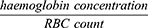Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Heamanetic Effects of a Dioxidovanadium(V) Complex in STZ-Induced Diabetic Male Sprague Dawley Rats
Authors Xulu N , Ngubane P , Khathi A, Booysen I, Sibiya N
Received 7 May 2019
Accepted for publication 28 November 2019
Published 19 October 2021 Volume 2021:14 Pages 4321—4333
DOI https://doi.org/10.2147/DMSO.S214726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Nombuso Xulu,1 Phikelelani Ngubane,1 Andile Khathi,1 Irvin Booysen,2 Ntethelelo Sibiya3
1School of Laboratory Medicine and Medical Sciences, University of KwaZulu Natal, Durban, South Africa; 2School of Chemistry and Physics, College of Agriculture, Engineering and Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa; 3Pharmacology Division, Faculty of Pharmacy, Rhodes University, Grahamstown, South Africa
Correspondence: Phikelelani Ngubane
Department of Human Physiology University of KwaZulu Natal, E-Block, Level 4, University Road, Chiltern Hills, Westville Campus, 3629 Private Bag X54001, Westville, Durban, 4000, South Africa
Email [email protected]
Background: Despite the effective maintenance of glucose homeostasis by insulin in type 1 diabetes mellitus, the drug has been implicated as one of the causes of haematological disturbances, which give rise to cardiovascular complications. As a result, research into alternative therapies for diabetes is needed. In our laboratory, an anti-hyperglycaemic novel vanadium complex has been synthesized using organic heterocyclic ligands. The complex has been shown and improve glycaemic control. The effects of this complex on haematological function, however, have not yet been established. Therefore, this study sought to investigate the haematological effects of dioxidovanadium(V) complex in (STZ)-induced diabetic rats.
Methods: Diabetic rats received vanadium complex (40 mg kg − 1 p.o), diabetic untreated (H2O) and insulin treated (0.175 mg kg− 1 s.c), groups acted as a negative and positive control, respectively. Vanadium complex was administered twice daily, and blood glucose concentration was monitored weekly for 5 weeks. Thereafter, the animals were sacrificed followed by blood and kidneys collection for haematological (full blood count and Annexin V), hormonal (EPO) and oxidative status (SOD and GPx) analysis.
Results: After 5 weeks, untreated diabetic rats presented with hyperglycaemia compared to non-diabetic rats which was attenuated by vanadium complex administration. Furthermore, vanadium treated groups presented with an augmented RBC count, haematocrit, haemoglobin concentration, MCHC, MCV, and (EPO) levels compared to diabetic control. An increase in annexin V expression hence cell survival was observed in vanadium complex treated rats. Lastly, the administration of the complex improved antioxidant status as evidenced by increases in SOD and GPx concentration in plasma and in the kidneys.
Conclusion: The administration of the anti-hyperglycaemic dioxidovanadium(V) complex improved haematological parameters, cell survival and the antioxidant status displayed by the diabetic rats. These results give an indication that the complex might be an effective alternative therapeutic drug for the treatment of hyperglycaemia in DM.
Keywords: hyperglycaemia, vanadium complex, haematological, cardiovascular
Graphical Abstract:
Introduction
Hyperglycaemia has been associated with the development of anaemia due to hyperglycaemia-induced haematological changes namely reduced erythrocyte membrane deformability, hyperosmolarity, increased erythrocyte haemolysis, clearance and destruction.1,2 Sustained hyperglycaemia induces haematological changes through elevated reactive oxygen species (ROS) formation, specifically superoxide (O2-) and hydrogen peroxide (H2O2) which cross the erythrocyte membrane barrier causing redox imbalances within the red blood cell (RBC) environment leading to heme-protein glycation, membrane polarity disruption, reduced membrane deformability. This causes an increase in membrane rigidity which leads to an increase in RBC clearance by the spleen.1 Furthermore, hyperglycaemia has also been associated with the reduction in kidney erythropoietin (EPO) producing potential, often leading to a sharp reduction in erythrocyte production associated with anaemia.3–5 Also, hyperglycaemia induces eryptosis (Red blood cell programmed death) by causing the translocation of phosphatidyl serine (PS) to the outer leaflet of the plasma membrane indicating apoptosis and cell senescence which can be quantified by using flow cytometry and a radio-labelled fluorescent conjugate, annexin V.6–8 These haematological changes are directly proportional to the decrease in red blood cell (RBC) count, haemoglobin (Hb) concentrations, haematocrit (Htc) levels, mean corpuscular volume (MCV) and mean corpuscular haemoglobin concentration (MCHC). These are all indications of anaemia which therefore results in the decreased perfusion of target tissues and cells, specifically the cardiomyocytes resulting in cardiac dysfunction.9,10
