Back to Journals » Journal of Pain Research » Volume 16
Head and Neck Cancer-Related Pain: A Descriptive Analysis of the Pain Phenotypes
Authors Khawaja S , Bavarian R , Abdul Rehman S , Hafeez H
Received 21 March 2023
Accepted for publication 18 August 2023
Published 25 August 2023 Volume 2023:16 Pages 2919—2927
DOI https://doi.org/10.2147/JPR.S411285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amitabh Gulati
Shehryar Khawaja,1– 3 Roxanne Bavarian,4 Summaiya Abdul Rehman,1 Haroon Hafeez1
1Department of Internal Medicine, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Punjab, Pakistan; 2Department of Diagnostic Sciences, Tufts University School of Dental Medicine, Boston, MA, USA; 3Department of Oral Diagnostic Sciences, University at Buffalo, School of Dental Medicine, Buffalo, NY, USA; 4Department of Oral and Maxillofacial Surgery, Harvard School of Dental Medicine, Boston, MA, USA
Correspondence: Shehryar Khawaja, Department of Internal Medicine, Shaukat Khanum Memorial Cancer Hospital and Research Centre, 7A Block R-3 M.A. Johar Town, Lahore, 54782, Pakistan, Tel +92 42 3590 5000 Ext 4452, Email [email protected]
Objective: This study aimed to describe the clinical characteristics and prevalence of different painful phenotypes in head and neck cancer (HNC)-related pain.
Materials and Methods: A cross-sectional study was conducted on 100 patients who presented with HNC-related pain. All patients underwent a comprehensive clinical assessment and were stratified in one or more painful phenotypes constructed based on the International Classification for Orofacial Pain, first edition, and International Classification for Headache Disorders, third edition.
Results: Among the participants included, 68% were male, and the mean age of the cohort was 49.71 ± 14.14 years. The most prevalent cancer sites were the tongue (29%) and buccal mucosa (24%). The average pain intensity was 5.88 ± 2.53 on an 11-point numeric verbal pain rating scale, where 0 was indicative of “no pain” and 10 was suggestive of “worst pain imaginable”. However, the worst pain intensity over the last month was 8.95 ± 1.53. The average number of pain sites per patient was 6, and the most common pain descriptors were dull ache, burning, and sharpness. Myofascial pain, jaw bone pain, and burning pain disorder were the most common phenotypes, and on average, three different phenotypes co-existed.
Conclusion: HNC-related pain has a varying and complex clinical profile, which may mirror the pain profiles of primary pain disorders, such as myofascial pain, jaw bone pain, or burning pain disorders, and often presents together as a cluster of phenotypes.
Plain Language Summary: The clinical presentation of head and neck cancer (HNC)-related pain varies considerably. This study assessed 100 patients with HNC-related pain. It stratified their symptoms into one or more known painful disorders based on the International Classification for Orofacial Pain, first edition and International Classification for Headache Disorders, third edition. It was found that HNC-related most commonly resembled myofascial pain, jaw bone pain, and burning pain disorder. On average, features of three different types of painful disorders co-existed. These results help illustrate the uniqueness and difficulty associated with managing HNC-related pain.
