Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Healthcare Costs and Resource Use of Patients with Dupuytren Contracture Treated with Collagenase Clostridium Histolyticum or Fasciectomy: A Propensity Matching Analysis
Authors Zah V , Pelivanovic J, Tatovic S, Vukicevic D , Imro M , Ruby J, Hurley D
Received 29 June 2020
Accepted for publication 6 October 2020
Published 4 November 2020 Volume 2020:12 Pages 635—643
DOI https://doi.org/10.2147/CEOR.S269957
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Vladimir Zah, 1 Jovana Pelivanovic, 1 Simona Tatovic, 1 Djurdja Vukicevic, 1 Martina Imro, 1 Jane Ruby, 2 David Hurley 2
1Health Economics and Outcomes Research Department, ZRx Outcomes Research Inc., Mississauga, ON, Canada; 2Medical Affairs, Endo Pharmaceuticals Inc., Malvern, PA, USA
Correspondence: Vladimir Zah
ZRx Outcomes Research Inc, Mississauga, ON, Canada
Tel +1 416-953-4427
Email [email protected]
Objective: Studies examining differences in US healthcare resource utilization (HCRU) and associated healthcare costs between collagenase clostridium histolyticum (CCH) and fasciectomy for Dupuytren contracture (DC) are limited. This study evaluated US HCRU and direct healthcare cost for the treatment of DC in privately insured patients using insurance claims.
Methods: This retrospective observational cohort study analyzed data from large nationwide insurance claims databases; it included individuals diagnosed with DC between July 1, 2011, and June 30, 2017, who were adults at index date (date of first treatment: CCH or fasciectomy). Participants had continuous health plan coverage 24 months pre-index and 12 months post-index date. All-cause and DC-related HCRU and healthcare costs from the payers’ perspective were compared between propensity score–matched cohorts. Generalized linear models assessed factors associated with all-cause total healthcare costs.
Results: Of 83,983 patients diagnosed with DC, 1932 adults receiving fasciectomy and 953 adults receiving CCH were included. The mean ± standard deviation total all-cause healthcare cost was significantly lower with CCH than with fasciectomy (US$11,897 ± US$14,633 versus US$15,528 ± US$22,254, respectively; P< 0.001). After propensity score matching, 702 and 999 patients remained in the CCH and fasciectomy cohorts, respectively. In this analysis, all-cause and DC-related total costs were significantly lower in the CCH cohort versus the fasciectomy cohort (all-cause: US$11,044 ± US$12,856 versus US$12,912 ± US$19,237, respectively, P=0.02; DC-specific: US$3417 ± US$3671 versus US$5800 ± US$4985, P< 0.001), mainly due to the lower frequency of outpatient visits. CCH treatment and the use of a consumer-driven healthcare plan were associated with lower healthcare costs.
Conclusion: Based on matched cohort data, adjusted 1-year healthcare costs for CCH-treated individuals were significantly lower compared with costs for fasciectomy-treated individuals.
Keywords: injection, surgery, insurance claims, retrospective database study
Corrigendum for this paper has been published
Plain Language Summary
- Dupuytren disease is characterized by thickening and shortening of the fibrous bands in the palm, ring finger, and little finger that may ultimately lead to permanent contracture of the fingers (Dupuytren contracture [DC])
- Dupuytren disease affects approximately 1% of adults in the United States, with a mean age at onset of approximately 60 years
- There is a paucity of comparative information available on healthcare resource use and costs between collagenase clostridium histolyticum (CCH; minimally invasive treatment option) and fasciectomy (surgical treatment option) treatments for DC in the United States
- This retrospective study evaluated claims of privately insured US patients for healthcare costs and resource expenditures related to DC treatment between July 1, 2011, and June 30, 2017
- Propensity score matching (PSM) is a broadly used technique in retrospective claims studies that is performed to minimize potential selection bias due to confounding factors and to ensure unbiased comparison between the study cohorts
- After PSM, 702 and 999 patients remained in the CCH and fasciectomy cohorts, respectively; all-cause and DC-related total costs were significantly lower in the CCH cohort versus the fasciectomy cohort (all-cause: US$11,044 ± US$12,856 versus US$12,912 ± US$19,237, respectively, P=0.02; DC-specific: US$3417 ± US$3671 versus US$5800 ± US$4985, P<0.001), mainly due to fewer outpatient visits
- Based on propensity-matched cohort data, total healthcare costs for CCH-treated individuals were significantly lower compared with costs for fasciectomy-treated individuals
Introduction