Cardiovascular disease remains one of the leading causes of morbidity and mortality in diabetes mellitus.11,12 The subcutaneous injections of insulin have been shown to cause hyperinsulinemia bringing about an increase in RBC production commonly known as secondary polycythaemia.11,12 The resultant accumulation of RBC’s makes diabetic patients more susceptible to cardiovascular complications.11 Similarly, other diabetic drugs such as metformin have been shown to inhibit the absorption of vitamin B12 in the gastro-intestinal tract resulting in anaemia.13 These challenges presented by conventional treatments warrant the continuous search for novel compounds that may provide glycaemic control and alleviate hyperglycaemia-induced cardiovascular complications associated with haematological changes.14
Vanadium compounds have been shown to act as effective hypoglycaemic agents however, their use has been associated with kidney, brain and liver toxicity.14,15 In our laboratory we have synthesized a novel vanadium complex such as dioxidovanadium(V) complex, cis-[VO2(obz)py] {Hobz=2-hydroxyphenyl-1H-benzimidazole and py =pyridine}] which is linked to organic heterocyclic ligands that provide thermodynamic stability and efficient vanadium transport to target tissues, rendering the complex safer, more potent and stable for use.13–15 Previous studies in our laboratory have shown that the administration of this vanadium complex attenuates hyperglycaemia in streptozotocin-induced diabetic rats.13 Moreover, the administration of this compound showed no hepatic hazards as evidenced by normal ALT and AST, suggesting non-toxicological effects.13 These observations are very crucial moving forward, considering toxicity reported with other vanadium complexes.13,15 In light of these findings, we sought to advance further, and investigate the effect of dioxidovanadium(V) complex on selected haematological markers in in STZ-induced diabetic male Sprague Dawley rats.
Methods and Materials
Drugs and Chemicals
All chemicals and reagents used were purchased from standard pharmaceutical suppliers and were of analytical grade.
Vanadium Complex Synthesis
Vanadium complexes were synthesized in the Department of Chemistry at the University of KwaZulu-Natal, Pietermaritzburg, South Africa. The novel dioxidovanadium(V) complex, cis-[VO2(obz)py] {Hobz=2-hydroxyphenyl-1H-benzimidazole and py =pyridine}] was successfully synthesized and verified using the UV–Vis, Emission, EPR, IR, V- and H NMR spectroscopy and crystal X-ray diffraction.
Vanadium Complex Benefits
- There was no marked toxicity observed on hepatic or renal function.
- The complex corrected hyperglycemia and impaired hepatic glycolysis of diabetic rats more safely and potently than other vanadium compounds. This is not simply due to improved intestinal absorption, indicating that the newly synthesized complex had more potent insulin-like properties.
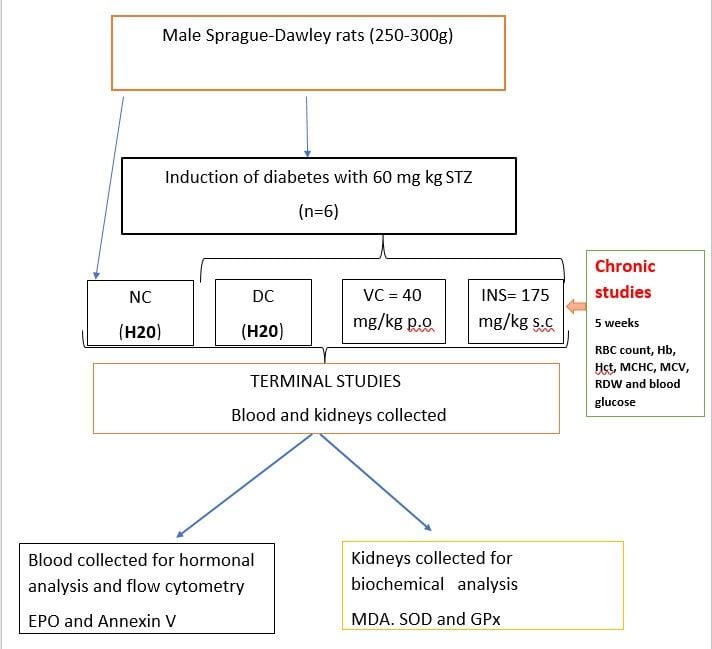
Animals and Housing
Male Sprague-Dawley rats (250–300 g) bred in the Biomedical Research Unit of the University of KwaZulu-Natal was used in this study. The animals were kept and maintained under standard laboratory conditions (for temperature and humidity) in a 12 h day: 12 h night cycle. The animals were allowed access to water ad libitum and were given standard rat chow (40 g) daily (Meadow Feeds, Pietermaritzburg, South Africa). The animals (n=6 in each group) were housed individually in Makrolon polycarbonate metabolic cages (Tecniplast, Labotec, South Africa). All animal experimentation was reviewed and approved by the Animal Research Ethics Committee of the University of KwaZulu-Natal (AREC/054/017D). The animals were monitored for pain, discomfort and distress using the criteria listed in the university’s Animal Research Ethics Committee’s humane endpoint document.