Keywords: head and neck cancer, pain, phenotypes, myofascial, neuralgia, neuropathic, trigeminal
Introduction
Head and neck cancers (HNCs) represent a heterogenous group of aerodigestive tract cancers, among which 90% are squamous cell carcinoma or variants thereof.1 The biological behavior of HNCs is aggressive and is associated with significant disease-related destruction.2,3
The prevalence of pain in all cancer types is around 50%.4 However, this is reported to be higher, averaging 70% among HNCs.3,4 Yet, there are no universally accepted diagnostic criteria or classifications for HNC-related pain.5,6 Instead, signs and symptoms of HNC-related pain have been categorized based on the underlying pathophysiological mechanism (nociceptive or inflammatory, neuropathic, or both), location (local or distant), or the protagonist of pain (cancer-related, treatment-related, or both).1,7,8
Prior studies have suggested that 43% to 80% of all patients with cancer-related pain receive inadequate pain management.3,9 A significant reason for this inefficacy is the diverse clinical signs and symptoms of HNC-related pain. The understanding of this variable clinical presentation is limited across the literature, and to date, no such data exists. It has been hypothesized that patients with HNC-related pain may present with clinical characteristics, or phenotypes, that may resemble primary pain disorders affecting the craniofacial and cervicogenic regions, such as myofascial orofacial pain, jaw bone pain or trigeminal neuralgia, which are defined in the International Classification of Orofacial Pain (ICOP-1) and International Classification for Headache Disorders (ICHD-3). It has also been reported that patients may have more than one painful phenotype.8
This study aimed to describe the clinical characteristics and prevalence of different painful phenotypes among patients with HNC-related pain based on modifications of the phenotypes described in the ICOP-1 and ICHD-3.5,10
Materials and Methods
This cross-sectional study was conducted in accordance with STROBE guidelines on adult patients who presented to the Orofacial Pain Clinic at the Shaukat Khanum Memorial Cancer Hospital and Research Centre in Pakistan with pain associated with HNC.11 Approval from the local Institutional Review Board was received (IRB-18-05) and written consent for study inclusion was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki.12
Patient Recruitment
The recruitment of participants started in January 2019, intending to enroll 100 participants (confidence interval of 95% and margin of error of 8.3%), and was completed in August 2022. Patients were referred to the Orofacial Pain Clinic in the Shaukat Khanum Memorial Cancer Hospital from various sources, including the head and neck oncology clinic, oral and maxillofacial surgery clinic, palliative medicine clinic, or emergency assessment room. All participants seen in the Orofacial Pain Clinic underwent a comprehensive clinical assessment consisting of a detailed history and thorough examination by an Orofacial Pain specialist, with additional imaging studies conducted when indicated.
Patient Eligibility
Inclusion criteria included all adult male and female patients who presented with HNC-related pain who consented to participate in the study. The diagnosis of HNC-related pain was made using the criteria outlined in Table 1. This is a modification of the ICHD-3 diagnostic criteria for headache attributed to intracranial neoplasm.6 HNC in this study was defined as primary tumors originating from the head and neck region or tumors derived from a different site in the body and then metastasized to the head and neck area (ICD-10 C76.0/3, C76.0/6, and C76.0/9), as diagnosed by a board-certified oncologist and in accordance with the American Joint Committee on Cancer (AJCC) Guidelines. Participants with oropharyngeal mucositis, a well characterized acute toxicity of chemoradiation, and post-operative pain disorder (a painful disorder that developed within four weeks of completion of the oncological surgery, chemotherapy, or radiation therapy and persisted for at least 3 months post-operatively) were excluded.13
 |
Table 1 Diagnostic Criteria for Head and Neck (HNC)-Related Pain |
Examination Protocol
During the consultation, participants were asked for the current, worst, and least pain intensity in the last month on an 11-point numeric verbal pain rating scale, where 0 was indicative of “no pain” and 10 was suggestive of “worst pain imaginable”. The participant provided details regarding pain site(s), laterality, chronicity, frequency, quality, and duration of pain. A clinical examination was performed by an orofacial pain specialist. The musculoskeletal assessment was performed using the examination principles defined in the Diagnostic Criteria for Temporomandibular Disorders (DC-TMD). For the neurological and cervicogenic examination, other guidelines were followed.14,15 The site of pain was assessed across different locations in the head and neck. These included intraoral sites (tongue, buccal mucosa, alveolar process of the maxilla and mandible, and teeth), extraoral orofacial sites (temporal, masseteric, auricular, mandibular body, posterior auricular, submandibular, zygomatic, and infraorbital regions), cervical region (anterior, lateral, and posterior regions), and cranium (supraorbital, orbital, parietal, vertex, and occipital regions). In addition, data on the subjective presence of dry mouth (xerostomia), taste alteration (dysgeusia), and swallowing difficulties (dysphagia) was recorded. Similarly, if the interincisal distance on maximum mouth opening was less than 20 millimeters, it was noted as trismus.
