extracted by two independent reviewers (A.R. and T.N.). All conflicts were resolved through discussion by the reviewers. All identified studies were exported to Rayyan, and an automatic duplicate finder was applied. References to relevant studies are also reviewed to identify additional manuscripts. Data from the studies were transferred to a standardized Excel sheet recording study title, study design, author, year of publication, number of subjects, mean patient age, proportion of male subjects, study aim and outcome, protocol for measurement, analysis and reporting of HRV parameters, and quality assessment. We identified the following clinical confounding factors: age, sex, mean HR, BMI. Studies that incorporated clinical confounders in their analysis were noted in our analysis of quality.
Risk of Bias and Study Quality Assessment
The quality of each publication was assessed based on selection bias, performance bias, attrition, detection, and reporting bias, based on the Downs and Blacks assessment tool. Questions 4, 8, 14, 15, 19, 23, 24, and 27 were removed as they asked about medications/treatment interventions that were specific to randomized controlled trials. Since our final selection of studies mainly included cohort and case–control studies, these questions did not apply for our review.
Results
Study Selection
We identified 1647 studies via title, abstract, and full-text searches pertaining to heart rate variability in patients diagnosed with DSM V psychiatric disorders. Inclusion criteria consisted of adult populations investigating heart rate variability as a diagnostic or prognostic factor in patients with psychiatric diagnosis. From the 1647 studies, inclusion was narrowed to studies that focused on heart rate variability as an outcome measure. First, studies were evaluated with an abstract and title screen. Studies that enrolled adolescents or children and studies that used animal models were excluded, narrowing down the initial 1647 studies to 239 studies. Of this subset, only 182 studies had a full-text manuscript published. A full-text screen was conducted on these 182 studies using additional exclusion criteria against studies that were primarily intervention focused or reported psychiatric symptoms only as secondary diagnosis to another primary diagnosis. These criteria were applied to exclude any studies that focused on HRV in patients with primary diagnoses other than psychiatric ones, as well as studies that analyzed HRV in the context of response to psychotropic medications rather than primary disease processes. From the full-text screen and based on the above criteria, we identified 31 studies consisting of 5496 patients were included for final review.
Study Characteristics
All of the included studies were either case-control or cohort studies, focusing on a wide variety of psychiatric populations, including depression, anxiety, PTSD, schizophrenia, and panic disorder. Eighteen out of thirty-one studies were case–control studies that recruited controls as well as patients with psychiatric conditions. Demographic information for each of the studies can be found in Table 1. The most common psychiatric diagnosis studied was PTSD, with seven studies (23%) investigating this disorder. The study cohort populations varied greatly in size, with the largest study recruiting 2430 participants compared to the smallest study enrolling 28 participants. Jacobson et al recruited participants with the youngest average age, at 19.56, while the study with the oldest participants was Ha et al with an average age of 65.2.13,14 The sex distribution of studies varied greatly, with eight studies reporting a breakdown of 50% ± 5% between male and female participants. Ishizawa et al, Carnevali et al, and Krause-Utz et al recruited only female participants, while Reinertsen et al and Minassian et al enrolled only male participants.15–19
 |
Table 1 Study Characteristics |
HRV Detection and Analysis
All studies calculated HRV parameters, although some focused on time-domain measurements, which include R–R wave intervals (RRI), root mean square of successive differences between normal heartbeats (RMSSD), mean of the standard deviation for all N–N intervals (SDNN), as well as frequency-domain measurements, such as high frequency (HF) HRV, and low frequency (LF) HRV as obtained by different heart rate monitors (Table 2). Additionally, some studies reported power, which is derived from frequency domain and can be measured in ms2/Hz for absolute power or as a percentage for relative power.5
 |
Table 2 HRV Detection and Analysis |
As studies were conducted over a wide range of time, the earliest being Kawachi et al in 1995, and the most recent paper, Jacobson et al published in 2022, there was a broad variety of the types of technology and techniques used for HRV measurement and analysis.13,20 Thirteen out of 31 studies used standard ECG monitoring with electrodes to record heart rate, while the remaining used various wearable or ambulatory monitoring devices such as Holter monitors. One study, Jacobson et al, used a smartphone camera to obtain vital measurements.13 Three studies used PPG readings to derive HRV parameters from.19,37,40 Twenty-two studies named the specific analysis software used to breakdown HRV into time-domain and frequency domain components; the most commonly used software was KUBIOS, which was used in nine of the studies reviewed. Similarly, the frequency at which HRV analysis was run varied between studies, with only 14 studies listing the exact number. Out of these, six studies ran analyses at frequencies higher than or equal to 1 kHz. Additionally, time of HRV measurement was not widely reported with other HRV parameters, which may result in variations between studies as it is used in calculating each derived HRV variable.
Presence of artifact in HRV recordings is relatively common but can significantly distort both time-domain and frequency-domain measures derived from recordings. Twenty-four studies in this review manually inspected and removed artifact and corrected for missed beats (Table 2). Studies that did not specify or clearly state whether there was an artifact removal process were marked as “n”. The artifact removal process as described by Valenza et al involves visual inspection of the signal, along with an algorithm designed to detect artifact using simple statistical thresholds as a cutoff point to distinguish the real signal.27
Furthermore, studies recorded HRV with varying measurement settings/protocols. In the study by Valenza et al, measurements were taken during the evening both at rest and while performing daily activities. In other studies, such as Kang et al, measurements of HRV were taken over a five-minute period during rest in a seated position. Due to differences in study designs, HRV reporting, study methodology, confounding, comparator groups, and data reporting, a meta-analysis was not performed.
