Back to Journals » Infection and Drug Resistance » Volume 17
Haemophilus aphrophilus and Eikenella corrodens Coinfection of Brain: An Unusual Case from China
Authors Yuan L, Lai LM, Zhu X, Rui Z, Liu Y, Chen Q
Received 4 January 2024
Accepted for publication 3 April 2024
Published 12 April 2024 Volume 2024:17 Pages 1439—1445
DOI https://doi.org/10.2147/IDR.S458020
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lei Yuan,* Lan Min Lai,* Xinyu Zhu, Zhao Rui, Yang Liu, Qiang Chen
Department of Clinical laboratory, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Chen, Email [email protected]
Background: The HACEK group comprises Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae, are Gram-negative bacteria that are slow-growing and fastidious. These organisms are common causes of culture-negative endocarditis. However, brain abscesses caused by Haemophilus aphrophilus and E. corrodens have been rarely reported. The case we describe, which was promptly identified and successfully treated, will be meaningful for the diagnosis and treatment of such infectious diseases.
Case Presentation: Herein, we report a case of brain abscess in a young man who was infected with Haemophilus aphrophilus and E. corrodens. The patient was admitted to the hospital with sudden onset of vomiting, coma, and fever. Magnetic resonance imaging (MRI) of the brain and cerebrospinal fluid cell counts suggested cerebral abscess, he underwent drainage of the abscess and empirical antimicrobial therapy of meropenem (2 g every 8 hours) and linezolid (0.6 g every 12 hours) for more than 10 days without significant improvement. Metagenomic next-generation sequencing (mNGS) of drainage fluid and matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) detection for isolated bacteria from samples suggested the presence of H. aphrophilus and E. corrodens. After 7 weeks of ceftriaxone (2 g every 12 hours) and meropenem (2 g every 8 hours) intravenously, the patient was discharged with a normal temperature and brain MRI showed improvement of the lesion.
Conclusion: Similar cases reported in previous studies were always associated with bacterial blood dissemination after dental surgery or myocarditis; however, the patient in our case had no any associated risk factors. As far as we know, this is the only case of central nervous system infection caused by H. aphrophilus and E. corrodens that has utilized combined mNGS and MALDI-TOF MS in the diagnosis.
Keywords: Haemophilus aphrophilus, Eikenella corrodens, HACEK, brain abscess
Introduction
The acronym HACEK refers to a group of fussy-growing Gram-negative bacteria, including Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae. HACEK group bacteria are common colonizers of the oropharyngeal, respiratory, gastrointestinal, and genitourinary tracts, and cause a majority of Gram-negative bacterial endocarditis: 1.2%–3% of all infective endocarditis.1 However, its fussy growth environment renders identification using conventional microbiological cultures inefficient, and this usually leads to delayed diagnosis and disease aggravation. H. aphrophilus and E. corrodens are microaerobic Gram-negative bacillusi, mostly colonize the oral cavity, and trigger invasive infections, including infective endocarditis, intracranial infections, liver abscess, and lung infections,2 under specific circumstances, such as immunosuppression, trauma, and others. Brain abscesses of these organisms commonly result from the spread of neighboring infections, with approximately a third associated with odontogenic infections, the remainder being severe otitis media, sinus tract infections, or mastoiditis. Patients usually present with headache, brain-nerve damage, and fever.3 Intracranial infections secondary to H. aphrophilus and E. corrodens are unusual. Existing reports suggest that they are mostly related to dental surgery, travel, drug abuse, contact with pets, or spinal surgery.4–7 Imaging can be a effective diagnostic tool, but final treatment mainly relies on pathogenetic diagnosis. Our study retrospectively analyzed a case of brain abscess infected with HACEK-group bacteria to investigate the diagnostic and therapeutic course, emphasizing the importance of hypothesis-independent molecular techniques such as metagenomic next-generation sequencing (mNGS) and matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) in rapid diagnosis.