Induction of Diabetes
Type 1 diabetes was induced using a well-established protocol.16 Briefly, animals were given a single intra-peritoneal injection of streptozotocin (60mg/kg) freshly prepared in 0.1 M citrate buffer (pH 4.5). The non-diabetic control group received the vehicle, citrate buffer through the same route. Animals showing glucosuria after 24 hours following a urine strip test (Rapidmed Diagnostics, Sandton, South Africa) were considered diabetic. After 7 days, blood glucose concentrations greater than 20 mmol/L in streptozotocin (STZ)-induced rats were considered to show stable diabetes.
Experimental Design
The haematological effects of the novel vanadium complex were investigated acutely and short- termly in STZ induced male Sprague-Dawley rats.
Acute (OGTT)
The diabetic (DC), insulin (INS) and vanadium complex (VAN) rat groups were used in the OGT. Insulin (0.175 mg kg-1 s.c), and three doses of novel vanadium complex (10, 20, 40 mg/kg, p.o) were administered to the rats via oral gavage and thereafter there was administration of 2g/kg of glucose orally to each rat group. We then collected blood samples through tail vein at 0, 30, 60, 90, 120 minutes (2hrs) to measure blood glucose concentrations.
Short-Term Effects
Post-induction, the short-term haemanetic effects of the novel vanadium complex (40 mg kg – 1 p.o) were investigated in STZ-induced diabetic rats. The experimental animals were divided into the following groups; non-diabetic (ND) diabetic control (DC), novel vanadium complex (40 mg kg −1 p.o) (VAN) and insulin (0.175 mg/kg-1, s.c) (INS) treated animals that served as positive control. All animals (n=6 in each group) were housed individually in Makrolon polycarbonate metabolic cages (Tecniplast, Labotec, South Africa). The vanadium complex was administered twice every third day at 09h00 AM and 15h00PM by means of an 18-gauge gavage needle (Kyron Laboratories (Pty) LTD, Benrose, South Africa). The diabetic group which received DMSO/saline (3mL kg-1, P.O.) and insulin group (0.175 mg kg-1 s.c) acted as a negative control and positive control, respectively. Over the period of 5 weeks the RBC count, haematocrit (Hct), haemoglobin (Hb), MCV, MCH, MCHC and blood glucose concentrations, were monitored every third day using a haemocytometer (Beckman Coulter, Indianapolis, United States) and OneTouch select glucometer (Lifescan, Mosta, Malta, United Kingdom) respectively.
Terminal Studies
At the end of the 5-week experimental period, all the animals were sacrificed by exposing them to halothane via a gas anaesthetic chamber (100 mg kg-1) for 3 minutes (Biomedical Resource Unit, UKZN, and Durban, South Africa). Thereafter, blood (100 µL) was collected by cardiac puncture (RBCP) into individual pre-cooled EDTA tubes to assess the effects of novel vanadium complexes on red blood cells, mean corpuscular volume, mean corpuscular haemoglobin concentration and haematocrit using a haemocytometer (Beckman Coulter, Indianapolis, United States). Thereafter, more blood was collected via cardiac puncture into pre-cooled heparinize containers and centrifuged (Eppendorf centrifuge 5403, Germany) at 4 °C, 503 g for 15 minutes for hormonal analysis. The kidneys were removed and weighed before freezing in liquid nitrogen. Thereafter, the kidneys were stored in an Ultra Bio Freezer (Snijders Scientific, Tilburg, Netherlands) at −80°C.