Study Qualities
Roughly half of the studies (53%) compared patients with a psychiatric diagnosis to healthy controls, while the remaining focused on a cohort of patients with a specific diagnosis. Clinical confounders that may affect HRV include age, sex, BMI, and resting HR, which have been previously shown to influence HRV.43 However, many studies recruited healthy controls without matching for age or sex. Only 15 studies (48%) provided a complete list of clinical confounders that were acknowledged between comparator groups. Five studies included only women or only men to eliminate the confounding impact of sex on heart rate variability measurements.15–19 Only six studies recruited healthy controls that were age-matched to the group with psychiatric diagnoses. None of the studies matched participants based on BMI or HR, suggesting that these confounders may play a role in affecting the outcomes of HRV that were reported.
External validity analysis was conducted using the Downs and Black assessment tool, with some questions removed that asked about interventions, directed at randomized controlled trials rather than case-control or cohort studies (Table 3). The Downs and Black score can be broken down into sub-scales as follows: reporting, external validity, bias, confounding, and power. For this review, although questions that were not relevant were removed from each section, we can still compare strengths and limitations of the studies based on their sub-scores. For the external validity sub-score, only two studies scored a 2/2 for achieving random sampling of a population, determined by reporting the proportion of the total population that participated in the study, as indicated by the Downs and Black paper. The remaining studies received a 0/2 in this sub-score for not specifying whether patients were randomly sampled or for recruiting consecutive patients. Similarly, only two studies reached a 3/3 score on the selection bias sub-category for succeeding to provide a list of clinical confounders, include them in the analyses, and specify the time-period in which participants in the control vs treatment group were recruited. This indicates that the remaining studies either failed to report this information or did not include confounders in their analysis of data. Twenty-one out of 31 studies reported an exact probability value in their statistical analysis section, while the remaining either reported a range such as p<0.05 or did not report numbers.
 |
Table 3 Downs and Blacks Quality Assessment Tool |
Study Outcomes and Key Findings
The main purpose of this review is to examine how HRV may be used clinically as a diagnostic tool in various psychiatric conditions. Twenty-eight out of 31 studies report a significant association between a component of HRV and the psychiatric condition they focused on. The remaining three studies, Valenza et al, Gaul-Alacova et al, and Jacobson et al, either reported descriptive statistics or did not find a statistically significant association.13,22,27 Out of the seven studies focusing on PTSD, D’Souza et al, Krause-Utz et al, Pyne et al, Dennis et al, and Minassian et al reported significantly lower HF-HRV in patients with PTSD, while Reinertsen et al and Dennis et al reported significantly lower LF-HRV in PTSD patients.16,18,19,23,29,30,44 Hopper et al found a significant difference in resting heart rate between PTSD patients and control patients; however, they did not report a significant change in LF-HRV.23
Similarly, of the six studies investigating HRV in patients with anxiety, Kawachi et al and Alvares et al found that SDNN, a time component variable of HRV, was significantly lower in the group of patients with anxiety compared to healthy controls.6,13,17,20,22,40,41 Forte et al demonstrated a negative association between LF and HF HRV components and anxiety.41 Results of the Carnevali et al study showed that RMSSD was decreased at rest in patients with generalized anxiety disorder compared to controls.17 Gaul-Alacova et al found a decreased total score of HRV in patients with anxiety compared to healthy controls, suggesting a problem with autonomic dysregulation, although not statistically significant.22 Jacobson et al reported findings of a deep learning model designed to analyze HRV in patients with anxiety that was able to predict the majority of variations correlating with anxiety symptoms; however, p-values did not meet statistical significance.13
Six studies enrolled patients with depression to investigate the relationship between HRV and depressive symptom severity and disease progression.14,19,24,28,31,36 Sung et al reported higher HF-HRV in patients with depression and traumatic brain injury compared to controls, while Minassian et al, Drago et al, Tessier et al, and Ha et al found lower levels of HF-HRV in patients with depression compared to healthy controls.14,19,24,28,31 Additionally, Tessier et al demonstrated a lower LF/HF ratio in patients with depression.31 Byun et al found decreased RRI, a time-domain measurement of HRV, to be associated with increased depressive symptom severity.36
The remaining studies focused on other psychiatric populations including patients with psychosis, schizophrenia, anorexia nervosa, and panic disorder, with significant findings summarized in Table 4.15,21,25,27,32,33,35,40,42
 |
Table 4 Study Objectives, Key Findings, Relevant Statistics |
As discussed previously, LF/HF ratio as an outcome variable has been suggested to represent sympatho-vagal balance of the autonomic system. Byun et al, Hartmann et al, Minassian et al, Sung et al, Valenza et al, and Tessier et al collected LF/HF ratio as an outcome variable.19,28,31,36 Sung et al, Byun et al, Hartmann et al, and Minassian et al reported a significant decrease in the LF/HF ratio in their patients with psychiatric diagnoses including depression and PTSD, respectively.19,28,34,36 These findings suggest a potential in monitoring trends of HRV in order to diagnose patients and to monitor treatment efficacy in psychiatric conditions.