Classifying Phenotypes of HNC-Related Pain
The subjective history of present illness combined with objective clinical and radiographic findings were then used to stratify participants’ symptomatology into one or more types of painful phenotypes. These phenotypes were jaw bone pain, myofascial pain disorder (orofacial or cervical), neuralgia, persistent pain disorder (facial or dentoalveolar) and burning disorder (mouth or face). The operational criteria of these clinical phenotypes are outlined in Tables 2–6 and were based upon the ICOP-1 and ICHD-3. If the signs and symptoms of the participants could not be categorized into one of these painful phenotypes, they were given a clinical phenotype of “Unknown” .For participants presenting with symptoms of a headache disorder, the clinical features of the headache were categorized using diagnostic guidelines for primary headache disorders as per the ICHD-3.6
 |
Table 2 Diagnostic Criteria for Jaw Bone Pain Phenotype |
 |
Table 3 Diagnostic Criteria for Myofascial Orofacial or Cervical Pain Disorder Phenotype |
 |
Table 4 Diagnostic Criteria for Neuralgia Phenotype |
 |
Table 5 Diagnostic Criteria for Persistent Facial or Dentoalveolar Pain Disorder Phenotype |
 |
Table 6 Diagnostic Criteria for Burning Mouth or Face Disorder Phenotype |
Data Collection and Analysis
Medical charts were reviewed to extract data regarding participant demographics, medical history, cancer characteristics, prior and concurrent anti-cancer therapies, and information about the recurrence or metastasis of the disease. Data analysis was performed using SPSS version 22.0 software (SPSS Inc. Chicago, IL, USA). Descriptive statistics were computed for each variable.
Results
A total of 100 participants were enrolled in the study. Of these, 68% were male. The mean age of the participants was 49.71 ± 14.14 years, and 56.6% identified themselves as Punjabi in ethnicity. The most common cancer type was oral squamous cell carcinoma (81%), followed by adenoid cystic carcinoma (6%). Other histopathologic diagnoses consisted of medullary thyroid carcinoma (3%) and breast cancer (1%) metastasized into the nasopharynx, leiomyosarcoma (1%) and osteosarcoma (1%) of the mandible, mammary analogue secretary carcinoma of the parotid gland (1%), salivary duct carcinoma of the submandibular gland, and in 3% of the cases, primary tumor origin was unknown. All participants had stage IV disease according to American Joint Committee on Cancer Tumor-Node-Metastasis (AJCC-TNM) classification. The most prevalent primary sites of HNC were the tongue (29%) and buccal mucosa (24%). Most participants had previously received radiation therapy (80%). However, the most common concurrent oncological treatment participants received was chemotherapy (45%) (Table 7). All patients were referred from within the cancer hospital, with the majority (51%) being from the Head and Neck Cancer Clinic (51%). Other referral sources include the Emergency Assessment Room (25%), Palliative Medicine Clinic (16%), Pain Medicine Clinic (4%), or other specialty clinics such as Pulmonology and Infectious Disease (4%).
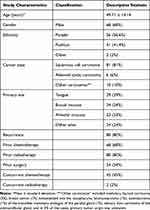 |
Table 7 Demographics and Cancer-Related Characteristics of the Study Population (n = 100) |
The average pain intensity at the time of the evaluation was 5.88 ± 2.53. However, the worst pain severity over the last month was an average of 8.95 ± 1.53 in intensity. In most participants, the pain was unilateral in distribution (65%) and constant in periodicity (93%). The most common descriptors of pain were dull ache (86%), burning (57%), and sharp (29%), and the most prevalent associated signs and symptoms with pain were dysphagia (56%) and trismus (45%) (Table 8).
 |
Table 8 Head and Neck Cancer-Related Pain Characteristics of the Study Population (n = 100) |
The pain site was assessed across 19 locations in the head, face, mouth, and neck. On average, each participant had approximately six sites of pain. Among these, the most common sites were lateral (65%) and posterior cervical regions (64%), the body of the mandible (60%), and temporal region (60%).
