Back to Journals » OncoTargets and Therapy » Volume 16
GW4869 Can Inhibit Epithelial-Mesenchymal Transition and Extracellular HSP90α in Gefitinib-Sensitive NSCLC Cells
Authors Wan X, Fang Y, Du J, Cai S, Dong H
Received 10 August 2023
Accepted for publication 13 October 2023
Published 8 November 2023 Volume 2023:16 Pages 913—922
DOI https://doi.org/10.2147/OTT.S428707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr John Maher
Xuan Wan,1,* Yuting Fang,2,* Jiangzhou Du,1 Shaoxi Cai,1 Hangming Dong1
1Chronic Airways Diseases Laboratory, Department of Respiratory and Critical Care Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong Province, 510515, People’s Republic of China; 2BSL-3 Laboratory, Guangdong Provincial Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University, Guangzhou, Guangdong Province, 510515, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaoxi Cai; Hangming Dong, Email [email protected]; [email protected]
Objective: GW4869 is an exosomal inhibitor. It is necessary to delay the occurrence of gefitinib resistance during non-small-cell lung cancer (NSCLC) treatment. This study aimed to investigate the anti-tumor effects of GW4869 on epithelial-mesenchymal transition (EMT) and expression of extracellular heat shock protein 90α (eHSP90α) that contributes to acquired resisitance. Our study provides a new sight into the treatment of EGFR-mutated NSCLC.
Materials and Methods: We performed western blotting to detect levels of EMT and eHSP90α. Wound healing and transwell assays were performed to evaluate the behavioral dynamics of EMT. A nude mouse model of HCC827 was established in vivo.
Results: GW4869 inhibited the expression of eHSP90α, EMT, invasion and migration abilities of HCC827 and PC9. GW4869 enhanced sensitivity to gefitinib in BALB/c nude mice bearing tumors of HCC827.
Conclusion: These studies suggest that GW4869 can inhibit EMT and extracellular HSP90α, providing new strategies for enhancing gefitinib sensitivity in NSCLC.
Keywords: GW4869, EMT, gefitinib-sensitive NSCLC, eHSP90α
Introduction
Lung cancer is the second most commonly diagnosed cancer with an estimated 2.2 million new cancer cases and 1.8 million deaths per year.1 Histologically, 85% of lung cancer cases are classified as non-small-cell lung cancer (NSCLC). In Asia, approximately 50% of NSCLC patients harbor mutations in the epidermal growth factor receptor (EGFR),2 mainly in exons 18, 19, and 21.
Gefitinib has been approved as a first-line treatment for patients with NSCLC with EGFR mutations.3 The initial treatment effect of EGFR-tyrosine kinase inhibitors (EGFR-TKIs) is satisfactory; however, the majority of patients eventually demonstrate disease progression due to the development of acquired resistance to TKI.4–6 Therefore, additional treatment options are needed to address the development of acquired resistance to gefitinib in later patients.
GW4869 is a cell-permeable symmetrical dihydroimidazolamide compound that can be used as an effective, specific, and noncompetitive inhibitor of membrane-neutral sphingomyelinase (nSMase2), which prevents ceramide-modulated inward budding of multivesicular bodies (MVBs) and subsequent release of exosomes from MVBs.7 GW4869 has been reported to reduce exosome release and is often used as an exosome inhibitor to suppress the increase in exosome secretion in response to gefitinib and chemoresistance in colorectal, pancreatic, and ovarian cancer cells.8,9 GW4869 may be a helpful strategy to overcome the antagonistic effects via inhibition of exosome and miRNA secretion when EGFR-TKIs and chemotherapeutic agents are co-administered.10 However, the mechanisms underlying its anti-tumor and antiresistance effects are not fully understood.
Epithelial-mesenchymal transition (EMT) is a vital process that drives the development of drug resistance in NSCLC.11 Previous studies have shown that tumor cells with EMT are more likely to develop drug resistance.12 In addition to secondary mutations in EGFR or EGFR amplifications, EMT is also one of the main resistance mechanisms to EGFR-TKIs, with alterations in EMT gene signatures and specific markers of the mesenchymal phenotype.13 During the EMT process, epithelial cells lose their adhesion capacity and acquire mesenchymal features as well as increased behavioral-dynamic abilities of migration and invasion,14 represented by the downregulation of E-cadherin and upregulation of vimentin and N-cadherin.15 Exosomes also play a critical role in EMT and tumor metastasis.16 Therefore, it was not difficult to envision how GW4869 influences the progression of cancer.