The statistics column in Table 4 highlights relevant p-values that each study reported supporting their main findings (Table 4). As mentioned above, three out of the 31 studies did not report any statistical probability values, either due to not having statistical significance or due to using alternative methods in evaluating their data, such as relying on descriptive statistics.13,22,27 Out of the remaining twenty-eight studies, twenty-four studies used exact numbers for p-value reporting, while Byun et al, Kimhy et al, Sung et al, and Hopper et al used ranges such as p <0.05 to indicate statistical significance.23,28,32,36 The p-values listed in this column were extracted from the results section and were related to the main findings reported in the paper. For example, Ahs et al investigated the relationship between the HF component of HRV and regional blood flow in patients with social phobia.25 They reported a significant association between HF-HRV and regional cerebral blood blow in the area of the anterior cingulate cortex, with a significant p-value = 0.037. Similarly, other studies that compared patients with psychiatric conditions to healthy controls reported p-values in their comparison between groups. Another example of reporting results using two concurrent psychiatric diagnoses was in Krause-Utz et al, where they compared patients with both BPD and PTSD to healthy controls and found a significantly lower HF-HRV in the patients with both diagnoses compared to BPD alone and compared to healthy controls, with a p value of 0.012 and 0.022, respectively.16 Methods of statistical analysis varied across studies, most commonly with ANOVA and multiple regression analyses; however, for simplicity and comparison purposes, p-values are included in Table 4 to highlight which studies reported results that met statistical significance.
Discussion
This review aims to understand the role of HRV in psychiatric conditions, with which we hope to demonstrate its potential as a clinical diagnostic tool. As previous studies have demonstrated, HRV analysis may provide a useful perspective into monitoring chronically ill patients and predicting adverse outcomes.45 Current standard tools for diagnosis of psychiatric conditions include thorough history-taking, behavioral assessment, and self-guided questionnaires in accordance with established guidelines such as the DSM 5.46 However, the main roadblock that clinicians currently face is in identifying and diagnosing psychiatric conditions prior to the onset of acute symptomatic crisis, or predicting prognostic factors that may warrant additional treatment measures. HRV monitoring may be a useful addition to current standards in predicting psychiatric patients’ prognoses and response to treatment.
The most reported psychiatric condition in the studies reviewed was PTSD (21%), followed by depression and anxiety (15%). Identification and diagnosis of these conditions varies greatly, especially in the setting of acute mental health emergencies. Although the diagnosis of psychiatric conditions relies on specific criteria outlined by the DSM V, there remains variability and uncertainties regarding disease severity and prognosis. Given that collateral information as well as the specific history obtained often have a tremendous impact on a psychiatrist’s assessment of a patient’s condition, the addition of more objective parameters such as vitals or lab values obtained in real-world settings might aid a physician in both diagnosing their patients and personalizing patients’ treatment plans.
In a study of veterans diagnosed with PTSD, Sadeghi et al analyzed heart rate data and found that PTSD hyperarousal events can be identified by unique heart rate patterns.47 This is consistent with the findings of our systematic review in demonstrating an association with HRV, but diagnostic power has still not been demonstrated. For example, Hopper et al demonstrated an elevated basal HR in patients with PTSD.23 Although Hopper et al did not appreciate a significant difference in LF-HRV or RMSSD, Minassian et al and Dennis et al demonstrated lower HF-HRV and LF-HRV in patients with PTSD. Specifically, Minassian et al showed that high LF/HF ratio prior to deployment was significantly associated with post-deployment diagnosis of PTSD in active-duty marines. A better understanding of the effect of PTSD on the sympathetic and parasympathetic nervous systems is needed, and studying HRV might be useful in uncovering this association. Although the focus of this systematic review was on the role of HRV in the diagnosis and understanding of specific DSM V disorders, it is critical to understand the potential that HRV holds in helping physicians evaluate the necessity and efficacy of alternate treatment options for patients with suspected PTSD. An example of a targeted PTSD therapy based on the interaction between the autonomic nervous system and its effects on heart rate was studied by Breit et al, who report preliminary evidence that vagus nerve stimulation can aid outcomes for treatment-refractory depression and PTSD.48 Additional studies into the treatment of PTSD and its effect on HRV would allow clinicians to utilize HRV monitoring to evaluate response to targeted therapies when other refractory agents have failed.
Few systematic reviews in the current literature have evaluated the relationship between HRV and the natural disease course of major depressive disorder. Our review shows that results from the studies included suggest that patients with depression have higher baseline HR and lower HRV. Additionally, there is a correlation between lower HRV and depression symptom severity, which can be very useful as a clinical prognostic factor to create adequate treatment regimen to prevent acute symptom crisis. Several studies have evaluated whether HRV might predict treatment response for patients with depression. In a paper by Choi et al, the study aimed to evaluate whether baseline HRV parameters could predict response to various antidepressant medications such as SSRI, SNRI, and TCA; they found that the change in low frequency to high frequency was positively associated with 12-week treatment response.49 This supports clinical use of HRV to not only predict the natural course of psychiatric conditions such as major depressive disorder but also to measure response to treatment with first-line agents such as SSRI medications.
