Back to Journals » Journal of Inflammation Research » Volume 15
HBV Infection Status Does Not Influence the Initial Metastatic Pattern and the Prognosis of Breast Cancer Patients with de novo and Relapsed Metastatic Disease
Authors Zhang N, Tao D, Lei H, Shao Q , Liu Y, Long H, Zeng X
Received 22 December 2021
Accepted for publication 13 April 2022
Published 21 April 2022 Volume 2022:15 Pages 2509—2521
DOI https://doi.org/10.2147/JIR.S355301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Ningning Zhang,1 Dan Tao,2 Haike Lei,3 Qing Shao,1 Yumin Liu,4 Hua Long,4 Xiaohua Zeng1
1Department of Breast Cancer Center, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 2Department of Radiation Oncology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 3Department of Appointment and Follow-up Center, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 4Department of Medical Record, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China
Correspondence: Xiaohua Zeng, Department of Breast Cancer Center, Chongqing University Cancer Hospital, 181 Han Yu Road, Shapingba District, Chongqing, 400030, People’s Republic of China, Tel/Fax +86-23-65310859, Email [email protected]
Purpose: This study aimed to evaluate the influence of hepatitis B virus (HBV) infection status on the initial metastatic pattern and prognosis in metastatic breast cancer (MBC).
Methods: MBC patients admitted to Chongqing University Cancer Hospital between January 2011 and December 2019 were enrolled. The association of HBV infection status with clinicopathological features was analyzed. The impact of HBV infection status on initial metastatic pattern and survival was evaluated.
Results: A total of 1124 patients with MBC, including 310 with de novo (cohort A) and 814 with relapsed metastatic disease (cohort B), were eligible for this study. Seropositive HBsAg was identified in 28 (9.0%) and 68 (8.4%) patients in cohort A and B, respectively. The clinicopathological features are similar between HBsAg-positive and HBsAg-negative patients. There was no significant association of HBV infection status with the rate of metastasis at each site in de novo and relapsed MBC. HBsAg-positive patients tended to have longer metastasis-free survival (MFS) and/or overall survival (OS) time, but it was not the independent prognostic factor.
Conclusion: In conclusion, HBV infection status does not influence the initial metastatic pattern and the prognosis of MBC patients.
Keywords: hepatitis B virus, infection status, metastatic breast cancer, initial metastatic pattern, prognosis
Introduction
The incidence rates of breast cancer continue to increase by about 0.5% per year,1 and breast cancer has become the first cancer worldwide.2 Of these, about 5% to 15% of breast cancer patients present with distant metastasis at diagnosis.3 However, surgery-based comprehensive treatment can cure some patients with early-stage breast cancer. Recurrence and metastasis are still unavoidable and are the leading causes of death for breast cancer patients.4–6 Therefore, exploring breast cancer metastasis factors can provide ideas and scientific basis for clinical intervention.
Hepatitis B virus (HBV) infection is a significant public health problem worldwide. The infection rate is especially high in some developing countries, such as China.7 HBV infection has long been known to play a role in the development of cholangiocarcinoma and hepatocellular carcinoma (HCC).8,9 Furthermore, increasing studies show that HBV exists in extrahepatic tissues, including the colon, kidneys, pancreas, lymph nodes, bone marrow, vessel walls, and skin.10,11 Previous studies indicated that HBV infection was related to a higher risk of extrahepatic malignancies, including pancreatic cancer, non-Hodgkin’s lymphoma and breast cancer.12–18 Some studies also proposed a hypothetical mechanism for the development of breast cancer as a result of HBV infection: persistence of occult infection and continuous replication of HBV lead to long-term subtle liver damage, and long-term necro-inflammatory damage to the liver may result in persistently high levels of estrogen, which is mainly deactivated in the liver and is a dominant risk factor for breast cancer.17,19 HBV may also directly affect the breast cells through its cis and trans effects of HBx, which may act as oncoprotein.19
Some studies have reported the impact of HBV infection on metastasis patterns of HCC, colorectal cancer (CRC), and pancreatic cancer.20–23 In HCC with HBV infection, intrahepatic metastasis happened less frequently than extrahepatic metastasis.24 Moreover, HBV infection decreases the risk of liver metastasis in patients with CRC.22,23 These findings suggest that HBV infection may serve an essential role in the development of certain non-liver malignancies. However, pancreatic cancer patients who were HBsAg positive had significantly more liver metastases than those who were HBsAg negative.21 Meanwhile, HBV infection was found to affect the prognosis of HCC, CRC, pancreatic cancer, and nasopharyngeal carcinoma patients,20–23,25–27 but these studies reported inconsistent conclusions in different tumors. Notably, there was little research on the influence of HBV infection on the initial metastatic pattern of breast cancer, and the impact of different HBV infection statuses on breast cancer survival has not been adequately investigated. Thus, we embarked on this study to explore whether HBV infection was associated with initial metastatic pattern and survival in metastatic breast cancer (MBC).