Heat shock protein 90α (HSP90α) is a highly conserved molecular chaperone that mediates the stability of protein structures by participating in the correct folding of the target protein and ensuring its normal physiological function.Structurally, HSP90 comes in two main sub-types, HSP90α and HSP90β.17 In addition to being localized intracellularly, HSP90α can be excreted into the extracellular environment, called extracellular HSP90α (eHSP90α), in tumor cells.18 Extracellular HSP90 (eHSP90) is secreted by exosomes and may be a useful target for tumor therapy.19,20 Cell motility and invasion have been found to be induced by eHSP90 in several cancer cell lines and preclinical models.21 Studies have revealed that the sensitivity of cancer cells to HSP90 inhibitors is due to the inhibition of eHSP90 rather than intracellular HSP90. Only the group of “eHSP90-dependent” cancer cells is sensitive to HSP90 inhibitors owing to the utilization of eHSP90 in motility, invasion, and metastasis.22 Notably, it has been shown that heat shock proteins (HSPs) and vesicles were co-released and HSPs served as mediators of resistance-associated secretory phenotype (RASP).23 Heat Shock Protein-rich extracellular vesicles (EVs) can promote cancer progression by enhancing EMT, migration, invasion, heterogeneity, metastasis, drug resistance, and angiogenesis in cancer cells.23 Co-release of EVs and eHSP90 from high oral metastatic cancer and castration-resistant prostate cancer cells induce tumorigenicity and EMT;24 however, in addition to inhibiting exosome secretion, the effects of GW4869 on HSP90α expression and EMT in gefitinib-sensitive NSCLC cells remain unclear.
Therefore, we investigated whether GW4869 could inhibit EMT and eHSP90α in gefitinib-sensitive NSCLC cells.
Materials and Methods
Cell Lines and Reagents
HCC827 (EGFR exon 19 deletions) was obtained from American Type Culture Collection (USA). PC9 (EGFR exon 19 deletions) was from the Guangdong Lung Cancer Institute (China). Gefitinib (ZD1839) and GW4869 (S7609) were purchased from Selleck Chemicals (Houston, TX, USA). Recombinant transforming growth factor-β1 (TGF-β1) was purchased from R&D Systems (Minneapolis, MN, USA). Human recombinant HSP90α (hrHSP90α) was obtained from StressMarq Biosciences, Inc. (Victoria, BC, Canada).
Western Blot
Protein lysate supernatants from the radioimmunoprecipitation assay (RIPA) were collected and mixed with SDS-PAGE loading buffer. Total proteins in the loading buffer were separated on 10% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Sigma, USA). The membrane was blocked with 5% nonfat dried milk in Tris-buffered saline and polysorbate 20 (TBST) and probed with antibodies against HSP90α, E-cadherin, N-cadherin (Cell Signaling Technology, Danvers, MA, USA), vimentin (Abcam, Cambridge, UK), and β-actin (Proteintech, Chicago, IL, USA).
Wound Healing Assay
Cells were seeded at an optimal density (5×105/mL) in a six-well cell culture plate and incubated for 24 h to 80% confluency. A 200 μL pipette tip was used to create a gap in the cell monolayer in each well. The monolayer was washed with the culture medium to remove cell debris, and the cells were allowed to migrate for 24 h. Each gap is measured using an optical microscope. The ratio of the gap size after 24 h to the gap size at baseline was treated as the relative migration rate and analyzed using ImageJ software.
Transwell Invasion Assay
Matrigel (Corning, USA) was melted at 4°C and diluted in the growth medium at a ratio of 1:8. Diluted matrigel was then added to coat the upper surface of the Transwell chambers (Corning, USA) at 30 μL per well. Cells (7 × 104/mL) were seeded on the coated membrane, and the chambers were placed into a Costar Transwell chamber plate containing growth medium. After 24 h, cells on the upper side of the membrane were removed prior to staining. The Transwell chambers were removed, and the medium was discarded. The chambers were washed twice with PBS and pre-cooled to 4°C. PBS was discarded, and the chambers were placed in 4% paraformaldehyde for 20 min and then washed twice with PBS. Cells on the submembrane surface were stained with 0.1% crystal violet. Cells were counted in three randomly selected fields and images were obtained under a microscope at 10× magnification. The number of cells that passed through the membrane was calculated.