With regard to schizophrenia, Kimhy et al found a significant negative association between HF-HRV and the severity of auditory hallucinations.32 Hattori et al and Haigh et al demonstrated reduced HRV, analyzed with different methodologies, in patients with schizophrenia.33,42 Our review findings are consistent with the current literature on HRV and schizophrenia, which have previously reported on associations between increased resting heart rate and reduced vagal modulation as reflected by decreased HRV. In a study by Refisch et al, researchers analyzed a genetic variant, the HCN1 gene, that predisposes patients to developing schizophrenia, and found an increased association in HRV deviations in patients that were homozygous compared to heterozygous for the variant.50 Another study by Wang et al found that SDNN was significantly associated with increased aggression in hospitalized patients with schizophrenia, suggesting utility in HRV as a monitoring tool for inpatient episodes of both verbal and physical aggression.51 Prognosis and prediction of symptom severity in patients with schizophrenia remains a current limitation that clinicians face while managing acute inpatient episodes, as well as preventing future episodes. As discussed previously in the management of patients with depression and PTSD, HRV may also be useful in monitoring treatment response in schizophrenia. In a study comparing HRV response between unmedicated patients with schizophrenia to patients on olanzapine or haloperidol, there was a significant difference in LF-HRV, suggesting an effect on autonomic regulation as a result of treatment in these patients.52 These findings reflect a potential role for HRV as a monitoring tool for diagnosis, symptom severity, and response to antipsychotic medications; further studies would allow for a better understanding of both the benefits and limitations of HRV in this setting. Additionally, the relationship between schizophrenia and the development of heart failure has been studied in the past, which suggests another practical implication of prognosis and monitoring schizophrenia using HRV beyond the field of psychiatry and into assessment of overall cardiovascular mortality.53
In the studies selected in this review, patients were enrolled and consented to HRV monitoring via a variety of methods and selection criteria. As HRV data is often obtained from wearable devices and entails routine monitoring beyond an outpatient office visit, ethical sourcing of data should be ensured with the patient’s informed consent and participation, along with a discussion of potential data privacy issues and risks. For example, Jacobson et al utilized smartphone sensors to monitor HRV in patients with anxiety.13 Patients were asked to download an application on their device that recorded information including GPS location and number of outgoing phone calls to correlate anxiety symptom severity with social interactions, exposure to light, and environmental factors. Participants were informed of the frequency and duration of device monitoring (once per hour through the app), as well as potential risks such as data security and privacy. Privacy must be an important ethical consideration in HRV monitoring with study designs involving at-home wearable devices and use of personal smartphones, with a proper discussion of the risks and benefits with each participant prior to their enrollment, which Jacobson et al demonstrated in their study protocol. Similarly, methods used for data handling and security after HRV variables are initially collected should be explicitly stated in study design protocols. The majority of studies outlined their respective study protocols for ensuring data security during analysis.
Although variability existed in the method of analysis and patient population in the included studies, certain trends were apparent even across diagnoses. For example, patients with social anxiety disorder demonstrated changes in HF-HRV, which correlated to blood flow in the anterior cingulate cortex according to Ahs et al. Similarly, per Alvares et al, patients with symptoms of phobic anxiety had lower SDNN and a smaller range of HR values.6 Generally speaking, higher heart rates have been associated with lower HRV. Thus, ideally, the association between HRV with a disease should be analyzed after controlling for HR. Overall, patients with psychiatric disorders seem to have lower variability in HR with a higher basal HR. It is important to note that a smaller range of HR values has been associated with increased risk of cardiac conditions, including heart failure and sudden cardiac death. Thus, it is critical to better understand and characterize this trend in heart rate variability.54,55
Studies were selected and narrowed down via the search method outlined in Figure 1, using MEDLINE, Cochrane, EMBASE, and PsycInfo library databases. However, it should be noted that the selection of studies was limited by publication bias, as there may have been additional contributions to the field that were not published in these databases. Future inclusion of studies beyond those that are published would provide a more comprehensive review of the current literature.
Potential sources of bias in the studies reviewed may come from methods in recording HRV. Measurement techniques for HRV varied widely, with some using ambulatory monitoring devices, while others used standard lead ECG readings. One study even included a smartphone camera as the device used to record HRV, which is much less reliable than a wearable device or other method of measurement. Additionally, among the studies reviewed, HRV analysis was conducted via different platforms, with 27% using the KUBIOS HRV package compared to others using MATLAB or SAS. The frequency of analysis may impact HRV reporting. Past research has noted that a reliable frequency range is between 250 and 500 Hz or higher, but as no recent work has been done to confirm this, there remains uncertainty about this topic.56 In our review, two studies reported frequencies less than 200, which may alter the precision of their results. Furthermore, a major source of bias is length of recording, with shorter recordings being much more affected by activity during recording. Finally, reporting of statistical analyses varied across studies, which should be acknowledged as a factor that limited the robustness of this review. In order to identify which studies had significant findings, discussion of statistics was limited to p-value reporting and highlighting studies that met significance with p < 0.05. However, a future review with a more in-depth analysis of the specific statistical tests that were run in each study could add additional nuance and robustness to the interpretation of HRV findings.