Case Presentation
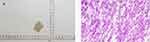
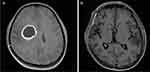
The patient was admitted to our hospital due to a sudden outbreak of vomiting and coma for 5 hours on October 23, 2022. Brain MRI suggested rounded nodular abnormal-density foci in the right frontoparietal lobe. The size of the largest one was about 2.9×3.6 cm, and brain abscess was considered. The physical examinations showed that his general situation was well. The patient had developed weakness of the left side of the limbs, fever, and headache 3 months prior, and recovered gradually after being treated with linezolid empiric anti-infective therapy. This admission was considered for abscess recurrence. It is worth noting that the patient had no history of pet ownership, travel, drug abuse, dental disease, or surgery. He underwent “transcranial endoscopic brain abscess incision and drainage combined right frontotemporal parietal decompression of cranial bone flap” under general anesthesia on the day of admission, and the operation went smoothly. Intraoperative histopathology of the brain abscess revealed large numbers of inflammatory cells (Figure 1).
He was then transferred to the emergency ICU for further treatment after the operation and given meropenem (2 g every 8 hours) and linezolid (0.6 g every 12 hours) to fight infection, antiepileptic treatment, dehydration treatment to lower the cranial pressure, and nutritional support. During the hospitalization, blood tests showed white blood cells 15.06×109/L (neutrophils 87%), hemoglobin 91 g/L, platelets 278×109 /L, CRP 18.48 mg/L, and PCT 0.28 ng/mL. Renal and liver function indices were within normal range. In order to continue CSF drainage, lumbar large pool drainage was performed, and three tubes drainage fluid were sent for conventional counting, biochemistry, bacterial culture, and mNGS examination. On routine CSF examination, total leukocyte count was 950/µL, of which neutrophilic lobulated nucleated granulocytes occupied 70% and lymphocytes occupied 30%. The Pandy test was positive, and CSF biochemical testing showed glucose was 2.34 mmol/L, chloride 111.33 mmol/L, and total protein 641.61 mg/dL. The mNGS results were suggestive of H. aphrophilus and E. corrodens sequences.
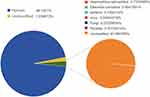
Meanwhile, CSF samples were inoculated with 5% sheep blood, chocolate agar, MacConkey agar, and Sabouraud dextrose agar. These were all incubated at 37°C for 48 h, and the chocolate agar plate was inoculated in a CO2 environment at a concentration of 5%. Figures 2 and 3 show the colony morphology on the chocolate plate and the microscopic bacterial morphology, respectively. Samples were then analyzed by MALDI-TOF MS (Vitek MS Biomedical Sciences), and the results suggested consistency with the mNGS. The taxonomic profile is shown in Figure 4. Following consultation with the pharmacy department, ceftriaxone (2 g every 12 hours) and meropenem (2 g every 8 hours) were given against H. aphrophilus and E. corrodens. The patient was discharged in good general condition after a total of 7 weeks of antibiotic treatment. Brain MRI showed that the lesion had disappeared. Images taken before and after treatment are shown in Figure 5. The diagnosis and treatment details are listed in Figure 6.
 |
Figure 6 Timeline of patient’s diagnosis and treatment. Abbreviations: CRO, ceftriaxone; MEM, meropenem. |
Discussion
Herein, we have presented a case of previously healthy young man with a complex brain abscess with both H. aphrophilus with E. corrodens infection. According to the literature, HACEK-group bacteria are fussy-growing Gram-negative organisms frequently seen in patients with infective endocarditis, as well as in periodontal infections, abscesses, and nonendocarditis bacteremia secondary to focal infections. Both H. aphrophilus and E. corrodens are considered commensal organisms of the oropharynx that are encountered in brain abscesses infrequently. No cases of these two bacteria infecting the brain had been reported in China until April 2023, when Lo Biundo et al8 published a case of brain infection resulting from H. aphrophilus and E. corrodens in a 26-year-old woman admitted to hospital with a history of left buccal margin excursion and ipsilateral monoplegia of the upper limb for 7 days. That was the only case reported with mixed infection by these two pathogens until now. MRI confirmed the presence of brain abscess and revealed a hypodense lesion in the left frontal lobe with extensive perifocal edema. The two pathogens were identified using MALDI-TOF MS. Similar to our report, there was no history of relevant risk factor exposure, as mentioned in previous literature.9 However, our case had limb weakness and headache up to 3 months prior to hospitalization successfully diagnosed by mNGS and MALDI-TOF MS. This might have been due to a failure in identifying the pathogens and improper use of antibiotics that allowed the bacteria to remain in the brain tissue, which would have led to the proliferation of the bacteria and worsening infection when the patient’s immune system became compromised. What is noteworthy is the site of abscess, both located in the frontal lobe in the two cases, which rationally explains their similar behavioral changes and weakness and paralysis of the lateral limbs, as neurological signs depend on the location of lesions.