Xenograft Model
BALB/C nude mice (male, 15–20 g, 4–6 weeks old) were purchased from the Laboratory Animal Center, Southern Medical University, and housed in a specific specific-pathogen-free (SPF) facility. Mice were kept on a 12 h light/dark cycle in an atmosphere of 40–70% humidity and an average temperature of approximately 24°C. All animal experiments in this study complied with the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines 40, as well as were approved by the Ethics Committee of Nanfang Hospital (Guangdong, China) (Medical Ethics No. NFEC-2019-065) and carried out under the rules of Laboratory animals and animal experiments of Nanfang Hospital of Southern Medical University. HCC827 cells (5×106/mL) were subcutaneously injected into the left flanks of the mice. After the tumor size reached 50 mm3, the mice were randomly divided into three groups (n = 5 each): (1) the control group (treated with 0.5% CMC-Na, which was used as a vehicle for GW4869 and gefitinib); (2) Gefitinib (20 mg/kg); (3) combined GW4869 (12 μg/g) + gefitinib (20 mg/kg) group. The mice received all the aforementioned drugs intragastrically (i.g.) once a day. On day 28 the mice were sacrificed. Blood samples were obtained from the eyes of the mice. Immunohistochemistry (IHC) was performed on tumor tissues to assess the levels of E-cadherin and vimentin.
Statistical Analysis
All data analyses were performed independently using GraphPad Prism software (version 5.0; GraphPad Software, USA). All data are expressed as the mean ± standard deviation (SD). One-way ANOVA–Newman–Keults test was used to determine the statistical significance. Statistical significance was set at P < 0.05.
Results
GW4869 Suppressed the Expression of eHSP90α and EMT Induced by TGF-β1
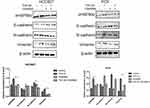
To study the effects of GW4869, cells were treated with GW4869. With increasing concentrations of GW4869, we found that the expression of eHSP90α decreased significantly compared with the control group (P<0.05) when the GW4869 concentration was 1 nM both in HCC827 and PC9 (Figure 1), accompanied by an increase in E-cadherin and a decrease in N-cadherin and vimentin (P<0.05). Meanwhile, being co-treated with TGF-β1 of 40 ng/mL and GW4869 (1nM), we found that GW4869 inhibited the TGF-β1-induced increase in eHSP90α, and reversed the EMT process induced by TGF-β1 in HCC827. Such pheonomenon was also observed in PC9. (Figure 2). These results suggest that the exosome inhibitor GW4869 inhibits eHSP90α and EMT in TGF-β1-stimulated cells.
GW4869 Inhibited Migration and Invasion in TGF-β1-Stimulated NSCLC Cells
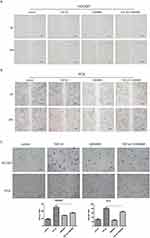
To further study the role of GW4869 on EMT, we showed that the cell mobility of TGF-β1 treatment combined with GW4869 (28.33±0.98%) was lower than TGF-β1 treatment (59.04±0.99%) alone through wound healing assay in HCC827. Also in PC9, the migration rate of GW4869 and TGF-β1 group (15.19±1.06%) was lower than the group pf TGF-β1 (46.48±2.00%). These results suggested that GW4869 can inhibit the migration stimulated by TGF-β1 (Figure 3). The transwell assay showed that the cell invasion number of GW4869 group (13.33±1.52) was significantly lower than that in the control (18.66±1.52) in HCC827, while the cell invasion number of TGF-β1 combined with GW4869 group (35±3.00) was significantly lower than that in the TGF-β1 alone group (50.66±8.14). As well, the cell invasion number of TGF-β1 combined with the GW4869 group (65.33±4.61) was significantly lower than that of TGF-β1 alone group (80.66±7.02) in PC9. These indicate that GW4869 can inhibit the migration (Figure 3A and B) and invasion (Figure 3C) of TGF-β 1-stimulated NSCLC cells. Taken together, GW4869 inhibited the migration and invasion of TGF-β 1-stimulated NSCLC cells.