Dupuytren disease is a fibrotic disorder characterized by thickening and shortening of the fibrous bands in the palm and fingers that may ultimately lead to permanent contracture of the fingers (Dupuytren contracture [DC]).1,2 Dupuytren disease most commonly involves the metacarpophalangeal and proximal interphalangeal joints of the ring and little fingers.1,2 This disease affects approximately 1% of adults in the United States,3 with a mean age at onset of approximately 60 years.2 Although the etiology of Dupuytren disease is not clearly understood, several potential risk factors have been identified, including diabetes, smoking, and alcohol abuse.4–6
Dupuytren disease can severely impair hand function, resulting in difficulties with activities of daily living, reduced work productivity, and diminished quality of life.3,7,8 These functional impairments may be sufficiently severe to warrant treatment.9 Current treatment options for DC include surgical excision (fasciectomy) or transection (fasciotomy) of the fibrous cords, percutaneous needle aponeurotomy, and injection of collagenase clostridium histolyticum (CCH).10 CCH is a mixture of 2 types of collagenase (class I and class II) in a defined mass ratio.11 The 2 enzymes cleave collagen molecules at different sites, resulting in enzymatic disruption of collagen cords.10,12 CCH was approved in 2010 by the US Food and Drug Administration for the treatment of DC with a palpable cord in adults.12 It is injected into the fibrous cord, and the treated joint is manipulated approximately 24–72 hours after injection if contracture persists to allow finger extension and cord rupture. The efficacy of CCH in the treatment of DC has been demonstrated in Phase 3, randomized, controlled trials, which have shown significant reductions in contractures and improvements in range of motion, compared with placebo.13,14 Besides, some studies conducted in the United States and Europe have reported that CCH treatment for DC is also associated with less use of healthcare resources and lower healthcare costs, compared with fasciectomy;15–20 however, few studies have examined differences in real-world healthcare resource utilization (HCRU) and associated healthcare costs with CCH versus fasciectomy for the treatment of DC in the United States. Hence, the objective of the current study was to analyze data from a large claims database to compare US HCRU and direct healthcare costs between CCH and fasciectomy for the treatment of DC in a privately insured population.
Materials and Methods
Data Source and Study Population
This was a retrospective observational cohort study conducted using data from the US IBM® MarketScan® Commercial Claims and Encounters Database. This database contains anonymized information on approximately 240 million patients from more than 300 employers and a wide range of health plans across all healthcare settings nationwide in the United States. The data collected include demographics, health insurance plan details, and individual HCRU and expenditures. Institutional review board or ethics committee approval was not required for this study because only anonymized data were used. All data used in this analysis are compliant with the specifications of the 1996 Health Insurance Portability and Accountability Act.
The current analysis included adults who had at least 1 administrative claim for DC (ie, an International Classification of Diseases 9th or 10th Revision [ICD-9/10] code 728.6 or M72.0) between July 1, 2011, and June 30, 2017. CCH-treated patients were identified by medication claims with a Healthcare Common Procedure Coding System (HCPCS) code J0775 or a National Drug Code (NDC) 66887-0003-01; also, patients were required to have a Current Procedural Terminology (CPT) code claim for CCH injection into the palm (CPT code 20527) within 90 days after the medication claim. Patients undergoing fasciectomy were identified by CPT codes 26121, 26123, and 26125. The date of first treatment with CCH or fasciectomy for DC was defined as the index date. Patients were required to have had continuous health plan coverage for 24 months prior to the index date and 12 months after the index date (post-index period). Patients who were <18 years old on the index date, had a diagnosis of Peyronie’s disease or had evidence of the use of a DC-associated treatment other than the index treatment (fasciotomy or needle aponeurotomy) during the study period were excluded from the analysis. To assess real-world healthcare costs during the 1-year course of the disease rather than a single-episode cost, patients were allowed to have additional treatments during the post-index period. Demographic characteristics (age, gender, geographic region, and type of insurance plan) were recorded at the index date, and comorbidities and risk factors were assessed over the 24 months preceding the index date.