Haematological Analysis
Red blood cell (RBC) count was measured in blood collected from all the rat groups using the haematocytometer (Beckman Coulter, Indianapolis, United States). Estimation of Mean Cell Haemoglobin (MCH) was calculated as reported by Cheesbrough (2004), using the following formula:
The mean cell haemoglobin concentration (MCHC) was calculated as reported by Cheesbrough (2004), using the following formula:
The mean cell volume (MCV) was calculated as reported by Cheesbrough (2004) using the following formula:
Packed RBC volume: RBC’s multiplied by the factor of ten.
Hormonal and Biochemical Analysis
EPO SOD and GPx Analysis
For plasma erythropoietin analysis, an ELISA kit (Elabscience and Biotechnology, Wuhan) was used following manufacturer’s instruction.
Flow Cytometry
A comparison of the percentage of red blood cells (RBC) expressing Annexin-V to the percentage of red blood cells without annexin-V expression among untreated and treated STZ-induced diabetic animals. This assay is used to count the number of cells that have undergone apoptosis.
Red Blood Cell Membrane Analysis
Instrument set up: BD FACS Canto-II flow cytometer and BD FACSdiva software (BD Biosciences, San Jose, CA) were used to acquire data.
Measurement of apoptotic red blood cell levels: Annexin-V FITC was used as this antibody binds to the translocated phosphatidylserine (PS) from the inner leaflet of the plasma membrane to the outer leaflet, consequently exposing PS to the external environment. Briefly, 50 µL of heparinized RBCs were stained with Annexin-V FITC (1:10) antibody and incubated in the dark for 20 minutes at room temperature; samples were then suspended in 500 µL of PBS and analysed immediately.
Statistical Analysis
Data was expressed as means ± standard error of means (SEM). Statistical analysis was conducted using GraphPad Prism Instat Software (version 5.00, GraphPad Software, San Diego, California, USA) using one-way ANOVA and two-way ANOVA for OGT and blood glucose followed by Tukey-Kramer multiple comparison test to simultaneously determine statistical differences between the means of the two groups. A value of p<0.05 was considered statistically significant.
Results
OGT and AUC
Figure 1 Shows the OGT responses over a period of 120 minutes (2 hours) in non-diabetic, diabetic control, as well as insulin and dioxidovanadium(V) Complex vanadium (10, 20 and 40 mg/kg) treated diabetic rats (n=6). The STZ-diabetic animals showed high blood glucose concentration throughout the two-hour OGTT period. All doses of vanadium complex significantly decreased blood glucose concentration in comparison to the STZ-untreated diabetic control (p<0.05). Insulin, also significantly decreased blood glucose concentration in comparison to STZ diabetic rats (p<0.05).
Figure 2 To verify the OGT findings we performed an area under the curve (AUC) test. The AUC of the diabetic control group increased significantly compared to non-diabetic rats (Figure 1). Treating STZ-induced diabetic rats with vanadium (10, 20, 40 mg/kg) significantly reduced blood glucose concentration during the OGT protocol. In addition, the AUC glucose was smaller in vanadium (40 mg/kg) treated diabetic animals in comparison with vanadium (10, 20 mg/kg) treated diabetic rats. Therefore, this dose was used in the 5-week study.
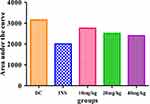 |
Figure 2 The area under the curve (AUC) of diabetic control (DC), insulin (INS) and vanadium (10, 20, and 40 mg/kg) treated diabetic rats. Values are expressed as mean ± SEM (n= 6 in each group). |
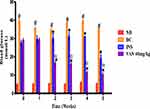
Figure 3 presents weekly blood glucose concentration in non-diabetic, diabetic control, as well as insulin and various doses of vanadium (40mg/kg) treated diabetic rats (n=6) over a 5-week experimental period. The untreated STZ-induced diabetic animals showed significantly high glucose concentrations over a period of 5 weeks in comparison with non-diabetic control (p=0.001) Treatment with vanadium complex and insulin showed a significant decrease in blood glucose by comparison with the diabetic control (p<0.05). Interestingly, however, the vanadium complex significantly (p<0.05) decreased blood glucose by comparison to insulin in weeks 4 and 5 of the experimental period.