GW4869 Leads to Additional Antitumor Effect via EMT and eHSP90α in vivo
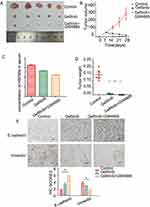
To further evaluate the effects of GW4869 on the EMT and eHSP9α expression, we performed an in vivo study. The HCC827 cells were subcutaneously injected into BALB/c nude mice. After the tumor size reached approximately 50 mm3, the mice were divided into three groups: control, gefitinib, gefitinib+GW4869 (Figure 4A). By day 28, there was a significant reduction in tumor size in the groups treated with gefitinib and gefitinib+GW4869.Tumor size in the GW4869 + gefitinib group was the smallest (Figure 4B). The gefitinib + GW4869 group had the lowest serum HSP90α level among the three groups (Figure 4C). The same trend was observed for tumor weight (Figure 4D). IHC analysis also showed that the expression of N-cadherin was significantly lower in the gefitinib+GW4869 group than in the single-drug group, whereas E-cadherin expression showed the opposite pattern and was calculated using IHC scores (Figure 4E). To summarize, these results suggest that the combination of gefitinib and GW4869 enhances the anti-tumor effect in vivo.
Discussion
EGFR-TKIs have been administered for years and have been reported to improve response rates, time-to-progression, and overall survival. Unfortunately, patients with EGFR-mutant lung cancer develop disease progression after a median of 10–14 months of treatment with EGFR-TKI.5 Drug resistance can be divided into two conditions: one is primary drug resistance, which is not sensitive to molecular targeted drug therapy, and considers more radiotherapy and chemotherapy; the other one is acquired drug resistance, which is initially sensitive to molecular targeted drugs for more than one month, but drug resistance appears during the treatment. The mechanisms of acquired drug resistance are complicated and can be divided into three categories: Changes in EGFR (T790M mutation), activation of alternative bypass (MET-amplification) or downstream pathways, and changes in the phenotype(EMT).25 Emerging evidence suggests that EMT is associated with acquired resistance to EGFR-TKIs in EGFR-mutation NSCLC.26
Currently, EMT is considered a key candidate for drug discovery against tumors. Cyclin-dependent kinase 7 (CDK7) inhibitors play a role in EMT-mediated EGFR-TKI resistance in NSCLC.27 A diphenyl urea derivative (DUD) inhibited lung cancer cell migration by reversing EMT via Wnt/β-catenin and PI3K/Akt signaling and decreasing MMPs.28 EMT endows tumor cells with the ability to invade, migrate, and resist. Wound healing and transwell assays showed that the invasion and migration of the TGF-β1-induced EMT model were inhibited by GW4869. These results also suggest that GW4869 hampers tumor invasion and migration, which may have a potential effect on EGFR-mutated invasion and migration. We treated nude mice with gefitinib and GW4869 and preliminarily concluded that GW4869 plays an additional role in inhibiting tumor growth. This inhibition of tumor growth may be explained by a reduction in the level of eHSP90α and an altered expression of EMT proteins.
Even though HSP90a has two subtypes include HSP90α and HSP90β. Knocking out HSP90β cells can not survive and HSP90α not, so we focus on HSP90α, especially eHSP90α.20 eHSP90 is secreted by exosomes and co-released with exosomes.29 Inhibition of eHSP90 release may overcome EMT-mediated resistance to EGFR-TKIs. GW4869 is often used to block exosome production and inhibit exosome release while it has been found to reverse gefitinib resistance and even overcome the antagonistic effects of co-administration of cisplatin in NSCLC; however, its mechanism remains to be further studied.30 Moreover, HSP90 membrane-deforming ability promotes exosome release in vitro and in vivo, whereas eHSP90- and HSP90-rich EVs function in the process of EMT.11 Of note, GW4869 inhibits EMT in lung cancer cells induced by cancer-associated fibroblasts (CAFs).31 In addition, the combination of GW4869 and PD-L1 antibody has the potential to improve the clinical antitumor efficacy.32 Therefore, we tested whether eHSP90α could be involved in EMT, invasion, and migration by inhibiting exosome release with GW4869. We found that both eHSP90α and EMT protein markers were regulated by GW4869 in a dose-dependent manner. GW4869 inhibited TGF-β1-induced eHSP90α expression and EMT protein markers, and suppressed TGF-β1-induced cell invasion and migration. Altogether, the exosome inhibitor GW4869 inhibited the expression of eHSP90α, EMT, and invasion and migration of HCC827 and PC9 cells, suggesting that GW4869 may block the release of eHSP90α and reverse tumor invasion and migration caused by EMT in gefitinib-sensitive cells.