Materials and Methods
Study Population
Data of patients treated at Breast Cancer Center of Chongqing University Cancer Hospital (Chongqing, China) between January 2011 and December 2019 who were diagnosed with breast cancer were retrospectively screened. This cancer center is one of the largest in southwest China (covering a population of 32.05 million residents who live in an approximately 82,402.95 km2 area). The following eligibility criteria were used to recruit patients: female patients with a definitive diagnosis of breast cancer, with at least one distant site metastasis, with complete information of molecular typing and available test results for HBV infection. Patients with bilateral breast cancer and other tumor diseases were excluded. Occult breast cancer (OBC) is a type of breast cancer that presents as metastatic axillary lymph node with undetectable breast lesions on all diagnostic modalities. Due to the low incidence and unique clinical manifestations, the diagnosis, treatment, and prognosis of OBC are still unclear. Moreover, the prognosis and prognostic factors for OBC remain controversial.28 Thus, we excluded OBC from our analysis in the present study to reduce some confusion. Patients have signed an informed consent about allow their information to be stored and used in the hospital database. This study was performed in accordance with the Declaration of Helsinki and approved by the medical ethics committee of the Chongqing University Cancer Hospital.
Data Collection
Information including demographic data, clinical and tumor characteristics, and treatment scheme were obtained from patient electronic medical records. Data on survival were collected from this center’s follow-up registry. The TNM stages were evaluated in accordance with the guidelines of the seventh Edition of the American Joint Committee on Cancer (AJCC).29 Histopathology was based on the World Health Organization (WHO) international histological classification.30 The modified Scarff-Bloom and Richardson system (mSBR) was used to determine the histological grade of the primary tumor.31 If staining of the nuclear tumor cells was more than 1% of the total tumor cells, estrogen receptor (ER) and progesterone receptor (PR) were confirmed to be positive. If the IHC staining was 3+ and IHC 2+, human epidermal growth factor receptor 2 (HER2) was considered positive, and ambiguous results were considered positive if the fluorescence in situ hybridization (FISH) showed amplification. Some patients with HER2 IHC 2+ but with the absence of FISH results were considered HER2 negative.
The time with the diagnosis of distant organ metastasis, including bone, lung/pleura, liver, and central nervous system (CNS), was recorded. CNS metastasis was defined as those with either metastasis in the brain parenchyma and/or metastasis in the leptomeninges. Metastatic status was evidenced with imaging/pathology examinations and/or radiologic abnormalities confirmed by computed tomography (CT) imaging or magnetic resonance imaging (MRI) or whole-body bone scan. Oligometastasis and polymetastasis were respectively defined as only one and ≥2 organ metastasis. Metastasis-free survival (MFS) was defined as the time from the initial diagnosis of breast cancer to the date with the diagnosis of distant metastasis. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death or last follow-up.
Information and Category of HBV Infection
Serum samples were collected and separated to test HBV by enzyme-linked immunosorbent assay at the Clinical Laboratories of Chongqing University Cancer Hospital. HBV serological markers including hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antibody (HBeAb) and hepatitis B core antibody (HBcAb) were recorded at the time of patients’ first hospitalization in our hospital. Based on the HBV infection status, patients were divided into active HBV group, resolved HBV group, and HBV negative group. Active HBV group was characterized by HBsAg positive, no matter of the status for HBeAg. Resolved HBV was defined as HBsAg negative and at least one of HBeAb and HBcAb positive, which indicated previous HBV infection, but the virus had previously been eradicated. HBV negative referred to HBsAg, HBeAg, HBeAb, and HBcAb negative, regardless of the status of HBsAb, and implied no history of HBV infection based on the blood test result.32,33 A further categorization classified patients into two groups, HBsAg positive (HBsAg+) and HBsAg negative (resolved HBV + HBV negative).