The young man had the symptoms of headache, fever, and weakness of the left limbs, with a maximum temperature of 39.5°C and positive signs of meningeal irritation. CSF cell count showed a leukocyte level in the CSF of 950/µL, decreased glucose and chloride, and significantly increased intracranial pressure; therefore, the initial clinical diagnosis was purulent meningitis. The clinician excluded intracranial tumor based on MRI and histopathologic examination, and H. aphrophilus and E. corrodens were ultimately confirmed based on CSF mNGS and MALDI-TOF MS. HACEK-group bacteria are Gram-negative with high nutritional requirements. H. aphrophilus grows slowly on chocolate plates in the CO2 environment with a concentration of 5% instead of ordinary plates,10 thus adding to the difficulty in identifying this organism. Our case highlights the importance of these two novel diagnostic technologies in the identification of pathogens that are difficult to culture.
MALDI-TOF MS shows good performance in identifying slow-growing caustic bacteria, providing a rapid and accurate method.11,12 In contrast, mNGS is a high-throughput technology13 that sequences DNA or RNA nucleic acids directly13 and does not require culture. This allows mNGS to identify multiple pathogens, including exceptional, newly discovered, and mixed-infection bacteria with high accuracy and efficiency.14 Numerous studies have shown that mNGS improves the diagnostic efficiency of culture-negative cases when patients have been treated with antimicrobial drugs,15 and demonstrated strong diagnostic ability in central nervous system infection, with an overall positive-detection rate of 15.7%-57%, sensitivity of 73%-92%, and specificity of 96–99% according to previous reports.16,17 Traditional tests for isolation of H. aphrophilus and E. corrodens are time-consuming and laborious. We innovatively combined MALDI-TOF with mNGS and identified the pathogens complemently and accurately in this case. This means that MALDI-TOF MS and mNGS are alternative diagnostic tools for microbiological evidence to prevent further aggravation of infections with shorter turnaround.
Therapeutic methods for brain abscesses consist mainly of neurosurgical aspiration and antibiotic therapy. The empirical antibiotic regimen is usually third-generation cephalosporins combined with metronidazole.18 However, commonly drugs in infectious cases of H. aphrophilus or E. corrodens are triple cephalosporins, meropenem, and vancomycin.19,20 So far, there have been few cases, and the drug selection needs further study. A recommended drug following antibiotic-susceptibility testing is usually the first choice, yet most hospitals have not developed antibiotic-susceptibility testing for these scarce bacteria,21,22 which makes the treatment difficult and can lead to an unsatisfactory treatment result. The patient in this case received incision drainage of the brain abscess, intravenous anti-infection drugs, high-flow oxygen, and nutritional support. In terms of antibiotic use, the initial treatment regimen was meropenem combined with linezolid in order to cover most Gram-negative and Gram-positive bacteria, though it did not work well. After the pathogens had been clarified, the symptoms significantly improved after 7 weeks of adjusted antibiotic treatment of a tertiary cephalosporin–ceftriaxone–meropenem combination. The patient continued oral linezolid treatment for 2 weeks after discharge from hospital, and MRI suggested the abscess was almost absorbed and his condition was stable with no recurrence at follow-up 2 months later.