The most common clinical phenotype among participants were myofascial pain disorder affecting the masticatory and cervical muscles (81%), jaw bone pain disorder (53%), and burning pain disorder (40%). Furthermore, 18% of the participants had a headache disorder, among which the most common phenotype was migraine headaches (9%). The mean number of clinical phenotypes identified per participant were 3.27 ± 1.40. In 16%, the cluster of symptoms could not be compartmentalized into a known clinical phenotype (Table 9).
 |
Table 9 Head and Neck Cancer-Related Pain Clinical Phenotypes of the Study Population (n = 100) |
Discussion
The purpose of this study was to describe the clinical characteristics and prevalence of various painful phenotypes among patients with HNC-related pain. The average pain intensity at the time of assessment was moderate (5.88 ± 2.53). However, in the 30 days preceding the evaluation, the average maximum pain severity was rated around nine. On the contrary, the least pain intensity felt by the participants was 3.29 ± 2.59. Regarding frequency, 93% of the participants had constant pain. This suggests that most patients will always have some degree of pain, and pain intensity may vary drastically throughout the illness. In a prior pain study, authors reported a similar observation in which the intensity of pain varied over time.7 It was reported to be highest prior to initiating cancer treatment and least towards the end of the treatment. Authors hypothesized this phenomenon to be associated with eradicating the underlying disease process. However, in the present study, all patients had active disease, most had a recurrence, and in many, it was deemed resistant to oncological therapies.
Subjective symptoms of xerostomia, dysphagia, dysgeusia, and trismus were common among the participants. These are known symptoms of advanced stage-HNC and expected side effects of radiation therapy, chemotherapy, and oncological surgery.3,16,17 In the present study, 80% of the participants had undergone radiation therapy, and 68% had previously received chemotherapy. The presence of non-painful supplementary symptoms often makes the management of HNC-related pain difficult, as they negatively impact the overall health quality of life, pain, and masticatory and digestive functions.16,17 Similarly, they may convolute painful characteristics, which may make the identification of correct painful phenotype and, subsequently, management challenging and complex.
The participants chose several descriptors to describe pain quality. In a prior pain study that examined patients with HNC, authors reported tender, sore, dull, and throbbing as the most frequent nociceptive descriptors of pain and burning and aching as the most common descriptors of neuropathic pain.7 Similar descriptors were reported in the present study as well. Most participants described painful symptoms as dull ache, burning, sharp, or throbbing pain. It is relevant to note that tender, sore, and dull were considered “dull ache” in the present study. In Urdu, the national language of the country, the translation of these words is the same.18
Dull ache and sharp pain descriptors are often associated with musculoskeletal pain disorders.19 Correspondingly, the most common pain sites were lateral and posterior cervical regions, the body of the mandible, and temporal regions. These sites are generally associated with myofascial and jaw bone pain phenotypes. Each participant, on average, reported pain across six sites. On the contrary, a much lower number has been reported in prior studies.7,20 This disparity is likely due to the widespread nature of the disease in the present cohort. All participants had stage IV disease, and most had a disease recurrence.
The clinical symptoms of HNC-related pain were stratified into various clinical phenotypes created in accordance with the ICOP-1 and ICHD-3. Among these, myofascial pain disorder, jaw bone pain disorder, and burning pain disorder had the highest prevalence, and neuralgia and persistent pain phenotypes were relatively rare. Furthermore, on average, each participant had nearly three concurrent painful phenotypes. These are novel and noteworthy findings, which corroborate the divergent clinical characteristics of HNC-related pain and help explain the complexities associated with classifying and managing HNC-related pain.
The pathophysiology of HNC-related pain is intricate. The clinical phenotype of pain is a consequence of a complex interaction between cancer cells, the cancer microenvironment (consisting of non-cancerous cells such as the tissue matrix, blood vessels, immune cells, and extracellular matrix), and systemic physiological processes overlapping during various phases of carcinogenesis.2,8,21 The regional tissue, muscular, bone, and nerve damage can result in a cluster of symptoms, which may resemble primary pain disorders. These interactions continue to evolve through illness, therapy, and survivorship, which may explain why a patient may have concurrent pain phenotypes.
In 16% of the participants, symptoms could not be categorized into a pre-established clinical phenotype. Interestingly, in all of these patients presenting with an unknown phenotype, their pain presented as neuropathic in nature, but were ultimately unable to fulfill the criteria for a distinct phenotype. Within this cohort, seven had probable persistent facial or dentoalveolar pain phenotype, six had probable burning face or mouth disorder, and four had probable neuralgia pain.