Statistical Analyses
The statistical analyses were performed by SPSS version 17.0 software (SPSS, Chicago, IL). The chi-square test, Student’s t-test, or Kruskal–Wallis H-test were used as appropriate to assess differences in baseline values between the HBsAg-positive and -negative groups. A two tailed p value <0.05 was considered significant, and a Bonferroni correction for multiple testing was performed. The Kaplan–Meier method was used to plot survival curves, and the Log rank test was used to assess the differences. For both univariate and multivariate analysis, a Cox regression was used. The Cox proportional-hazards model was used to calculate the hazard ratio (HR) and 95% confidence interval (95% CI). Significant variables in the univariate analysis were included for the multivariable analysis using the forward stepwise method.
Results
Baseline Characteristics
After preliminary screening, 1398 breast cancer patients with distant metastasis were further screened, of whom 13 were excluded because of the male gender, 101 patients were eliminated due to non-available HBV infection information, and three patients were excluded with hepatitis C virus (HCV) infection. Forty-three patients were eliminated with insufficient pathological diagnosis or molecular typing information. Other 114 patients were further excluded for combined with other primary cancer. Finally, a total of 1124 cases were qualified for the analyses, including 310 (27.6%) patients with distant metastasis at diagnosis (termed as cohort A) and the remaining 814 (72.4%) patients with relapsed distant metastasis (termed as cohort B). The flowchart of the patient selection process is shown in Figure 1.
 |
Figure 1 Flow diagram of patient selection into the study cohort. |
In the whole population, seropositive HBsAg was identified in 96 (8.5%) patients. Resolved HBV (HBsAg negative and either HBeAb or HBcAb positive) was identified in 530 (47.2%) patients (143 in cohort A and 387 in cohort B). HBV negative (HBsAg, HBeAb, and HBcAb negative) was identified in 498 (44.3%) patients (139 in cohort A and 359 in cohort B). Thus, 96 patients (28 in cohort A and 68 in cohort B) were assigned to the HBsAg-positive group, while 1028 patients (282 in cohort A and 746 in cohort B) were assigned to the HBsAg-negative group (resolved HBV + HBV negative). Due to previous studies34–36 and our data analysis (data not shown) indicating that de novo and recurrent MBC represent different MBC populations, we further analyzed the distribution of the rate of metastasis at each site according to HBV infection status in breast cancer patients with de novo and relapsed distant metastasis. The results are shown in Table 1 and Figure 2. As the comparisons of baseline characteristics listed in Table 1, HBsAg-positive and HBsAg-negative patients are similar in most clinicopathological features. However, for cohort A, the results showed that the index of HER2 and body mass index (BMI) were significantly associated with HBV infection status. These differences might be a chance finding due to the imbalance in the baseline characteristics and sample size between the groups. In addition, HBsAg-positive patients tended to be younger at diagnosis, but the difference was not significant. Moreover, there were no additional significant differences in patient characteristics between the HBsAg-positive and -negative groups.