In a previous study, a treatment course of 6 weeks or more was necessary due to the recurrent nature of these bacterial infections,23 recommending that antimicrobials be taken for at least 4–6 weeks and the duration of antibiotic treatment should be extended depending on the patient’s condition. Despite advances in diagnosis and treatment, central nervous system infections retain high morbidity and mortality rates.23 Early diagnosis and treatment are crucial, especially in cases of rare pathogen infections. To alleviate the pain of the patient, shorten the duration of the illness, and improve their prognosis, we should apply CSF mNGS in the early stages of onset. Antibiotic-susceptibility testing for HACEK-group bacteria is impractical for many laboratories because of fastidiousness of these organisms’ growth, and hence published reports and guidelines frequently provide the only guidance for antimicrobial selection.8,24,25 The therapeutic procedure and dosing regimen in this report are valuable for the treatment of HACEK-group bacteria infections, especially in immunocompetent patients. Additional antibacterial drugs and aggressive interventions, such as abscess drainage and nutritional support, could improve the effectiveness of treatment.
Conclusion
This case of brain abscess caused by coinfection with H. aphrophilus and E. corrodens without apparent incentive is the first to be reported in a patient in China. The patient was discharged in good condition after prompt and appropriate anti-infective treatment. The diagnosis and treatment process of this report is thus informative for similar cases. To avoid serious consequences of aggravation, clinicians should consider the possibility of infection with rare pathogens in cases of difficult diagnosis. Furthermore, this case highlights the significant advantages of mNGS and MALDI-TOF MS in detecting unique and mixed infections.
Abbreviations
MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; mNGS, metagenomic next-generation sequencing; MALDI-TOF MS, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry.
Data Sharing
All the data that support the findings of the case report have been provided in the article, and are available from the corresponding author upon reasonable request.
Ethics and Consent
Written informed consent to have the case details and any accompanying images published was provided by the patient. The Ethics Committee of the First Affiliated Hospital of Nanchang University approved the waiver in this case report based on Chinese ethics guidelines for clinical research to publish the case details.
Acknowledgments
We thank the patient and his parents for provision of clinical data.
This paper has been uploaded to ResearchGate as a preprint: https://www.researchgate.net/publication/377519464_Haemophilus_aphrophilus_and_Eikenella_corrodens_co-infection_of_brain_a_unusual_case_from_china.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This work was supported by a Jiangxi Provincial Department of Education project (GJJ200220) and the Jiangxi Provincial Traditional Chinese Medicine Science and Technology Program (2023B1262). The funding supported the publishing fees.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Revest M, Egmann G, Cattoir V, Tattevin P. HACEK endocarditis: state-of-The-art. Expert Rev Anti Infect Ther. 2016;14(5):523–530. doi:10.1586/14787210.2016.1164032
2. Nørskov-Lauritsen N. Classification, identification, and clinical significance of Haemophilus and aggregatibacter species with host specificity for humans. Clin Microbiol Rev. 2014;27(2):214–240. doi:10.1128/CMR.00103-13
3. Liao Y, Luo CY, Ko WC, Chen PL. Aggregatibacter aphrophilus culture-negative endocarditis diagnosed by 16S rRNA gene sequencing in excised mitral valve - A case report. J Microbiol Immun Infect. 2017;50(4):557–558. doi:10.1016/j.jmii.2016.10.002
4. Miller DC, Fung M, Carbo A. A furry friend’s dirty mouth: brain abscess due to aggregatibacter (haemophilus) Aphrophilus. Am J Med. 2017;130(10):e447–e448. doi:10.1016/j.amjmed.2017.04.020
5. Yamashita J, Bone FJ, Hitchcock E. Brain abscess due to Haemophilus Aphrophilus: case report. J Neurol Neurosurg Psychiatry. 1972;35(6):909–911. doi:10.1136/jnnp.35.6.909
6. Abla AA, Maroon JC, Slifkin M. Brain abscess due to Haemophilus aphrophilus: possible canine transmission. Neurosurgery. 1986;19(1):123–124. doi:10.1227/00006123-198607000-00021
7. Tok S, Neidert MC, Bloemberg G, Sürücü O. Aggregatibacter aphrophilus ventriculitis following C1-C2 transarticular screw fixation. Neurol Neurochir Pol. 2016;50(1):63–68. doi:10.1016/j.pjnns.2015.11.005
8. Lo Biundo C, Bongiovanni A, Tumbiolo S, et al. Aggregatibacter aphrophilus and Eikenella corrodens: a case of brain abscess. New Microbiol. 2023;46(2):216–218.