Effects of Vanadium Complex on Haematological Parameters
Table 1 Shows haematological parameters of non-diabetic rats, diabetic control rats, as well as insulin and vanadium (40mg/kg) treated diabetic rats (n=6) over a 5-week experimental period. Diabetic control rats exhibited a decrease in haematological parameters, namely red blood cell count (RBC), haemoglobin (HB), haematocrit (Hct) and mean corpuscular haemoglobin concentration (MCHC) in comparison to non-diabetic control rats (p<0.05). Interestingly, the administration of the vanadium complex significantly (p<0.05) increased haematological parameters such as RBC count, Hb, Hct and MCHC by comparison to STZ-induced diabetic control rats at the end of the 5-week experimental period (p<0.05). Similar results were obtained following insulin administration.
 |
Table 1 Shows the Effects of the Vanadium Complex and the Positive Control Insulin on Various Hematological Parameters |
Effects of Vanadium Complex on Erythropoietin
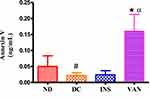
Figure 4 shows plasma erythropoietin (EPO) concentrations in non-diabetic, diabetic, diabetic vanadium and insulin treated groups at the end of the 5-week experimental period. STZ-induced diabetic animals’ EPO concentrations were significantly decreased in comparison with non-diabetic control group. Interestingly, the administration of vanadium (40 mg/kg, p.o) attenuated the diabetic-associated decrease in EPO concentration and significantly (p<0.05) increased EPO concentrations by comparison to STZ-induced diabetic control rats at the end of the 5-week experimental period. Insulin also increased EPO concentrations.
Effects of Vanadium Complex on Oxidative Stress
Table 2 shows malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx) concentrations in non-diabetic, diabetic, diabetic vanadium and insulin treated groups at the end of the 5-week experimental period. Diabetic control rats had significantly (p<0.05) increased kidney MDA concentrations and had a reduced expression of SOD and GPx in comparison with non-diabetic control. STZ-diabetic rats treated with vanadium and insulin showed significantly (p<0.05) decreased MDA concentration and increased expression of SOD and GPx in kidneys in comparison to STZ-induced diabetic control rats.
 |
Table 2 Shows the Effects of the Vanadium Complex and the Positive Control Insulin on Kidney (MDA) Concentrations, (SOD) and (GPx) Expressions in STZ-Induced Diabetic Rats Over a 5-Week Period |
Annexin V Expression
(Figure 5) Shows annexin V expression in non-diabetic, diabetic, diabetic vanadium and insulin treated groups at the end of the 5-week experimental period. STZ-induced diabetic animals’ annexin V was significantly decreased in comparison with non-diabetic control group. Interestingly, the administration of the highest dose of vanadium (40 mg/kg, p.o) significantly increased annexin V concentrations signalling an amelioration in the diabetic-associated decrease in red blood cell survival. Insulin also showed a slight but non-significant increase in annexinV concentrations.
Correlation Co-Efficient of MCV vs Annexin V
Figure 6 represents scatter plot graphs clearly showing the degree of correlation between MCV and positive annexin V staining cells. Scatter plot A) which is the normal control group and scatter plot C) which represents the insulin group exhibit no correlation and weak correlation. Whilst the diabetic control (ANOVA p-value 0.0136; r −0.9864) and vanadium group scatter plot B) and D), respectively, exhibit negative and positive strong collinear correlation.
Discussion
The red blood cell count (RBC), haemoglobin (Hb), haematocrit (Htc), mean cell volume (MCV) and mean corpuscular haemoglobin concentration (MCHC) are all haematological indices used to assess for the development of anaemia.17,18 Diabetes has been shown to be associated with the development of anaemia and cardiovascular dysfunction as a result of sustained hyperglycaemia-induced haematological changes, oxidative stress and RBC death.19 Hyperinsulinemia in type 1 diabetes, as a result of the bolus intravenous injection of insulin, leads to an increased blood viscosity and the pathophysiological elevation of the MCV and RBC count as shown by the results in Table 1 which depict an increase in both the MCV and RBC count above the physiological range, potentiating an increased susceptibility to cardiovascular myopathies.25 The unwarranted RBC production causes diabetic patients to be more susceptible to cardiovascular complications.25 Insulin, however, acts as an important co-factor in erythropoiesis due to the presence of insulin receptor R on the surface of RBCs, and it also increases the concentration of hypoxic inducible factor 1 (HIF-1) which promotes erythropoiesis, and in turn increases blood viscosity resulting in diminished oxygen delivery and cardiovascular complications.26 These two mechanisms explain the increased cardiovascular mortality that is often associated with insulin and warrant research into alternative treatments that will lower hyperglycaemia and improve glycaemic control.24