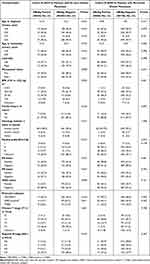 |
Table 1 Comparison of Baseline Characteristics Between HBsAg-Positive and HBsAg-Negative Group in de novo and Relapsed Metastatic Breast Cancer |
HBV Infection Status and Metastatic Pattern
In cohort A, CNS, liver, lung/pleura, bone and polymetastasis metastasis were respectively found in 3 (10.7%), 6 (21.4%), 11 (39.3%), 18 (64.3%) and 14 (50.0%) patients in active HBV group, 10 (7.0%), 36 (25.2%), 53 (37.1%), 66 (46.2%) and 49 (34.3%) patients in resolved HBV group, and 15 (10.8%), 33 (23.7%), 54 (38.8%), 75 (54.0%) and 58 (41.7%) patients in HBV negative group (Figure 2A). In cohort B, CNS, liver, lung/pleura, bone and polymetastasis metastasis were respectively found in 6 (8.8%), 15 (22.1%), 35 (51.5%), 36 (52.9%) and 31 (45.6%) patients in active HBV group, 31 (8.0%), 109 (28.2%), 178 (46.0%), 165 (42.6%) and 159 (41.1%) patients in resolved HBV group, and 22 (6.1%), 90 (25.1%), 142 (39.6%), 168 (46.8%) and 134 (37.3%) patients in HBV negative group (Figure 2B). No significant difference in intrahepatic and extrahepatic metastasis rate at each site was found between active HBV group, resolved HBV group, and HBV negative group (Figure 2A and B). We further compared the rate of metastasis at each site between HBsAg-positive group and HBsAg-negative group. However, no significant difference was observed. The detailed information is shown in Figure 2C and D.
The Associations Between HBV Infection Status and Survival
At the time of this analysis (September 17, 2021), 176 (56.8%) of the patients with de novo MBC and 454 (55.8%) of the patients with relapsed MBC had died. In cohort A population, active HBV group and resolved HBV group tended to have longer median OS time than HBV negative group (38.27 vs 25.20 months, P=0.0317; 34.53 vs 25.20 months, P=0.0308, respectively, P<0.05/3=0.017 was considered as significant; Table 2 and Figure 3A). However, in the multivariate analysis, significant difference was not found (Table 3). In cohort B population, the median OS time for patients with active HBV, resolved HBV, and HBV negative were 83.27 months, 72.03 months, and 75.13 months, respectively. However, significant difference in OS among these three groups was not found (Figure 3B). When the cohort was separated into two groups, both in cohort A and cohort B, the median OS of patients in HBsAg-positive group was longer than that in HBsAg-negative group, but the difference was not significant (38.27 vs 28.70 months, P = 0.115; 83.27 vs 73.00 months P = 0.360, respectively, Figure 3C and D). In cohort B, patients with three HBV infection statuses, including active HBV, resolved HBV, and HBV negative, had no significant difference in the median MFS time (30.0 months, 25 months, and 27 months, respectively, Figure 3E). Although HBsAg-positive patients had longer median MFS time than HBsAg-negative patients, the difference was not significant (30.0 vs 25.0 months, P = 0.265, Figure 3F). Table 3 summarizes the prognostic factors associated with OS. For patients with de novo MBC, nuclear grade (HR: 3.496, 95% CI: 1.394–8.767, P=0.008), liver metastasis (HR: 9.742, 95% CI: 2.060–46.080, P=0.004) and CNS metastasis (HR: 23.070, 95% CI: 2.827–188.278, P=0.003) at initial diagnosis were identified to be independent prognostic factors that increased the death risk. For patients with relapsed MBC, later primary T staging was an independent risk factor for poor prognosis (HR: 1.517, 95% CI: 1.021–2.253, P=0.039). However, breast surgery independently decreased the death risk (HR: 0.028, 95% CI: 0.003–0.243, P<0.0001), and patients with longer MFS time had a favorable prognosis (HR: 0.364, 95% CI: 0.272–0.488, P<0.0001) (Table 3).