The studies included in this review were assessed for quality using the Downs and Black assessment tool, which was previously validated for evaluating study quality, external validity, bias, confounding and selection bias, as well as study power. In evaluating external validity based on this tool, only two out of 31 studies met criteria for reporting specific methods for random sampling of patients, which should be noted as a source of bias in the interpretation of the study results. Similarly, confounding and selection bias was present in the majority of studies, as they failed to report clinical confounders such as sex, BMI, and age, or control for these variables in their analysis. Prior studies have demonstrated that these limitations should be considered in interpreting study results and further support the need for additional studies to be conducted to assess the utility of HRV in psychiatric conditions. Future studies can better control for these differences by enrolling patients of the same sex, or by controlling sex and age-matched healthy controls to compare groups with confounder analysis.
As previously studied, physiological parameters such as respiratory rate and heart rate can also greatly affect the variance of HRV even in healthy patients.57 The majority of studies did not incorporate adjustment for respiratory rate with heart rate reported alongside HRV parameters. This may be increasingly relevant in patients who are hospitalized for mental health crises and may be monitored for rapidly fluctuating heart rates, which can affect overall HRV; however, it may not be as important of a factor to consider in standard outpatient psychiatric care where HR may be more stabilized. In addition, several studies did not report results due to proprietary reasons, which is another limitation in this space.
Similarly, prior studies have demonstrated the impact of sex-related differences in HRV in cohorts matched for other variables such as age and other comorbidities. Previous work suggests parasympathetic activity may be a dominant contributor to autonomic signaling in women compared to men, resulting in altered HRV over time.58 In this review, five studies eliminated sex as a confounding variable by only enrolling women or only enrolling men as participants. However, the remainder of the studies did not account for sex-related differences in HRV, which should be taken into consideration for future studies and may influence the significance of their results.
Additional confounders that may contribute to variations in HRV include BMI, potentially due to its role in cardiovascular health and increased atherosclerotic disease risk. Prior studies have demonstrated a link between increased visceral adiposity and increased sympathetic activity in obese adults, as well as decreased vagal responsivity.59 However, these effects on HRV may still be reversible, as shown in the LIGHT randomized trial studying healthy lifestyle changes in adults with increased cardiovascular risk.60 The trial showed improvements in both time domain and frequency domain measurements of HRV in patients with diabetes at 1-year follow-up after initiation of lifestyle changes such as improved diet, increased step count, and weight reduction. Although our current review excluded articles that focused on cardiovascular risk or other comorbid diagnoses to focus on psychiatric conditions and their relationship with HRV, these confounding variables may play a role even in patients with primarily psychiatric symptoms. For future investigation on how these confounders may impact HRV, participants should be matched based on similar BMI as well as similar overall cardiovascular risk factors.
Comparison to healthy controls was used in approximately half of the studies reviewed, providing insight into a normalized value of HRV in some cases. However, only a fraction of the studies matched participants to controls based on age and gender, which are known factors that can potentially impact the overall measurement of HRV and may introduce a source of bias in analysis of significance. Studies have also suggested that keeping an individual as their own control, or measuring intra-individual rather than inter-individual heart rate variability, might be a useful parameter and potentially helpful in determining if a patient’s condition has worsened with time.61 This has been studied in relation to patients diagnosed with COVID-19 and suggests that change in heart rate variability is more critical than its absolute value.62,63 Future studies might be able to use intra-individual heart rate variability to better shed light on the severity of certain psychiatric diseases. It is critical to note that in several of these studies, the study design might themselves have been confounding factors to the data. Future studies must develop standardized measurement protocols to ensure repeatability and validity of conclusions.
It is important to note that there exists diversity of thought in this field regarding the utility of HRV. Given the variation in quality of publications as well as study methodology and design, there exist studies that have both shown a significant association between HRV and specific psychiatric disorders as well as those that have found no significance. This makes it particularly difficult to draw definitive and practical insights regarding this topic, something that we hope future studies can help improve. A thorough surveillance of the current literature shows the least variability in association between schizophrenia and HRV, yet there remains a significant variety of opinions in the discussion of depression and PTSD as mentioned above.
Additionally, there are still gaps in our current understanding of HRV as well as limitations that must be addressed when considering the use of HRV as a clinical prognostic tool. Ethical considerations surrounding data privacy should be mentioned, as HRV is typically derived from continuous monitoring of heart rate and can be analyzed from devices such as smartwatches or smartphones, resulting in access to personal data. Applications that download and store patient data should outline data security methodology explicitly in order for patients to fully understand potential risks and benefits prior to providing informed consent. As discussed previously, there are imitations of HRV, with confounding variables such as age, sex, BMI, and cardiovascular risk factors that may influence the interpretation of HRV analysis. However, our review of the current literature demonstrates that significant associations between HRV and psychiatric conditions do exist, and future studies are needed to assess HRV as a clinical tool.