A limitation of this study is the use of a non-validated methodology for assessing pain quality and clinical phenotypes, which may have led to observation bias. However, in the present investigation, a single board-certified Orofacial Pain specialist assessed all patients using a standard protocol. Moreover, criteria for different phenotypes of pain were established using internationally accepted diagnostic guidelines, the ICOP-1 and the ICHD-3.5,10 Likewise, to prevent any confirmation bias, if the symptoms could not be indexed against pre-determined phenotypes, they were classified as unknown. Nevertheless, these results should be interpreted with caution and necessitate replication.
In conclusion, these findings suggest that HNC-related pain has a varying and complex clinical profile. Signs and symptoms may mirror the pain profiles of primary pain disorders, such as myofascial pain, jaw bone pain, or burning pain disorders, and often present together as a cluster of phenotypes. The presence of each or multiple clinical phenotypes in a patient with HNC-related pain is clinically relevant, as each phenotype has its own underlying pathophysiology and evidence-based treatment. Future studies using the clinical phenotype model for tailoring the management of HNC-related pain patients are advocated. Furthermore, the inclusion of HNC-related pain as a diagnostic category in the upcoming versions of head and neck pain classification systems is recommended.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Epstein JB, Elad S, Eliav E, Jurevic R, Benoliel R. Orofacial pain in cancer: part II—clinical perspectives and management. J Dent Res. 2007;86(6):506–518. doi:10.1177/154405910708600605
2. Benoliel R, Epstein J, Eliav E, Jurevic R, Elad S. Orofacial pain in cancer: part I--mechanisms. J Dent Res. 2007;86(6):491–505. doi:10.1177/154405910708600604
3. Yarom N, Sroussi H, Elad S. Orofacial pain in patients with cancer and mucosal diseases. In: Farah CS, Balasubramaniam R, McCullough MJ, editors. Contemporary Oral Medicine. Springer International Publishing; 2019:2187–2212.
4. van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi:10.1093/annonc/mdm056
5. International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia. 2020;40(2):129–221.
6. Olesen J. International Classification of Headache Disorders. Lancet Neurol. 2018;17(5):396–397. doi:10.1016/S1474-4422(18)30085-1
7. Epstein JB, Wilkie DJ, Fischer DJ, Kim YO, Villines D. Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol. 2009;1:26. doi:10.1186/1758-3284-1-26
8. Khawaja SN, Scrivani SJ. Head and neck cancer-related pain. Dent Clin N Am. 2022;2022:1.
9. Apolone G, Corli O, Caraceni A, et al. Pattern and quality of care of cancer pain management. Results from the cancer pain outcome research study group. Br J Cancer. 2009;100(10):1566–1574. doi:10.1038/sj.bjc.6605053
10. Olesen J. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia. 2018;38(1)1–211.
11. Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int Surg J. 2014;12(12):1495–1499.
12. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
13. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
14. Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and orofacial pain special interest group. J Oral Facial Pain Headache. 2014;28(1):6–27. doi:10.11607/jop.1151
15. Sharav Y, Benoliel R, eds. Orofacial Pain and Headache. Mosby; 2008.
16. Elad S, Zadik Y, Yarom N. Oral Complications of Nonsurgical Cancer Therapies. Atlas Oral Maxillofac Surg Clin North Am. 2017;25(2):133–147. doi:10.1016/j.cxom.2017.04.006
17. Jensen SB, Pedersen AML, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18(8):1039–1060. doi:10.1007/s00520-010-0827-8
18. Salim M, Mansab AC. Urdu pain words. J Coll Physicians Surg Pak. 1999;9(10):463–466.
19. Sarlani E, Balciunas BA, Grace EG. Orofacial pain—part I: assessment and management of musculoskeletal and neuropathic causes. AACN Clin Issues. 2005;16(3):333–346. doi:10.1097/00044067-200507000-00007
20. Nasir KS, Hafeez H, Jamshed A, Hussain R. Effectiveness of nerve blocks for management of head and neck cancer associated neuropathic pain disorders; a retrospective study. J Cancer Allied Spec. 2020;6(2). doi:10.37029/jcas.v6i2.367
21. Schmidt BL. The neurobiology of cancer pain. J Maxillofac Surg. 2015;73(12):S132–S135. doi:10.1016/j.joms.2015.04.045
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