Exosomes can promote the occurrence and development of tumors, so inhibiting the biosynthesis and secretion of exosomes can restrain the occurrence of tumor. GW4869 is one of the exosomes that inhibit biogenesis of exosomes and also is the first reported noncompetitive nSMase2 inhibitor. It is worth noting that ceramides are widely considered to be a lipid tumor suppressor gene. nSMase2 simultaneously inhibits cell proliferation and drug-resistant cancers.33,34 Compared to other exosome inhibitors, GW4869 is the most widely used in tumor research, including breast cancer, prostate cancer, melanoma, and glioma. Studies have shown that the nano unit is composed of GW4869 and the ferroptosis inducer, Fe3+. It can inhibit the secretion of tumor-derived exosomes and weaken the immunosuppression induced by exosome PD-L1, thus sensitizing anti-PD-L1 therapy efficacy.35–37 In addition to GW4869, several other nSMase2 inhibitors have clinical potential, such as 2,6-dimethoxy4-(5-phenyl-4-thiophen-2-yl-1H-imidazol-2-yl)-phenol(DPTIP).38,39 Screening of exosomes can be decreased by sulfasalazine to restrain bortezomib resistance in multiple myeloma.40 Therefore, exosomes may play a role in drug resistance in tumors. At the same time, studies have shown that exosomes inhibitors dimethyl carbamide urea (DMA), which significantly reduces the secretion of eHSP90. It has been shown that eHSP90α and EMT promote tumor development.41 In our experiments, gefitinib had a very good anti-tumor effect. The tumor volume and weight of GW4869 combined with gefitinib were smaller than those in the gefitinib alone group, suggesting that GW4869 may have a synergistic effect on gefitinib. GW4869 is an inhibitor of multiple vesicle components. Probably to improve tumor cell sensitivity to gefitinib by inhibiting eHSP90α and EMT. However, no study has investigated whether GW4869 reduces eHSP90α expression and EMT. In this study, we found that GW4869 reduced the expression of eHSP90α, which was accompanied by upregulation of E-cadherin and downregulation of vimentin. In addition, GW4869 was found to enhance the antitumor effects in combination with gefitinib in vivo. GW4869 has the potential to block the progression of gefitinib-resistant NSCLC. However, the mechanism underlying the GW4869 inhibits of eHSP90α release remains unclear. Whether GW4869 combined with gefitinib benefits patients with gefitinib-resistant NSCLC requires further investigation.
Therefore, we evaluated the anti-tumor effect of the exosome inhibitor GW4869 in gefitinib-sensitive NSCLC. We found that GW4869 can inhibit EMT, cell invasion, and migration and has an additional benefit in combination with gefitinib, which may provide a new idea for acquired resistance management in EGFR mutation-positive NSCLC.
Conclusions
In conclusion, our results demonstrated that GW4869 can inhibit EMT and eHSP90α in gefitinib-sensitive NSCLC cells. Thus, these findings may provide new strategies for delaying the development of acquired resistance to gefitinib, and a novel application of the exosome inhibitor GW4869 in NSCLC.
Abbreviations
HSP90α, heat shock protein alpha; EMT, epithelial-mesenchymal transition; NSCLC, non-small-cell lung cancer; EGFR, epidermal growth factor receptor; EGFR-TKI, EGFR-tyrosine kinase inhibitor; MVBs, multivesicular bodies; RASP, resistance-associated secretory phenotype; EV, extracellular vesicles; hrHSP90α, human recombinant HSP90α; RIPA, radioimmunoprecipitation assay; PVDF, polyvinylidene difluoride.
Ethical Statement
All animal experiments in this study complied with the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines 4, as well as were approved by the Ethics Committee of Nanfang Hospital (Guangdong, China) (Medical Ethics No. NFEC-2019-065).
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81470228 and 81670026) and Natural Science Foundation of Guangdong Province (2017A030313849).Sincerely thanks to the generous donation of PC9 from the Guangdong Lung Cancer Institute.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hyuna S, Jacques F, Me RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Ke E, Zhou Q, Wu Y. Emerging Paradigms in Targeted Treatments for Asian Patients with NSCLC. Expert Opin Pharmacother. 2015;16(8):1167–1176. doi:10.1517/14656566.2015.1040391