Healthcare Resource Utilization and Cost Outcomes
To examine the overall and DC-related economic burden, all-cause and DC-specific HCRU and costs were determined during the 1-year post-index period. All-cause resource utilization and costs were calculated based on any claims recorded downstream, while DC-specific resource utilization and costs were calculated based on claims with a diagnosis code for DC. Healthcare resource utilization was calculated based on events captured in specific claims (outpatient visits, inpatient admissions, and emergency department visits). Healthcare costs were calculated based on insurance plan payments for medical and pharmaceutical services provided. All treatment courses occurring during the 12-month follow-up period were included in the analyses and the respective costs of re-interventions were also evaluated if they occurred during the follow-up period.
Statistical Analysis
Demographic and clinical characteristics for the CCH and fasciectomy cohorts were analyzed using χ2 tests for categorical variables and independent t-tests for continuous variables. To minimize study selection bias, patients categorized into treatment cohorts were subjected to propensity score matching. A propensity score is a probability that a patient with certain demographic or baseline characteristics will be assigned to a particular treatment; propensity score matching in cohort studies allows one to balance treatment groups across measured covariates. The nearest neighbor matching algorithm21 was used to match patients treated with CCH to fasciectomy-treated patients in a 1:2 ratio.
Total all-cause costs were calculated by summing the adjudicated amounts paid by insurance for medical and pharmaceutical services during the post-index period. A generalized linear model with gamma log link distribution was used to analyze differences in total healthcare costs between the 2 cohorts, after adjustment for confounding variables. Confounding variables and effect modifiers that showed significant correlations with outcome or treatment choice were included in the base model. Variables were retained in the final model if statistically significant, and this model was used for cost adjustment. Statistical analyses were performed using the software package IBM® SPSS® Statistics for Windows, version 23.0 (IBM Corp, Armonk, NY). P values <0.05 were considered significant.
Results
Patient Characteristics
Between July 1, 2011, and June 30, 2017, 83,983 patients in the database had a diagnosis of DC, of whom 18,942 had ≥1 claim for CCH or fasciectomy and 2885 met all the inclusion criteria (CCH, n=953; fasciectomy, n=1932; Figure 1). In the unmatched sample, the characteristics of the CCH and fasciectomy cohorts were similar, except for a higher percentage of males in the CCH cohort and some differences in patient geographic distributions (Table 1). Most patients were Charlson Comorbidity Index weight group score 0 in the CCH (62.9%) and fasciectomy (59.1%) cohorts, and the percentage of patients with medical conditions that comprise the Charlson Comorbidity Index were generally similar between the 2 cohorts (Supplementary Table). Also, patients in the fasciectomy cohort had a higher frequency of trigger finger syndrome, carpal tunnel syndrome, and tobacco use disorder versus those in the CCH cohort (Table 1). After propensity score matching, 702 patients remained in the CCH cohort and 999 remained in the fasciectomy cohort. Overall, the matched cohorts were well balanced across demographic and clinical characteristics.
 |
Table 1 Baseline Demographic and Clinical Characteristics for Unmatched Cohorts |
 |
Figure 1 Study flow. |
Healthcare Costs and Resource Utilization
In the unmatched sample, the mean total all-cause healthcare cost was significantly lower with CCH versus fasciectomy (US$11,897 ± US$14,633 vs US$15,528 ± US$22,254, respectively; P<0.001), mainly due to lower costs of outpatient care (Table 2). After matching, significant differences between CCH and fasciectomy were noted in several components of both all-cause (Figure 2A) and DC-related (Figure 2B) costs in the post-index period. Overall, all-cause and DC-related total costs were significantly lower in the CCH cohort than in the fasciectomy cohort (all-cause: US$11,044 ± US$12,856 vs US$12,912 ± US$19,237, respectively, P=0.02; DC-specific: US$3417 ± US$3671 vs US$5800 ± US$4985, respectively, P<0.001), despite the higher prescription costs in the CCH cohort (US$3600 ± US$6164 vs US$1806 ± US$7114, P<0.001). This difference was mainly driven by costs related to outpatient HCRU, as mean all-cause and DC-related outpatient visits were significantly lower in the CCH cohort compared with the fasciectomy cohort (P≤0.001; Table 3). No significant differences in hospitalization, emergency department visits, or outpatient medical prescriptions were found between the 2 treatment cohorts (Table 3).