 |
Table 2 Univariable Cox Regression Analysis for Overall Survival in Breast Cancer Patients with de novo and Recurrent Distant Metastasis |
 |
Table 3 Multivariable Cox Regression Analysis for Overall Survival in Breast Cancer Patients with de novo and Recurrent Distant Metastasis |
Discussion
To the best of our knowledge, the present study is the first large-scale study to investigate the influence of HBV infection on initial metastatic patterns and the prognosis of MBC patients with de novo and relapsed metastatic disease. The main finding of this study is that HBV infection status did not impact the initial metastatic patterns in MBC population, regardless of intrahepatic or extrahepatic metastasis. Moreover, although patients with HBV infection had a longer survival time, HBV infection was not the independent prognostic factor for MBC. Furthermore, we observed that in recurrent MBC, young BC patients (less than 50 years old) tended to account for a higher proportion of HBV infection patients than those who were 50 years old or above. To some extent, these results were consistent with the other study, which showed that young breast cancer was associated with an increased prevalence of HBV infection.16,17
HBV infection has also been connected to an increased risk of breast cancer morbidity, aside from HCC. Serum HBsAg monitoring is still one of the most critical elements in determining the status of HBV infection.37–39 Positivity for serum HBsAg identifies patients who have been exposed to HBV and have an acute or chronic HBV infection.38,40 China has the world’s highest rate of HBV infection, with an estimated 70 million HBsAg carriers and a prevalence of 5% to 6% in the general population.41,42 The prevalence of HBsAg in patients with MBC was 8.5% in the current study, which was consistent with earlier studies that found 8–15% in breast cancer patients16,43,44 and basically consistent with the general population in western China.45 Our findings revealed that MBC was not associated with elevated prevalence of HBV infection, which was in line with previous studies that HBV was not a breast cancer risk factor.12,43,46
HBV infection mainly injures the liver and causes necrosis and inflammation of liver cells.47 The impact of HBV infection on cancer metastatic patterns, such as CRC, HCC, and pancreatic cancer, has gotten a lot of attention in recent years, and the results have been inconsistent. Regarding breast cancer, the effect of HBV infection on liver metastasis was also inconsistent in different populations. Yu et al reported that HBV infection independently increased the risk of liver metastasis and thus worsened the hepatic metastasis-free survival (HMFS) of patients who received modified radical mastectomy or breast conserving surgery. However, HBV infection was not associated with extrahepatic metastases.48 On the contrary, Xiao et al found that HBV infection did not independently affect HMFS of patients with non-metastatic breast cancer.16 Li et al reported that, for very young patients with curatively resected breast cancer, HBsAg did not increase the rate of liver metastases.43 The results of current study provide the first evidence that HBV infection does not impact the rate of intrahepatic and extrahepatic metastasis in MBC. This was the first study to investigate the impact of HBV infection on MBC metastatic pattern. Thus, these findings and the results of our study imply that more research on whether HBV infection affects the likelihood of intrahepatic and extrahepatic metastases in breast cancer is warranted.
HBV infection has been proven to be associated with the survival of various malignancies. The association of HBV infection with poor prognosis was reported in nasopharyngeal carcinoma,27 lung cancer,49 pancreatic cancer,21 and ovarian cancer.50 HBV infection was an independent positive predictive factor for survival in patients with operable esophageal cancer.51 Previous investigations on the impact of HBV infection on CRC survival have reached to conflicting conclusions.22,25,26 This discrepancy could be explained in part by the diversity and heterogeneity of different cancers. Concerning the impact of HBV infection on breast cancer, Li et al recently reported that, in very young patients with curatively resected breast cancer, HBsAg is an independent negative prognostic factor for disease-free survival (DFS) and OS.43 Xiao et al demonstrated that chronic HBV infection predicts a worse prognosis in stage II/III BC patients, but not stage I BC.16 However, Gao et al52 and Yu et al48 respectively found that HBV infection had no association with DFS and OS of breast cancer patients. In the present study, we found that HBV infection did not influence the survival of MBC, no matter in patients with de novo or relapsed metastatic disease. However, a larger sample size study should be carried out to verify the influence of HBV infection on breast cancer prognosis, and the genetic or biological mechanisms underlying prognosis remain to be elucidated.
It is worth noting that these patients with previous HBV infection may receive less intensive therapy, resulting in a worse time to metastatic relapse. For patients with current or resolved HBV infection, HBV reactivation may occur when they are exposed to various anti-cancer therapies.53 HBV reactivation could lead to the interruption of the therapy schedule. The liver dysfunction attributed to HBV reactivation may also influence the choice of treatment, which makes the prognosis for cancer management uncertain.54 Due to the limitations of this investigation, there is a lack of data on the occurrence of HBV reactivation with the use of several systemic treatments for breast cancer, such as chemotherapy, endocrine therapy, and targeted therapy, which may affect patient management and survival.