9. Maraki S, Papadakis IS, Chronakis E, Panagopoulos D, Vakis A. Aggregatibacter aphrophilus brain abscess secondary to primary tooth extraction: case report and literature review. J Microbiol Immun Infect. 2016;49(1):119–122. doi:10.1016/j.jmii.2013.12.007
10. Khairat O. Haemophilus aphrophilus endocarditis. Br Med J. 1971;1(5751):728. doi:10.1136/bmj.1.5751.728-a
11. Belkacem A, Caseris M, Yazdanpanah Y. A case of aggregatibacter aphrophilus multiple abscess. Open Forum Infect Dis. 2015;2(2):ofv031. doi:10.1093/ofid/ofv031
12. van Belkum A, Welker M, Erhard M, Chatellier S. Biomedical mass spectrometry in today’s and tomorrow’s clinical microbiology laboratories. J Clin Microbiol. 2012;50(5):1513–1517. doi:10.1128/JCM.00420-12
13. Petersen CE, Valentine NB, Wahl KL. Characterization of microorganisms by MALDI mass spectrometry. Mass Spect Prot Pept. 2009;492:367–379.
14. Chen XF, Hou X, Xiao M, et al. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analysis for the identification of pathogenic microorganisms: a review. Microorganisms. 2021;9(7):1536. doi:10.3390/microorganisms9071536
15. van Veen SQ, Claas EC, Kuijper EJ. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol. 2010;48(3):900–907. doi:10.1128/JCM.02071-09
16. Dai Y, Chen L, Chang W, Lu H, Cui P, Ma X. Culture-negative streptococcus suis infection diagnosed by metagenomic next-generation sequencing. Front Public Health. 2019;7:379. doi:10.3389/fpubh.2019.00379
17. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi:10.1038/s41576-019-0113-7
18. Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29(5):831–842. doi:10.1101/gr.238170.118
19. Papa A, Kotrotsiou T, Papadopoulou E, Reusken C, GeurtsvanKessel C, Koopmans M. Challenges in laboratory diagnosis of acute viral central nervous system infections in the era of emerging infectious diseases: the syndromic approach. Expert Rev Anti Infect Ther. 2016;14(9):829–836. doi:10.1080/14787210.2016.1215914
20. Shruthi U, Prabhu Raj AR, Kumari HBV, Nagarathna C. Anaerobic bacteriological profile of brain abscess in a tertiary care center in southern India. Anaerobe. 2019;59:68–71. doi:10.1016/j.anaerobe.2019.05.012
21. Xing XW, Zhang JT, Ma YB, et al. Metagenomic next-generation sequencing for diagnosis of infectious encephalitis and meningitis: a large, prospective case series of 213 patients. Front Cell Infect Microbiol. 2020;10:88. doi:10.3389/fcimb.2020.00088
22. Wilson MR, Sample HA, Zorn KC, et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi:10.1056/NEJMoa1803396
23. Gonçalves RJ, Murinello A, Gomes da Silva S, Coelho JS, Lopes Santos A, Sá Damásio H. Hepatic abscess due to streptococcus anginosus and eikenella corrodens, secondary to gastric perforation by a fish bone. GE. Port J Gastroenterol. 2019;26(6):414–419. doi:10.1159/000497333
24. Jaramillo-Lanchero RD, Suarez-Alvarez P, Teheran-Sierra L. Effect of respiratory inhibitors and quinone analogues on the aerobic electron transport system of Eikenella corrodens. Sci Rep. 2021;11(1):8987. doi:10.1038/s41598-021-88388-0
25. Chien YC, Huang YT, Liao CH, Chien JY, Hsueh PR. Clinical characteristics of bacteremia caused by Haemophilus and aggregatibacter species and antimicrobial susceptibilities of the isolates. J Microbiol Immun Infect. 2021;54(6):1130–1138. doi:10.1016/j.jmii.2020.12.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.