Vanadium compounds have been shown to possess hypoglycaemic and insulin mimetic effects.20 El Karib et al suggests that vanadium is a phosphatase inhibitor and that can activate serine/threonine kinases which are distal to the insulin receptors by preventing the dephosphorylation of phosphatases and allowing for the subsequent activation of those insulin receptors resulting in an increased glucose uptake into the cell lowering the overall blood glucose. In our study, oxidovanadium (V) complex, cis-[VO2(obz)py] {Hobz=2- hydroxyphenyl-1H-benzimidazole and py =pyridine}] has been synthesized and linked to organic heterocyclic ligands, which eliminated toxicity and improved the complexes potency. This is important as the ligands also aid in the glucose lowering component of the complex as hyperglycaemia is the main instigator of complications associated with diabetes mellitus.21 Other studies have also shown that vanadium compounds induce the recruitment of vesicles containing GLUT 4, by stimulating the tyrosine kinase activity of the β-subunit of the insulin receptor and therefore allowing for increased cellular glucose entry and decreased blood glucose.21 Furthermore, vanadium was also shown to inhibit N-terminal kinase resulting in the improvement of insulin sensitivity causing a reduction in hyperglycaemia and hyperglycaemia associated complications in experimental diabetes.21
In this study, dioxidovanadium(V) complex, cis-[VO2(obz)py] {Hobz=2- hydroxyphenyl-1H-benzimidazole and py =pyridine}] has been synthesized and conjoined with organic heterocyclic ligands, which eliminate toxicity and improve the complexs' potency.13 . We have shown that the administration of this vanadium complex attenuates hyperglycaemia in streptozotocin-induced diabetic rats.13 Moreover, the administration of this compound showed no hepatic hazards as evidenced by normal ALT and AST, suggesting non-toxicological effects.13 These observations are very crucial moving forward, considering toxicity reported with other vanadium complexes.13 In light of these findings, we sought to advance further, and investigate the effects of dioxidovanadium(V) complex on selected haematological markers, in an effort to establish a holistic therapeutic appoach against diabetes.
One of the major causes of anaemia in DM is the formation of abnormal erythrocyte cell membranes due to hyperglycaemia.21 The high blood glucose concentrations cause abnormal surface area-to-volume ratio resulting in reduced deformability of erythrocytes membranes, RBC dysfunction and their consequent sequestration in the spleen leading to diabetic anaemia.22 The assessment of haematological parameters could be used to disclose the lethal effects of diabetes mellitus on the cardiovascular system and other systems of the body which depend on the efficient functioning of red blood cells to receive oxygen and undergo glycolysis to produce ATP.22 Oxygen plays a critical role in the body as it is used to oxidize/convert nutrients into usable energy.22 The primary function of RBCs is to carry oxygen from the lungs to the body tissues and remove carbon dioxide as a waste product. To efficiently perform this function, all the haematological indices, specifically the MCV and RBC count, need to be maintained within a narrow range.22,23 This physiological range depends on the maintenance of hormonal and biochemical factors, namely erythropoietin (EPO), SOD and GPx.22 Erythropoietin plays an important role in the production of RBCs as it is responsible for stimulating erythropoiesis in the bone marrow resulting in the normal production of RBCs. Hyperglycaemia has been shown to cause disturbances in EPO production by directly affecting RBC production which increases the risk for anaemia and the development of cardiovascular complications.23 This disturbance was also observed in this study as, STZ-diabetic rats showed a significant decrease in EPO concentration, which can be linked to the decrease in RBC count and other indices in Table 1. Oxidative stress is also a common hyperglycaemia-associated complication that results in RBC apoptosis and kidney tissue damage.22 To prevent oxidative stress, the body has specialized mechanisms to detoxify excessive ROS formation that involves the elevated production of antioxidants such as SOD and GPx.22 In our study, hyperglycaemia as a result of treatment with STZ caused excessive ROS formation and a significant reduction in the antioxidant enzymes SOD and GPX, leading to oxidative stress.22 The 5-week treatment with vanadium complex significantly decreased blood glucose, which resulted in the restoration of blood parameters, namely, RBC count, Hb, haematocrit, MCV and MCHC to within physiological ranges as observed in Table 1. The complex also resulted in a significant increase in EPO, SOD and GPX concentrations; this led to an improvement in red blood cell production as well as function since there was physiological restoration of parameters that directly affected the RBC count. Elevated RBC count increased oxygen carrying capacity which alleviated cardiovascular myopathies associated with hyperglycaemia. Similar mechanisms involving the reduction of elevated glucose could be how vanadium decreases ROS formation and improves haematological function in STZ diabetic animals. Hyperglycaemia increases electron transfer donors (NADH and FADH2) causing the inner mitochondrial membrane potential to rise above the threshold value. This leads to elevated reactive oxygen species (ROS) production and oxidative stress that brings about RBC haemolysis and clearance.