However, there are certain limitations to this study that should be mentioned. First, the number of HBsAg-positive group was far less than that of the HBsAg-negative group, as only 28 and 68 patients were HBsAg positive in de novo MBC and relapsed MBC group, respectively. The imbalances in baseline characteristics between two groups existed in original data, resulting in analytical bias and affecting the reliability of the results. In particular, the observed imbalance at HER2 status can also impact the time from diagnosis to metastasis or death, which will make the correlation analysis of HBV infection status with the prognosis bias. Second, HBcAb positive was assumed to denote a history of transient HBV infection, HBV-DNA which would show the severity of HBV infection was frequently detected in this crowd with HBcAb positive, but HBsAg negative.55 Besides, in pancreatic cancer, it had been reported that HBV infection with active replication could promote liver metastasis.21 However, we were unable to assess the effect of HBV-DNA levels on the metastatic pattern and prognosis of patients with breast cancer due to the retrospective nature of this investigation. Therefore, whether breast cancer patients with different HBV infection burdens have different metastatic patterns and survival remains unknown. Third, some patients with relapsed metastatic breast cancer did not consult a doctor in our hospital until their disease progressed. Thus, we were unable to collect the accurate HBV infection information during the time at initial diagnosis of breast cancer or between diagnosis and relapse, confusing some analysis results.
Conclusion
In conclusion, our study discovered that HBV infection status did not influence the initial metastatic pattern of MBC patients with de novo and relapsed metastatic disease. HBsAg-positive patients tended to have longer MFS and/or OS time, but it was not the independent prognostic factor for MBC. Further prospective studies with a large sample size are warranted to confirm the prognostic value of HBsAg status in MBC. Furthermore, other HBV infection indicators, such as HBV-DNA level and other possible clinicopathologic factors for breast cancer, should be taken into consideration.
Data Sharing Statement
All data included in this study are available upon request by contact with the corresponding author Xiaohua Zeng.
Ethical Approval and Informed Consent
This study was performed in accordance with the Declaration of Helsinki and approved by the medical ethics committee of the Chongqing University Cancer Hospital.
Funding
Xiaohua Zeng is supported by Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJZD-K202000104), Xiaohua Zeng, Qing Shao and Ningning Zhang is supported by Chongqing Science and Health Joint Medical Research Project (Grant No.2021MSXM085, 2021MSXM291, 2022MSXM004), respectively. Xiaohua Zeng is supported by Talent Program of Chongqing (Grant No. CQYC20200303137) and Chongqing Municipal Health and Health Commission (Grant No.2019NLTS005).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pfeiffer RM, Webb-Vargas Y, Wheeler W, Gail MH. Proportion of U.S. trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol Biomarkers Prev. 2018;27(10):1214–1222. doi:10.1158/1055-9965.EPI-18-0098
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.19323
4. Testa U, Castelli G, Pelosi E. Breast cancer: a molecularly heterogenous disease needing subtype-specific treatments. Med Sci. 2020;8(1):18. doi: 10.3390/medsci8010018.
5. Atalay G, Biganzoli L, Renard F, et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer. 2003;39(17):2439–2449. doi:10.1016/S0959-8049(03)00601-4
6. Rashid NS, Grible JM, Clevenger CV, Harrell JC. Breast cancer liver metastasis: current and future treatment approaches. Clin Exp Metastasis. 2021;38(3):263–277. doi:10.1007/s10585-021-10080-4
7. Lu FM, Li T, Liu S, Zhuang H. Epidemiology and prevention of hepatitis B virus infection in China. J Viral Hepat. 2010;17(Suppl 1):4–9. doi:10.1111/j.1365-2893.2010.01266.x