 |
Table 2 Mean (SD) All-Cause Healthcare Costs During the 12-Month Post-Index Period in Unmatched Cohorts |
 |
Table 3 Healthcare Resource Utilization During the 12-Month Post-Index Period in the Matched Cohorts |
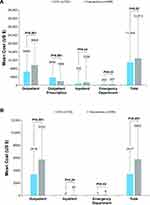 |
Figure 2 Mean (SD) all-cause (A) and DC-related (B) healthcare costs during the post-index period in the matched cohorts. |
Demographic and baseline clinical characteristics that correlated with outcome or treatment choice were age, gender, Charlson Comorbidity Index score, certain risk factors for DC, depression, some types of insurance, and geographic region (Table 1 and Supplementary Table). A generalized linear model was designed to identify key drivers of total healthcare costs in the matched cohorts. Factors independently associated with total all-cause healthcare costs were age, Charlson Comorbidity Index score, payer plan type, choice of treatment (CCH or fasciectomy), and the presence of depression or anxiety at baseline (Table 4). Treatment with CCH and the use of a consumer-driven healthcare plan were significant predictors of lower insurance company expenditures (ie, associated with lower costs). After adjustment for confounders, total healthcare costs remained significantly higher in the fasciectomy cohort than in the CCH cohort (US$9987 vs US$8934, respectively; P<0.001).
 |
Table 4 Key Drivers of Total All-Cause Healthcare Costs in Matched Cohorts |
Discussion
Studies examining differences in real-world HCRU and associated healthcare costs with CCH versus fasciectomy for the treatment of DC in the United States are limited. The current study demonstrated that utilization of healthcare resources and associated costs, reflected in this study as payments made by insurance companies, are lower in adults who receive CCH as therapy for DC compared with fasciectomy in the United States over a 1-year study period. The higher outpatient prescription costs in the CCH cohort were outweighed by the reduced total costs, mainly driven by fewer outpatient office visits.
These findings are consistent with results of another US claims database analysis, covering February 1, 2010, through December 31, 2011, in which patients with DC treated with CCH incurred significantly lower total healthcare costs and used fewer healthcare resources than patients undergoing fasciectomy.15 However, in that study, when analyzing the adjusted difference between DC-specific pre-index and post-index costs, no significant differences were observed between cohorts.15 By contrast, in the current study, both all-cause and DC-specific total costs were significantly lower with CCH than with fasciectomy. However, direct comparison between these 2 studies is limited by the different outcomes assessed (ie, total and DC-specific healthcare costs over the post-index period in the current study versus differences between pre-index and post-index DC-specific costs in the prior study). In another US claims database analysis, covering January 1, 2012, through June 30, 2016, fasciectomy for the treatment of DC was associated with a significantly higher total cost of care versus CCH treatment during the first 90 days post-treatment, with outpatient surgical visits as the main cost driver of this difference.20
Similar findings have been reported in studies from Europe. The mean cost of treatment for DC was substantially lower (~30–50%) with CCH versus fasciectomy in Spain.16,22 The cost savings ranged from 29% (CCH compared with fasciectomy with no subsequent physical therapy) to 51.5% (CCH compared with fasciectomy requiring subsequent hospitalization and physical therapy).16 Finally, cost savings in Australia were notably higher—reaching up to 64%—when treating DC with CCH instead of with fasciectomy.23 During the 12-month post-index period in the current study, DC-related costs for CCH treatment were 41% less than costs for fasciectomy. While healthcare costs and utilization can vary substantially across countries and clinical settings, this study confirms significant 1-year US insurance company cost savings for DC treatment with CCH compared with fasciectomy. On the other hand, a single-surgeon retrospective review found that time horizon may play a significant role when comparing the costs of treatment for DC.24 This study observed somewhat lower initial treatment window costs for CCH-treated patients (US$4189) than fasciectomy-treated patients (US$5291). However, when observed for 5 years, cumulative costs for CCH versus fasciectomy were similar (US$5952 vs US$5507). Statistical significance of the results was not reported in the study, thereby preventing our ability to draw a conclusion about the cost difference between the cohorts.24 The difference in the results of this study compared to ours stems from a multitude of factors. The Leafblad et al study was a one-institution, single-surgeon study versus the national level commercial data collection utilized in our study. Also, Leafblad et al performed the study in a substantially smaller cohort of patients (n=350) with quite different baseline characteristics, such as older age, and possibly different clinical stage of the disease compared to the patients included in our study’s cohorts.