In addition to oxidative stress and disruptions to membrane osmolarity, hyperglycaemia has also been shown to induce eryptosis and decreased cell survival.27 The specific binding of annexin V to phosphatidyl serine (PS) has greatly facilitated the measurement of exposed phospholipid on the outer membrane of cells, which is used to quantify/measure cell survival in RBCs.27 Previous studies have used flow cytometry and the increased concentration of the fluorescent conjugate (annexin V) to assess the level of cell senescence/aging.6,28 For cell survival to be concluded, the correlation of certain full blood count markers such as MCV must be assessed.6 This is done by assessing the correlation co-efficient between MCV and annexin V concentrations in the diabetic control vs the treatment group.28 Recent studies have associated the increase in annexin V in conjunction with a strong positive correlation between MCV and annexin V with cell senescence/survival.27,28 Figure 6 shows a strong negative correlation between MCV and annexin V of the diabetic control group. This is due to the differences in the relative size of the RBCs and annexin V binding. This indicative o increased hyperglycaemia-associated RBC destruction via ROS resulting in decreased RBC survival.6,28 The treatment group, however, has a strong positive collinear correlation that is suggestive of an elongated life span of the red blood cell in circulation.6 This change may be attributed to the vanadium complex’s ability lower hyperglycaemia, which in turn improved RBC parameters and the life span of erythrocytes. This results in a lowered risk to develop anaemia and cardiovascular complications that could arise from the lack of oxygen delivery in the system.6,28
There was also a significant reduction in annexin V positive cells in the diabetic control in comparison to the normal control group. This may be due to hyperglycaemia-induced RBC haemolysis via oxidative stress and other processes that decrease the overall RBC count. The lowered RBC count results in a limited number of viable red blood cells present within circulation (young and aged).6,28 This then explains the limited binding of annexin V to PS of red blood cells in the diabetic control group. Interestingly, there was a significant increase of annexin V binding in the vanadium complex treatment group in comparison to the diabetic control group. This alludes to the increased RBC survival and the number of viable RBC’s, which suggests that the vanadium complex may provide stability and mechanical support to the RBCs.6,28 In doing so, the complex can improve RBC resistance to shear stress brought about by hyperglycaemia and therefore prevent rapid clearance of the RBCs, improving RBC survival, and consequently prevent cardiovascular dysfunction.6
Conclusion
In conclusion, the administration of dioxidovanadium(V) complex, cis-[VO2(obz)py] {Hobz=2- hydroxyphenyl-1H-benzimidazole and py =pyridine}] (Figure 7) improved the haematological parameters, namely, RBC count, haematocrit, haemoglobin and mean corpuscular haemoglobin concentration.This may prevent the development of RBC clearance and anaemia observed diabetic animals. This was via lowering blood glucose concentrations, improving the kidney antioxidant status, increasing kidney EPO secretion as well as improving RBC survival.
Abbreviations
RBC, Red blood cell count; Hb, Haemoglobin; Htc, Haematocrit; MCV, Mean cell volume; MCHC, Mean corpuscular haemoglobin concentration; FBC, Full blood count; EPO, Erythropoietin; SOD, Superoxide dismutase; GPx, Glutathione Peroxidase; GLUT 4, Glucose transporter type 4; DM, Diabetes mellitus; STZ, Streptozotocin; OGT, Oral Glucose Tolerance Test; NC, Non-diabetic control; DC, Diabetic control; INS, Insulin; VAN, Vanadium; HIF, Hypoxia-Inducible Factor; ROS, Reactive oxygen species; NADH, Nicotinamide adenine dinucleotide; FADH2, Flavin adenine dinucleotide; RBCP, Cardiac puncture.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All animal experimentation was reviewed and approved by the Animal Research Ethics Committee of the University of KwaZulu-Natal (AREC/054/017D). The animals were monitored for pain, discomfort and distress using the criteria listed in the university’s Animal Research Ethics Committee’s humane endpoint document.
Acknowledgments
The authors would like to thank biomedical research unit personnel for their technical assistance. This study was partly funded by NRF South Africa and the University of KwaZulu- Natal, College of Health Sciences.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
NRF Funded- Funding was used to acquire animals (Sprague Dawley rats), equipment and machinery to perform analytical techniques (Blood analysis and ELISA’s).
Disclosure
The authors declare that they have no competing interests.
References
1. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1–14.