8. De Mitri MS, Cassini R, Bernardi M. Hepatitis B virus-related hepatocarcinogenesis: molecular oncogenic potential of clear or occult infections. Eur J Cancer. 2010;46(12):2178–2186. doi:10.1016/j.ejca.2010.03.034
9. Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5(10):1221–1228. doi:10.1016/j.cgh.2007.05.020
10. Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984;65(Pt 3):651–655. doi:10.1099/0022-1317-65-3-651
11. Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993;18(4):781–789. doi:10.1002/hep.1840180406
12. Song C, Lv J, Liu Y, et al. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw Open. 2019;2(6):e195718. doi:10.1001/jamanetworkopen.2019.5718
13. Dumitrascu T, Pineau P. Is hepatitis b virus a player in pancreatic cancer? Chirurgia. 2018;113(3):344–352. doi:10.21614/chirurgia.113.3.344
14. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-HODGKIN lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11(9):827–834. doi:10.1016/S1470-2045(10)70167-4
15. Wu H, Zhao C, Adhikari VP, et al. The prevalence and clinicopathological features of breast cancer patients with hepatitis B virus infection in China. Oncotarget. 2017;8(11):18185–18190. doi:10.18632/oncotarget.15305
16. Xiao W, Zhou Y, Yu P, et al. Prognostic value of chronic hepatitis B virus infection in patients with breast cancer in a hepatitis B virus endemic area. Ann Transl Med. 2020;8(5):180. doi:10.21037/atm.2020.01.97
17. Lu LJ, Adhikari VP, Zhao CX, et al. Clinical study on the relationship between hepatitis B virus infection and risk of breast cancer: a large sized case-control and single center study in southwest of China. Oncotarget. 2017;8(42):72044–72053. doi:10.18632/oncotarget.19132
18. Qin B, Zhao K, Wei J, et al. Novel evidence indicates the presence and replication of hepatitis B virus in breast cancer tissue. Oncol Rep. 2020;43(1):296–305. doi:10.3892/or.2019.7393
19. Adhikari VP, Lu LJ, Kong LQ. Does hepatitis B virus infection cause breast cancer? Chin Clin Oncol. 2016;5(6):81. doi:10.21037/cco.2016.08.04
20. Hatanaka K, Kudo M, Fukunaga T, et al. Clinical characteristics of NonBNonC- HCC: comparison with HBV and HCV related HCC. Intervirology. 2007;50(1):24–31. doi:10.1159/000096309
21. Wei XL, Qiu MZ, Chen WW, et al. The status of HBV infection influences metastatic pattern and survival in Chinese patients with pancreatic cancer. J Transl Med. 2013;11:249. doi:10.1186/1479-5876-11-249
22. Qiu HB, Zhang LY, Zeng ZL, et al. HBV infection decreases risk of liver metastasis in patients with colorectal cancer: a cohort study. World J Gastroenterol. 2011;17(6):804–808. doi:10.3748/wjg.v17.i6.804
23. Liu R, Kong W, Deng M, Lin G, Dai T, Ye L. Association between hepatitis B virus infection and colorectal liver metastasis: a meta-analysis. Bioengineered. 2021;12(1):736–744. doi:10.1080/21655979.2021.1890871
24. Wang J, Li Q, Sun Y, et al. Clinicopathologic features between multicentric occurence and intrahepatic metastasis of multiple hepatocellular carcinomas related to HBV. Surg Oncol. 2009;18(1):25–30. doi:10.1016/j.suronc.2008.05.009
25. Song E, Chen J, Ou Q, Su F. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. Am J Surg. 2001;181(6):529–533. doi:10.1016/S0002-9610(01)00634-1
26. Utsunomiya T, Saitsu H, Saku M, et al. Rare occurrence of colorectal cancer metastasis in livers infected with hepatitis B or C virus. Am J Surg. 1999;177(4):279–281. doi:10.1016/S0002-9610(99)00045-8
27. Liu X, Li X, Jiang N, et al. Prognostic value of chronic hepatitis B virus infection in patients with nasopharyngeal carcinoma: analysis of 1301 patients from an endemic area in China. Cancer. 2014;120(1):68–76. doi:10.1002/cncr.28377
28. Poulakaki F. Occult Breast Cancer[M]//Breast Cancer Essentials. Springer C; 2021:667–674.
29. Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
30. Sinn HP, Kreipe H, Brief A. Overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care. 2013;8(2):149–154. doi:10.1159/000350774
31. Genestie C, Zafrani B, Asselain B, et al. Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res. 1998;18(1B):571–576.
32. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507–539. doi:10.1002/hep.21513
33. Iloeje UH, Yang HI, Chen CJ. Natural history of chronic hepatitis B: what exactly has REVEAL revealed? Liver Int. 2012;32(9):1333–1341. doi:10.1111/j.1478-3231.2012.02805.x
34. Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112(9):1445–1451. doi:10.1038/bjc.2015.127
35. Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21(11):2169–2174. doi:10.1093/annonc/mdq220
36. Zhao W, Wu L, Zhao A, et al. A nomogram for predicting survival in patients with de novo metastatic breast cancer: a population-based study. BMC Cancer. 2020;20(1):982. doi:10.1186/s12885-020-07449-1
37. Coffin CS, Zhou K, Terrault NA. New and old biomarkers for diagnosis and management of chronic hepatitis B virus infection. Gastroenterology. 2019;156(2):355–368. doi:10.1053/j.gastro.2018.11.037
38. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
39. Thompson AJ, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51(6):1933–1944. doi:10.1002/hep.23571
40. Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013;10:239. doi:10.1186/1743-422X-10-239
41. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi:10.1002/hep.27406
42. Liu J, Zhang S, Wang Q, et al. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21–49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis. 2016;16(1):80–86. doi:10.1016/S1473-3099(15)00218-2
43. Li N, Zhong QQ, Yang XR, et al. Prognostic value of hepatitis b virus infection in very young patients with curatively resected breast cancer: analyses from an endemic area in China. Front Oncol. 2020;10:1403. doi:10.3389/fonc.2020.01403
44. Zhao Y, Li H, Zhang Y, et al. Oncoprotein HBXIP modulates abnormal lipid metabolism and growth of breast cancer cells by activating the LXRs/SREBP-1c/FAS signaling cascade. Cancer Res. 2016;76(16):4696–4707. doi:10.1158/0008-5472.CAN-15-1734
45. Wang H, Men P, Xiao Y, et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):811. doi:10.1186/s12879-019-4428-y
46. Su FH, Chang SN, Chen PC, Sung FC, Su CT, Yeh CC. Association between chronic viral hepatitis infection and breast cancer risk: a nationwide population-based case-control study. BMC Cancer. 2011;11:495. doi:10.1186/1471-2407-11-495
47. Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol. 2022;22(1):19–32. doi:10.1038/s41577-021-00549-4
48. Yu P, Liu P, Li N, et al. Hepatitis B virus infection specially increases risk of liver metastasis in breast cancer patients: a propensity-matched analysis. Transl Cancer Res. 2020;9(3):1506–1517. doi:10.21037/tcr.2020.01.63
49. Peng JW, Liu DY, Lin GN, Xiao JJ, Xia ZJ. Hepatitis B virus infection is associated with poor prognosis in patients with advanced non small cell lung cancer. Asian Pac J Cancer Prev. 2015;16(13):5285–5288. doi:10.7314/APJCP.2015.16.13.5285
50. Wong L, Cheung TH, SF Y, TT L. Prevalence and impact of hepatitis B virus infection in ovarian cancer patients in an endemic area-A retrospective cohort study. J Viral Hepat. 2020;27(5):520–525. doi:10.1111/jvh.13250
51. Zou J, Chen J, Xie X, et al. Hepatitis B virus infection is a prognostic biomarker for better survival in operable esophageal cancer: analysis of 2004 patients from an endemic area in China. Cancer Epidemiol Biomarkers Prev. 2019;28(6):1028–1035. doi:10.1158/1055-9965.EPI-18-1095
52. Gao D, Song J, Chen C, Zhu S, Wang Z, Sun S. Relationships of hepatitis B virus infection with clinicopathological features in breast cancer and survival outcomes in central China. Transl Cancer Res. 2020;9(4):2511–2517. doi:10.21037/tcr.2020.03.15
53. Chang Y, Jeong SW, Jang JY. Hepatitis B virus reactivation associated with therapeutic interventions. Front Med. 2021;8:770124. doi:10.3389/fmed.2021.770124
54. Liu Z, Jiang L, Liang G, et al. Hepatitis B virus reactivation in breast cancer patients undergoing chemotherapy: a review and meta-analysis of prophylaxis management. J Viral Hepat. 2017;24(7):561–572. doi:10.1111/jvh.12672
55. Marusawa H, Uemoto S, Hijikata M, et al. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31(2):488–495. doi:10.1002/hep.510310232
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