Strengths and Limitations
Strengths of the current study include the large data set, which can be considered representative of the privately-insured US patient population, and the use of propensity score matching, which is an established technique for balancing observational study cohorts.21 Limitations include the nature of the retrospective claims data that were collected primarily for administrative purposes, including a higher possibility of human error than in prospectively planned research. Although the claims underwent a rigorous data-cleaning process before the analysis was conducted, claims with negative monetary amounts or duplicate claims may remain in the dataset, leading to potential information bias. Also, the database used did not capture information on the severity of DC or the number of affected digit(s); thus, it was not possible to assess the effectiveness of treatment in terms of hand function. Although the observation period of the current study was longer than that of previously published studies assessing the health-economic burden of DC, the 1-year duration might be relatively short to capture the clinical course of the disease and/or recurrence. Also, treatment-related adverse events were not recorded. However, healthcare resource usage as assessed in this study reflects real-world treatment effectiveness, and thus, may serve as an indirect measure of the benefit-risk ratio of the different treatments. A further limitation is that while the incidence of DC increases with age,25 the study comprised only privately insured patients <65 years of age. At 65 years, patients are eligible for Medicare insurance. Thus, the results of this study might not be generalizable to the Medicare-covered US population.
When interpreting the study results, it is important to note that the prescription claims database did not include information on the diagnosis, thus preventing an accurate assessment of DC-related prescription costs. However, the study was primarily designed to assess all-cause costs in DC. Also, the cost of CCH injection was included in DC-specific outpatient and inpatient costs claimed by the HCPCS, along with DC diagnosis codes (Figure 2B).
Conclusion
Findings from this retrospective claims study suggest that treatment of DC with CCH is associated with lower healthcare costs, as reflected by payer spending, and less usage of healthcare resources than fasciectomy. Future studies should assess the costs associated with productivity loss, as well as the impact of different treatments on work productivity.
Acknowledgments
The data used in the analysis were extracted from the IBM® MarketScan® Commercial Claims and Encounters Database. The MarketScan Research Databases are under the license of IBM and are available from the authors upon reasonable request and with permission of IBM. Technical editorial and medical writing assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Michael Shaw, PhD, Synchrony Medical Communications, LLC, West Chester, PA.
Funding
Funding for this study was provided by Endo Pharmaceuticals Inc., Malvern, PA. Technical editorial and medical writing assistance, provided under the direction of the authors, was funded by Endo Pharmaceuticals Inc.
Disclosure
Vladimir Zah, Djurdja Vukicevic, and Martina Imro are employees of ZRx Outcomes Research Inc. (institution founded by Endo Pharmaceuticals to conduct the present analysis), and have received grants from Endo Pharmaceuticals Inc. Jovana Pelivanovic and Simona Tatovic were employed by ZRx Outcomes Research Inc. at the time of the study. Jane Ruby was employed by Endo Pharmaceuticals Inc. at the time of the study. David Hurley is an employee of Endo Pharmaceuticals Inc. The authors report no other potential conflicts of interest for this work.
References
1. Gudmundsson KG, Jónsson T, Arngrímsson R. Guillaume Dupuytren and finger contractures. Lancet. 2003;362(9378):165–168. doi:10.1016/S0140-6736(03)13871-8
2. Picardo NE, Khan WS. Advances in the understanding of the aetiology of Dupuytren’s disease. Surgeon. 2012;10(3):151–158.
3. DiBenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand (NY). 2011;6(2):149–158.
4. Burge P, Hoy G, Regan P, Milne R. Smoking, alcohol and the risk of Dupuytren’s contracture. J Bone Joint Surg Br. 1997;79(2):206–210.