2. Chawla A, Chawla R, Jaggi SJ. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546.
3. Manandhar N, Bhattarai KJ. Anemia in hospitalized patients: a cross-sectional study on different erythrocyte indices and their relationships. J Health Sci Res. 2018;3(1):1–10.
4. Omotayo T, Akinyemi G, Omololu P, et al. Possible involvement of membrane lipids peroxidation and oxidation of catalytically essential thiols of the cerebral transmembrane sodium pump as component mechanisms of iron-mediated oxidative stress-linked dysfunction of the pump’s activity. Redox Biol. 2015;4:234–241.
5. Gutteridge JM, Halliwell BJ. Invited review free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. 1993;19(3):141–158.
6. García-Roa M, del Carmen Vicente-Ayuso M, Bobes AM, et al. Red blood cell storage time and transfusion: current practice, concerns and future perspectives. Blood Transfus. 2017;15(3):222.
7. Pietkiewicz S, Schmidt JH, Lavrik IN. Quantification of apoptosis and necroptosis at the single cell level by a combination of imaging flow cytometry with classical annexin V/propidium iodide staining. J Immunol Methods. 2015;423:99–103.
8. Maćczak A, Cyrkler M, Bukowska B, Michałowicz JJ. Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study). J Hazard Mater. 2016;307:328–335.
9. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, Von Haehling SJ. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12(10):610.
10. Chowdary KR, Mounika GV, Chowdary CP, Velamanakki RS, Bakshi V, Gorle MT. Study on prognostic markers in chronic kidney disease patients as an independent risk factors and its management; 2018.
11. Bjornstad P, Snell-Bergeon JK, Nadeau KJ, Maahs DM. Insulin sensitivity and complications in type 1 diabetes: new insights. World J Diabetes. 2015;6(1):8.
12. Shih LY, Huang J-Y, Lee CT. Insulin-like growth factor I plays a role in regulating erythropoiesis in patients with end-stage renal disease and erythrocytosis. J Am Soc Nephrol. 1999;10(2):315–322.
13. Sibiya S, Msibi B, Khathi A, et al. The effect of dioxidovanadium complex (V) on hepatic function in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2019;(999):1–7.
14. Aroda VR, Edelstein SL, Goldberg RB, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016;101(4):1754–1761.
15. Irving E, Stoker AJM. Vanadium compounds as PTP inhibitors. Molecules. 2017;22(12):2269.
16. Booysen IN, Hlela T, Akerman MP, Xulu BJP. Mono-and polynuclear vanadium (IV) and-(V) compounds with 2-substituted phenyl/pyridyl heterocyclic chelates. Polyhedron. 2015;85:144–150.
17. Xulu S, Owira PM. Naringin ameliorates atherogenic dyslipidemia but not hyperglycemia in rats with type 1 diabetes. J Cardiovasc Pharmacol. 2012;59(2):133–141.
18. Conway BN, Badders AN, Costacou T, et al. Perfluoroalkyl substances and kidney function in chronic kidney disease, anemia, and diabetes. Diabetes Metab Syndr Obes. 2018;11:707. doi:10.2147/DMSO.S173809
19. Chalew S, Hamdan MJ. Racial disparity in HbA1c persists when fructosamine is used as a surrogate for mean blood glucose in youth with type 1 diabetes. Pediatr Diabetes. 2018;19(7):1243–1248.
20. Buttarello MJ. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol 2016;38:123–132.
21. Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek DJPB. Insulin-mimetic property of vanadium compounds. Postepy Biochem. 2016;62(1):60–65.
22. Niu X, Xiao R, Wang N, et al. The molecular mechanisms and rational design of anti-diabetic vanadium compounds. Curr Top Med Chem. 2016;16(8):811–822.
23. Safeukui I, Buffet PA, Deplaine G, et al. Sensing of red blood cells with decreased membrane deformability by the human spleen. Blood Adv. 2018;2(20):2581.
24. Hess JR, Solheim BGJ. Red blood cell metabolism, preservation, and oxygen delivery. Transfus Med. 2016:97–109.
25. Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144.
26. Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen EJ. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002;277(31):27975–27981.
27. Mozos IJ. Mechanisms linking red blood cell disorders and cardiovascular diseases. BioMed Res Int. 2015;2015. doi:10.1155/2015/682054
28. Alexander Bizjak D, Brinkmann C, Bloch W, Grau M. Increase in red blood cell- nitric oxide synthase dependent nitric oxide production during red blood cell aging in health and disease: a study on age dependent changes of rheologic and enzymatic properties in red blood cells. PLoS One. 2015:10(4)e0125206.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.