3. Rawluk J, Waller CF. Gefitinib. Recent Results Cancer Res. 2018;211:235–246.
4. Johnson M, Garassino MC, Mok T, et al. Treatment strategies and outcomes for patients with EGFR-mutant non-small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: focus on novel therapies. Lung Cancer. 2022;170:41–51. doi:10.1016/j.lungcan.2022.05.011
5. Wu SG, Shih JY. Management of Acquired Resistance to EGFR TKI-targeted Therapy in Advanced Non-Small Cell Lung Cancer. Mol Cancer. 2018;17:38. doi:10.1186/s12943-018-0777-1
6. Esposito AR, Pasquale R, Sacco A, et al. Liquid Biopsy Testing Can Improve Selection of Advanced Non-Small-Cell Lung Cancer Patients to Rechallenge with Gefitinib. Cancers. 2019;11(10):1431. doi:10.3390/cancers11101431
7. Li W, Tsen F, Sahu D, et al. Extracellular Hsp90 (eHsp90) as the Actual Target in Clinical Trials: intentionally Or Unintentionally. Int Rev Cell Mol Biol. 2013;303:203–235.
8. Eguchi T, Taha EA, Calderwood SK, et al. Novel Model of Cancer Drug Resistance: oncosomal Release of Cytotoxic and Antibody-Based Drugs. Biology. 2020;9(3):47. doi:10.3390/biology9030047
9. Catalano M, O’Driscoll L. Inhibiting Extracellular Vesicles Formation and Release: a Review of EV Inhibitors. J Extracell Vesicles. 2020;9:1703244.
10. Eguchi T, Sogawa C, Ono K, et al. Cell Stress Induced Stressome Release Including Damaged Membrane Vesicles and Extracellular HSP90 by Prostate Cancer Cells. Cells. 2020;10(1):9. doi:10.3390/cells10010009
11. Jakobsen KR, Demuth C, Sorensen BS, et al. The Role of Epithelial to Mesenchymal Transition in Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Transl Lung Cancer Res. 2016;5(2):172–182. doi:10.21037/tlcr.2016.04.07
12. Nowak E, Bednarek I. Aspects of the Epigenetic Regulation of EMT Related to Cancer Metastasis. Cells. 2021;
13. Jolly MK, Celia-Terrassa T. Dynamics of Phenotypic Heterogeneity Associated with EMT and Stemness during Cancer Progression. J Clin Med. 2019;9(1):8. doi:10.3390/jcm9010008
14. Shibue T, Weinberg RA. EMT, CSCs, and Drug Resistance: the Mechanistic Link and Clinical Implications. Nat Rev Clin Oncol. 2017;14(10):611–629. doi:10.1038/nrclinonc.2017.44
15. Li Y, Zhang T, Qin S, et al. Effects of UPF1 expression on EMT process by targeting E‑cadherin, N‑cadherin, Vimentin and Twist in a hepatocellular carcinoma cell line. Mol Med Rep. 2019;19(3):2137–2143. doi:10.3892/mmr.2019.9838
16. Xu K, Zhang C, Du T, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134:111111. doi:10.1016/j.biopha.2020.111111
17. Trepel J, Mollapour M, Giaccone G, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–549. doi:10.1038/nrc2887
18. Huang B, Pan J, Liu H, et al. High Expression of Plasma Extracellular HSP90α is Associated With the Poor Efficacy of Chemotherapy and Prognosis in Small Cell Lung Cancer. Front Mol Biosci. 2022;9:913043. doi:10.3389/fmolb.2022.913043
19. Lang BJ, Guerrero-Giménez ME, Prince TL, et al. Heat Shock Proteins are Essential Components in Transformation and Tumor Progression: cancer Cell Intrinsic Pathways and Beyond. Int J Mol Sci. 2019;20(18):4507. doi:10.3390/ijms20184507
20. Dong HM, Le YQ, Wang YH, et al. Extracellular Heat Shock Protein 90Alpha Mediates HDM-induced Bronchial Epithelial Barrier Dysfunction by Activating RhoA/MLC Signaling. Respir Res. 2017;18(1):111. doi:10.1186/s12931-017-0593-y
21. Hoter A, El-Sabban ME, Naim HY. The HSP90 Family: structure, Regulation, Function, and Implications in Health and Disease. Int J Mol Sci. 2018;19(9):2560. doi:10.3390/ijms19092560
22. Taha EA, Ono K, Eguchi T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int J Mol Sci. 2019;20(18):4588. doi:10.3390/ijms20184588