5. Gamstedt A, Holm-Glad J, Ohlson C-G, Sundström M. Hand abnormalities are strongly associated with the duration of diabetes mellitus. J Intern Med. 1993;234(2):189–193. doi:10.1111/j.1365-2796.1993.tb00729.x
6. Noble J, Heathcote JG, Cohen H. Diabetes mellitus in the aetiology of Dupuytren’s disease. J Bone Joint Surg Br. 1984;66(3):322–325. doi:10.1302/0301-620X.66B3.6725338
7. Wilburn J, McKenna SP, Perry-Hinsley D, Bayat A. The impact of Dupuytren disease on patient activity and quality of life. J Hand Surg Am. 2013;38(6):1209–1214. doi:10.1016/j.jhsa.2013.03.036
8. Engstrand C, Borén L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren’s contracture three months after fasciectomy and hand therapy interventions. J Hand Ther. 2009;22(1):
9. Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6(12):715–726. doi:10.1038/nrrheum.2010.180
10. Lipman MD, Carstensen SE, Deal DN. Trends in the treatment of Dupuytren disease in the United States between 2007 and 2014. Hand (N Y). 2017;12(1):13–20. doi:10.1177/1558944716647101
11. Brazzelli M, Cruickshank M, Tassie E, et al. Collagenase clostridium histolyticum for the treatment of Dupuytren’s contracture: systematic review and economic evaluation. Health Technol Assess. 2015;19(90):1–202. doi:10.3310/hta19900
12. Xiaflex® (collagenase clostridium histolyticum) for injection, for intralesional use [Package insert]. Malvern, PA: Endo Pharmaceuticals Inc; 2019.
13. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. doi:10.1056/NEJMoa0810866
14. Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35(12):2027–2038.
15. Donga P, DeKoven M, Kaplan FT, Tursi JP, Lee WC. Costs of collagenase clostridium histolyticum and fasciectomy for Dupuytren’s contracture. Am J Pharm Benefits. 2015;7(1):24–31.
16. Sanjuan Cerveró R, Ferrando NF, Jornet JP. Use of resources and costs associated with the treatment of Dupuytren’s contracture at an orthopedics and traumatology surgery department in Denia (Spain): collagenase clostridium hystolyticum versus subtotal fasciectomy. BMC Musculoskelet Disord. 2013;14:293. doi:10.1186/1471-2474-14-293
17. Povlsen B, Shields AM, Bhabra GS. Resource utilisation associated with single digit Dupuytren’s contracture treated with either surgery or injection of collagenase clostridium histolyticum. Hand Surg. 2014;19(2):205–209.
18. Mehta S, Belcher HJ. A single-centre cost comparison analysis of collagenase injection versus surgical fasciectomy for Dupuytren’s contracture of the hand. J Plast Reconstr Aesthet Surg. 2014;67(3):368–372.
19. Atroshi I, Strandberg E, Lauritzson A, Ahlgren E, Waldén M. Costs for collagenase injections compared with fasciectomy in the treatment of Dupuytren’s contracture: a retrospective cohort study. BMJ Open. 2014;4(1):e004166.
20. Camper SB, Divino V, Hurley D, DeKoven M. Cost per episode of care with Collagenase clostridium histolyticum versus fasciectomy for Dupuytren contracture: a real-world claims database analysis. J Hand Surg Glob Online. 2019;1(2):57–64.
21. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):69–80.
22. De Salas-Cansado M, Cuadros M, Del Cerro M, Arandes JM. Budget impact analysis in Spanish patients with Dupuytren’s contracture: fasciectomy vs. collagenase Clostridium histolyticum. Chir Main. 2013;32(2):68–73. doi:10.1016/j.main.2013.02.012
23. Sefton AK, Smith BJ, Stewart DA. Cost comparison of collagenase clostridium histolyticum and fasciectomy for treatment of Dupuytren’s contracture in the Australian Health System. J Hand Surg Asian Pac Vol. 2018;23(3):336–341. doi:10.1142/S2424835518500327
24. Leafblad ND, Wagner E, Wanderman NR, et al. Outcomes and direct costs of needle aponeurotomy, collagenase injection, and fasciectomy in the treatment of Dupuytren contracture. J Hand Surg Am. 2019;44(11):919–927.
25. Hindocha S. Risk factors, disease associations, and Dupuytren diathesis. Hand Clin. 2018;34(3):307–314.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