Conclusion
There exists a significant amount of literature regarding HRV and psychiatric disorders. Furthermore, COVID-19 has increased the prevalence of mental health disorders, which is another reason that a better understanding of the relationship between HRV and psychiatric disorders needs to be explored.64 If physicians are able to glean diagnostic/prognostic information from HRV, this could broadly enhance patient care by allowing for more personalized treatment strategies, early intervention, and a better understanding of a patient’s disease trajectory. For example, although this systematic review did not specifically explore the role of HRV in monitoring response to medications, it is one of many practical clinical applications of HRV as a tool. There is a current need to identify biomarkers for disease trajectory and treatment outcomes for psychiatric disorders. HRV has the potential to contribute to a precision medicine approach, wherein clinical and biologic assessments can be utilized to determine diagnosis, treatment, and prognosis prediction. The field will benefit from future studies that specifically explore the utility of HRV in each of these arms of disease management for major DSM V psychiatric disorders as well as ways in which an HRV assessment can be objectively and systematically incorporated in psychiatric clinical appointments. A longitudinal study that tracks HRV over an extended period of time for patients with various diagnoses will also prove extremely useful. Furthermore, it will be important to understand confounders including other medications and comorbidities that might be attached to a patient, which future studies might also explore.
However, as discussed, there is significant variability in the methodologies used to study this correlation, which makes it difficult to meaningfully interpret available data in a way that is directly clinically applicable. Furthermore, the quality of the available literature is lacking due to poor management of confounding factors including age, sex, comorbidities, and lifestyle. Given that these factors may heavily influence both the natural course of psychiatric disorders and HRV, future studies must ensure consistent randomization, matching, methodologies for HRV determination, and data analysis to appropriately account for confounders. Additionally, the use of standardized protocols and rigorous methodologies in future research may help establish the validity and clinical utility of HRV in psychiatry.
The presence of an association between HRV and the severity of various psychiatric disorders, however, remains promising. We hope that additional data in this space can pave the way for HRV parameters to enhance the diagnosis and prognosis of several psychiatric disorders.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Achten J, Jeukendrup AE. Heart rate monitoring: applications and limitations. Sports Med. 2003;33(7):517–538. doi:10.2165/00007256-200333070-00004
2. Costa T, Hill S, Taylor A, Green A, Black F, Watson S. autonomic dysregulation in individuals with psychiatric disorders and healthy. BJPsych Open. 2022;8(Suppl 1):186. doi:10.1192/bjo.2022.186
3. Electrophysiology TFOTESOCTNA. heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. doi:10.1161/01.CIR.93.5.1043
4. Dong J. (2016). The role of heart rate variability in sports physiology. Exp Ther Med, 11(5), 1531–1536. 10.3892/etm.2016.3104
5. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi:10.3389/fpubh.2017.00258
6. Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016;41(2):89–104. doi:10.1503/jpn.140217
7. Wilkinson DJ, Thompson JM, Lambert GW, et al. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry. 1998;55(6):511–520. doi:10.1001/archpsyc.55.6.511
8. Stogios N, Gdanski A, Gerretsen P, et al. Autonomic nervous system dysfunction in schizophrenia: impact on cognitive and metabolic health. NPJ Schizophr. 2021;7(1):22. doi:10.1038/s41537-021-00151-6
9. McGorry PD. Early clinical phenotypes, clinical staging, and strategic biomarker research: building blocks for personalized psychiatry. Biol Psychiatry. 2013;74(6):394–395. doi:10.1016/j.biopsych.2013.07.004
10. Gunnell D, Kidger J, Elvidge H. Adolescent mental health in crisis. BMJ. 2018;361:k2608. doi:10.1136/bmj.k2608
11. Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol. 2019;38(1):3. doi:10.1186/s40101-019-0193-2
12. Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248(1 Pt 2):H151–3. doi:10.1152/ajpheart.1985.248.1.H151
13. Jacobson NC, Bhattacharya S. Digital biomarkers of anxiety disorder symptom changes: personalized deep learning models using smartphone sensors accurately predict anxiety symptoms from ecological momentary assessments. Behav Res Ther. 2022;149:104013. doi:10.1016/j.brat.2021.104013
14. Ha JH, Park S, Yoon D, Kim B. Short-term heart rate variability in older patients with newly diagnosed depression. Psychiatry Res. 2015;226(2–3):484–488. doi:10.1016/j.psychres.2015.02.005
15. Ishizawa T, Yoshiuchi K, Takimoto Y, Yamamoto Y, Akabayashi A. Heart rate and blood pressure variability and baroreflex sensitivity in patients with anorexia nervosa. Psychosom Med. 2008;70(6):695–700. doi:10.1097/PSY.0b013e31817bb090
16. Krause-Utz A, Walther JC, Lis S, Schmahl C, Bohus M. Heart rate variability during a cognitive reappraisal task in female patients with borderline personality disorder: the role of comorbid posttraumatic stress disorder and dissociation. Psychol Med. 2019;49(11):1810–1821. doi:10.1017/S0033291718002489
17. Carnevali L, Mancini M, Koenig J, et al. Cortical morphometric predictors of autonomic dysfunction in generalized anxiety disorder. Auton Neurosci. 2019;217:41–48. doi:10.1016/j.autneu.2019.01.001
18. Reinertsen E, Nemati S, Vest AN, et al. Heart rate-based window segmentation improves accuracy of classifying posttraumatic stress disorder using heart rate variability measures. Physiol Meas. 2017;38(6):1061–1076. doi:10.1088/1361-6579/aa6e9c
19. Minassian A, Geyer MA, Baker DG, et al. Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med. 2014;76(4):292–301. doi:10.1097/PSY.0000000000000056
20. Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety (data from the Normative Aging Study). Am J Cardiol. 1995;75(14):882–885. doi:10.1016/s0002-9149(99)80680-8
21. Rao RK, Yeragani VK. Decreased chaos and increased nonlinearity of heart rate time series in patients with panic disorder. Auton Neurosci. 2001;88(1–2):99–108. doi:10.1016/S1566-0702(01)00219-3
22. Gaul-Alacova P, Boucek J, Stejskal P, Kryl M, Pastucha P, Pavlik F. Assessment of the influence of exercise on heart rate variability in anxiety patients. Neuro Endocrinol Lett. 2005;26(6):713–718.
23. Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 2006;60(1):83–90. doi:10.1016/j.jpsychores.2005.06.002
24. Drago S, Bergerone S, Anselmino M, et al. Depression in patients with acute myocardial infarction: influence on autonomic nervous system and prognostic role. Results of a five-year follow-up study. Int J Cardiol. 2007;115(1):46–51. doi:10.1016/j.ijcard.2006.04.029
25. Ahs F, Sollers JJ 3rd, Furmark T, Fredrikson M, Thayer JF. High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. Neuroimage. 2009;47(3):815–820. doi:10.1016/j.neuroimage.2009.05.091
26. Kang E, Lee I, Park J, Kim K, Yu B. (2010). Platelet serotonin transporter function and heart rate variability in patients with panic disorder. J Korean Med Sci, 25(4), 613–8. 10.3346/jkms.2010.25.4.613
27. Valenza G, Nardelli M, Lanata A, et al. Predicting mood changes in bipolar disorder through heartbeat nonlinear dynamics. IEEE J Biomed Health Inform. 2016;20(4):1034–1043. doi:10.1109/JBHI.2016.2554546
28. Sung CW, Lee HC, Chiang YH, et al. Early dysautonomia detected by heart rate variability predicts late depression in female patients following mild traumatic brain injury. Psychophysiology. 2016;53(4):455–464. doi:10.1111/psyp.12575
29. Pyne JM, Constans JI, Wiederhold MD, et al. Heart rate variability: pre-deployment predictor of post-deployment PTSD symptoms. Biol Psychol. 2016;121(Pt A):91–98. doi:10.1016/j.biopsycho.2016.10.008
30. Dennis PA, Dedert EA, Van Voorhees EE, et al. Examining the crux of autonomic dysfunction in posttraumatic stress disorder: whether chronic or situational distress underlies elevated heart rate and attenuated heart rate variability. Psychosom Med. 2016;78(7):805–809. doi:10.1097/PSY.0000000000000326
31. Tessier A, Sibon I, Poli M, Audiffren M, Allard M, Pfeuty M. Resting heart rate predicts depression and cognition early after ischemic stroke: a pilot study. J Stroke Cerebrovasc Dis. 2017;26(10):2435–2441. doi:10.1016/j.jstrokecerebrovasdis.2017.05.040
32. Kimhy D, Wall MM, Hansen MC, et al. Autonomic regulation and auditory hallucinations in individuals with schizophrenia: an experience sampling study. Schizophr Bull. 2017;43(4):754–763. doi:10.1093/schbul/sbw219
33. Hattori S, Suda A, Kishida I, et al. Association between dysfunction of autonomic nervous system activity and mortality in schizophrenia. Compr Psychiatry. 2018;86:119–122. doi:10.1016/j.comppsych.2018.08.002
34. Hartmann R, Schmidt FM, Sander C, Hegerl U. Heart rate variability as indicator of clinical state in depression. Front Psychiatry. 2018;9:735. doi:10.3389/fpsyt.2018.00735
35. Eddie D, Bates ME, Vaschillo EG, Lehrer PM, Retkwa M, Miuccio M. Rest, reactivity, and recovery: a psychophysiological assessment of borderline personality disorder. Front Psychiatry. 2018;9:505. doi:10.3389/fpsyt.2018.00505
36. Byun S, Kim AY, Jang EH, et al. Detection of major depressive disorder from linear and nonlinear heart rate variability features during mental task protocol. Comput Biol Med. 2019;112:103381. doi:10.1016/j.compbiomed.2019.103381
37. Clamor A, Sundag J, Lincoln TM. Specificity of resting-state heart rate variability in psychosis: a comparison with clinical high risk, anxiety, and healthy controls. Schizophr Res. 2019;206:89–95. doi:10.1016/j.schres.2018.12.009
38. D'Souza J M, Wardle M, Green C E, Lane S D, Schmitz J M, Vujanovic A A. (2019). Resting Heart Rate Variability: Exploring Associations With Symptom Severity in Adults With Substance Use Disorders and Posttraumatic Stress. J Dual Diagn, 15(1), 2–7. 10.1080/15504263.2018.1526431
39. Byun S, Kim A Young, Jang E Hye, Kim S, Choi K Woo, Yu H Young, Jeon H Jin. (2019). Entropy analysis of heart rate variability and its application to recognize major depressive disorder: A pilot study. Technol Health Care, 27(S1), 407–424. 10.3233/THC-199037
40. Clamor A, Ludwig L, Lincoln TM. Heart rate variability as an index of emotion (dys)regulation in psychosis? Int J Psychophysiol. 2020;158:310–317. doi:10.1016/j.ijpsycho.2020.08.016
41. Forte G, Favieri F, Oliha EO, Marotta A, Casagrande M. Anxiety and attentional processes: the role of resting heart rate variability. Brain Sci. 2021;11(4):480. doi:10.3390/brainsci11040480
42. Haigh SM, Walford TP, Brosseau P. Heart rate variability in schizophrenia and autism. Front Psychiatry. 2021;12:760396. doi:10.3389/fpsyt.2021.760396