23. Den RB, Lu B. Heat Shock Protein 90 Inhibition: rationale and Clinical Potential. Ther Adv Med Oncol. 2012;4(4):211–218. doi:10.1177/1758834012445574
24. Chen J, Rong N, Liu M, et al. The Exosome-Like Vesicles Derived From Androgen Exposed-Prostate Stromal Cells Promote Epithelial Cells Proliferation and Epithelial-Mesenchymal Transition. Toxicol Appl Pharmacol. 2021;411:115384. doi:10.1016/j.taap.2020.115384
25. Ruizhu S, Zhansheng H. Drug resistance mechanisms and progress in the treatment of EGFR-mutated lung adenocarcinoma. Oncol Lett. 2022;24(5):408. doi:10.3892/ol.2022.13528
26. Liao BC, Griesing S, Yang JC. Second-line treatment of EGFR T790M-negative non-small cell lung cancer patients. Ther Adv Med Oncol. 2019;11:1758835919890286. doi:10.1177/1758835919890286
27. Ji W, Choi YJ, Kang M-H. Efficacy of the CDK7 Inhibitor On EMT-Associated Resistance to 3Rd Generation EGFR-TKIs in Non-Small Cell Lung Cancer Cell Lines. Cells. 2020;9(12):2596. doi:10.3390/cells9122596
28. Dai B, Fan M, Yu R, et al. Novel Diphenyl Urea Derivative Serves as an Inhibitor On Human Lung Cancer Cell Migration by Disrupting EMT Via Wnt/beta-catenin and PI3K/Akt Signaling. Toxicol In Vitro. 2020;69:105000. doi:10.1016/j.tiv.2020.105000
29. Lauwers E, Wang YC, Gallardo R, et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol Cell. 2018;71(5):689–702. doi:10.1016/j.molcel.2018.07.016
30. Chen R, Qian Z, Xu X, et al. Exosomes-Transmitted miR-7 Reverses Gefitinib Resistance by Targeting YAP in Non-Small-Cell Lung Cancer. Pharmacol Res. 2021;165:105442. doi:10.1016/j.phrs.2021.105442
31. You J, Li M, Cao L, et al. Snail1-Dependent Cancer-Associated Fibroblasts Induce Epithelial-Mesenchymal Transition in Lung Cancer Cells Via Exosomes. QJM. 2019;112(8):581–590. doi:10.1093/qjmed/hcz093
32. Yang Y, Li CW, Chan LC, et al. Exosomal PD-L1 Harbors Active Defense Function to Suppress T Cell Killing of Breast Cancer Cells and Promote Tumor Growth. Cell Res. 2018;28(8):862–864. doi:10.1038/s41422-018-0060-4
33. Tallon C, Hollinger KR. Nipping disease in the bud: nSMase2 inhibitors as therapeutics in extracellular vesicle-mediated diseases. Drug Discov Today. 2021;26(7):1656–1668. doi:10.1016/j.drudis.2021.03.025
34. Ghandour B, Dbaibo G, Darwiche N. The unfolding role of ceramide in coordinating retinoid-based cancer therapy. Biochem J. 2021;478(19):3621–3642. doi:10.1042/BCJ20210368
35. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with antiPD-1 response. Nature. 2018;560(7718):382. doi:10.1038/s41586-018-0392-8
36. Poggio M, Hu T, Pai CC, et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell. 2019;177(2):414–27 e413. doi:10.1016/j.cell.2019.02.016
37. Wang G, Xie L, Li B, et al. A nanounit strategy reverses immune suppression of exosomal PD-L1 and is associated with enhanced ferroptosis. Nat Commun. 2021;12(1):5733. doi:10.1038/s41467-021-25990-w
38. Rojas C, Barnaeva E, Thomas AG, et al. DPTIP, a newly identifed potent brain penetrant neutral sphingomyelin- nase 2 inhibitor, regulates astrocyte-peripheral immune communica- tion following brain inflammation. Sci Rep. 2018;8(1):17715. doi:10.1038/s41598-018-36144-2
39. Rojas C, Sala M, Thomas AG, et al. A novel and potent brain penetrant inhibitor of extracellular vesicle release. Br J Pharmacol. 2019;176(19):3857–3870. doi:10.1111/bph.14789
40. Wang F, Oudaert I, Tu C, et al. System Xc inhibition blocks bone marrow-multiple myeloma exosomal crosstalk, thereby countering bortezomib resistance. Cancer Lett. 2022;535:215649. doi:10.1016/j.canlet.2022.215649
41. Wong DS, Jay DG. Emerging Roles of Extracellular Hsp90 in Cancer. Adv Cancer Res. 2016;129:141–163.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.