43. Karmali SN, Sciusco A, May SM, Ackland GL. Heart rate variability in critical care medicine: a systematic review. Intensive Care Med Exp. 2017;5(1):33. doi:10.1186/s40635-017-0146-1
44. D’Souza JM, Wardle M, Green CE, Lane SD, Schmitz JM, Vujanovic AA. Resting heart rate variability: exploring associations with symptom severity in adults with substance use disorders and posttraumatic stress. J Dual Diagn. 2019;15(1):2–7. doi:10.1080/15504263.2018.1526431
45. Mazzeo AT, La Monaca E, Di Leo R, Vita G, Santamaria LB. Heart rate variability: a diagnostic and prognostic tool in anesthesia and intensive care. Acta Anaesthesiol Scand. 2011;55(7):797–811. doi:10.1111/j.1399-6576.2011.02466.x
46. Martin-Key NA, Schei TS, Barker EJ, et al. The current state and diagnostic accuracy of digital mental health assessment tools for psychiatric disorders: protocol for a systematic review and meta-analysis. JMIR Res Protoc. 2021;10(1):e25382. doi:10.2196/25382
47. Sadeghi M, Sasangohar F, McDonald AD, Hegde S. Understanding heart rate reactions to Post-Traumatic Stress Disorder (PTSD) among veterans: a naturalistic study. Hum Factors. 2022;64(1):173–187. doi:10.1177/00187208211034024
48. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi:10.3389/fpsyt.2018.00044
49. Choi KW, Jeon HJ. Heart rate variability for the prediction of treatment response in major depressive disorder. Front Psychiatry. 2020;11:607. doi:10.3389/fpsyt.2020.00607
50. Refisch A, Chung HY, Komatsuzaki S, et al. A common variation in HCN1 is associated with heart rate variability in schizophrenia. Schizophr Res. 2021;229:73–79. doi:10.1016/j.schres.2020.11.017
51. Wang HT, Yang CM, Chen KR, Chueh KH. Relationship between heart rate variability and aggressive behavior among patients with schizophrenia hospitalized in acute wards. Perspect Psychiatr Care. 2020;56(2):321–329. doi:10.1111/ppc.12433
52. Hempel RJ, Tulen JH, van Beveren NJ, Roder CH, Hengeveld MW. Cardiovascular variability during treatment with haloperidol, olanzapine or risperidone in recent-onset schizophrenia. J Psychopharmacol. 2009;23(6):697–707. doi:10.1177/0269881108091254
53. Veeneman RR, Vermeulen JM, Abdellaoui A, et al. Exploring the relationship between schizophrenia and cardiovascular disease: a genetic correlation and multivariable Mendelian randomization study. Schizophr Bull. 2022;48(2):463–473. doi:10.1093/schbul/sbab132
54. Sessa F, Anna V, Messina G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging. 2018;10(2):166–177. doi:10.18632/aging.101386
55. La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107(4):565–570. doi:10.1161/01.cir.0000047275.25795.17
56. Malik M, Bigger JT, Camm AJ. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381. doi:10.1093/oxfordjournals.eurheartj.a014868
57. Monfredi O, Lyashkov AE, Johnsen AB, et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64(6):1334–1343. doi:10.1161/HYPERTENSIONAHA.114.03782
58. Evans JM, Ziegler MG, Patwardhan AR, et al. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91(6):2611–2618. doi:10.1152/jappl.2001.91.6.2611
59. Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration - a risk of CVD. Diabetes Metab Syndr Obes. 2017;10:57–64. doi:10.2147/DMSO.S123935
60. Hayiroglu MI, Cinier G, Yuksel G, et al. Effect of a mobile application and smart devices on heart rate variability in diabetic patients with high cardiovascular risk: a sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 2021;79(11):1239–1244. doi:10.33963/KP.a2021.0112
61. Quer G, Gouda P, Galarnyk M, Topol EJ, Steinhubl SR. Inter- and intraindividual variability in daily resting heart rate and its associations with age, sex, sleep, BMI, and time of year: retrospective, longitudinal cohort study of 92,457 adults. PLoS One. 2020;15(2):e0227709. doi:10.1371/journal.pone.0227709
62. Quer G, Radin JM, Gadaleta M, et al. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat Med. 2021;27(1):73–77. doi:10.1038/s41591-020-1123-x
63. Asarcikli LD, Hayiroglu MI, Osken A, Keskin K, Kolak Z, Aksu T. Heart rate variability and cardiac autonomic functions in post-COVID period. J Interv Card Electrophysiol. 2022;63(3):715–721. doi:10.1007/s10840-022-01138-8
64. Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–98. doi:10.1016/j.jad.2020.11.117
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
