Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Group-Based Symptom Trajectory of Intramuscular Administration of Scopolamine Augmentation in Moderate to Severe Major Depressive Disorder: A Post-Hoc Analysis
Authors Wang X, Zhu X, Ji X, Yang J, Zhou J
Received 3 March 2023
Accepted for publication 21 April 2023
Published 1 May 2023 Volume 2023:19 Pages 1043—1053
DOI https://doi.org/10.2147/NDT.S408794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xiao Wang,1,2,* Xuequan Zhu,1– 3,* Xiao Ji,1,2 Jian Yang,1– 3 Jingjing Zhou1– 3
1The National Clinical Research Center for Mental Disorders & Beijing Anding Hospital of Capital Medical University, Beijing, People’s Republic of China; 2Beijing Key Laboratory of Mental Disorders & Beijing Anding Hospital of Capital Medical University, Beijing, People’s Republic of China; 3Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jian Yang; Jingjing Zhou, The National Clinical Research Center for Mental Disorders & Beijing Anding Hospital of Capital Medical University, 5 Ankang Lane, Dewai Avenue, Xicheng District, Beijing, 100088, People’s Republic of China, Email [email protected]; [email protected]
Objective: Developing new strategies for rapid and sustained relief of depressive symptom has been the focus of research in the field of major depressive disorder (MDD). Scopolamine exerts rapid antidepressant effect in recent years but is controversial. Therefore, we aimed to identify a sensitive patient who may respond to intramuscular injections of scopolamine added to antidepressants based on distinct trajectory patterns.
Methods: We analyzed longitudinal post hoc data collected from 66 MDD patients at Beijing Anding Hospital, Capital Medical University, over a 4-week period. In addition to demographics, depressive symptoms were assessed using the 16-item Quick Inventory of Depressive Symptomatology and Self-Report (QIDS-SR16) Scale and 17-item Hamilton Rating Scale for Depression (HRSD-17) following an i.m. injection of scopolamine. We explored different longitudinal patterns of depressive symptoms using a group-based trajectory model (GBTM). We used multiple logistic regression models to help identify predictors of different depressive symptom trajectories.
Results: A two-class GBTM was identified as optimal for classifying depressive symptoms: high/rapidly declining (39.4%) and moderate/gradually declining depression trajectories (60.6%) were distinguished based on the HRSD-17. The high/rapidly declining depression trajectory was characterized by high initial depression followed by a rapid decrease at the end of the study. The moderate/gradual decline trajectory was dominated by moderate depression and gradual decline over 4 weeks. There were no significant associations of age, gender, education, or age of onset with the two trajectory groups.
Conclusion: Scopolamine added to antidepressants can effectively relieve the symptoms of patients with severe depression, and it decreases faster than patients with moderate depression.
Keywords: major depressive disorder, scopolamine, depressive symptom, group-based trajectory analysis
Introduction
Major depression (MDD) is a mental disorder with high prevalence, morbidity and mortality that affects more than 300 million people worldwide and is a leading cause of disability worldwide.1–3 At present, the commonly used antidepressants targeting the monoamine neurotransmitter system require several weeks to produce full therapeutic effect, and cannot relieve all symptoms of depression, which will significantly increase the risk of suicide and self-mutilation.4,5 Therefore, we need to find faster, effective and sustained treatment for depression.
The muscarinic cholinergic receptor system plays an important role in the pathophysiology of depression, and there is substantial evidence supporting the role of the muscarinic cholinergic system in mood regulation.6 Subsequently, numerous studies have now shown the rapid antidepressant effects of scopolamine.7–9 Conventional antidepressants are not fully efficacious produce effects only after weeks of daily dosing, scopolamine was found to have a rapid antidepressant effect (within 72 hours) in patients with unipolar and bipolar depression.10 A study suggests that scopolamine can produce rapid antidepressant effects even in treatment resistant MDD.11 Oral scopolamine added to antidepressants is a safe and effective drug for adjuvant treatment of moderate and severe depression.12 Previous studies13 have shown that scopolamine has no significant antidepressant effect on severe depression compared with placebo. Thus, questions remain regarding scopolamine’s antidepressant efficacy.
Therefore, we would like to further determine whether scopolamine has poor efficacy or is the population insensitive? Finding people who are effective for scopolamine and improving the ability of depressed patients to respond to treatment are key to helping improve depressive symptoms.14 Furey et al used neuroimaging to evaluate potential biomarkers of scopolamine, which enhanced anterior cingulate cortex activity and stronger oxygen-dependent signaling in the medial occipital cortex predicted scopolamine response during emotional facial processing prior to treatment.15 Similarly, the baseline response of the amygdala to explicit sad face stimuli was used to predict the antidepressant response of scopolamine, showing that the intensity of the blood oxygen level dependence signal in the amygdala was positively correlated with the antidepressant efficacy of scopolamine.16 Previous studies17,18 demonstrated the efficacy of scopolamine in patients with depression, especially in treatment-naïve and female patients. Previous studies have attempted to predict efficacy based on clinical features and functional magnetic resonance imaging results, but due to small sample sizes and complicated operations, it has been difficult to use widely in clinical practice. Prior to treatment, efforts have been made to identify patterns of symptoms and population characteristics, to help determine responses to the addition of scopolamine to antidepressants, and to identify patients who are sensitive to scopolamine. Populations sensitive to scopolamine must be identified to predict the treatment response and to help find more effective treatments for MDD patients.
However, little is known about the reasons for the difference in antidepressant efficacy when scopolamine is added to antidepressants. The group-based trajectory modeling is a person-centered (idiographic) method.19 This approach20,21 has been used to identify different symptom trajectories in the treatment of depression, forming different subgroups. Trajectory modeling facilitates a comprehensive understanding of intervention effects and helps to identify predictors of intervention response.22 However, to our knowledge, this modeling approach has never been used to predict the antidepressant effects of scopolamine, so we used this method to find patients who are sensitive to scopolamine added to antidepressants, which is helpful for further relevant large randomized controlled trials (RCTs).
In this study, group-based trajectory models were used to re-examine data from large RCTs to help predict the effect of scopolamine addition to antidepressants.23 Zhou et al have described this research protocol in more detail24 [ClinicalTrials.gov Identifier: NCT03131050]. Therefore, we conducted this study to determine (i) subgroups of different trajectories of depressive symptoms from baseline to 28 days, (ii) find out whether the groups (described below) was related to the trajectories, and in particular whether the trajectories depended on the dose of scopolamine, (iii) to find out predictors of trajectory membership, and to help predict the efficacy of scopolamine added to antidepressants.
Methods
Participants
This study analyzed secondary panel survey data from a Chinese longitudinal study of scopolamine added to antidepressants in moderate to severe major depressive disorder. Participants (N = 66) were recruited at Beijing Anding Hospital, Capital Medical University, we used Diagnostic and Statistical Manual IV (DSM-IV) standard structured clinical interviews and the 17-item Hamilton Depression Rating Scale (HRSD-17; Total score ≥20 indicates MDD) for diagnosis. Participants were randomly assigned to one of three groups: a high-dose group, a low-dose group or a placebo control group, with 22 subjects in each group. The study is a single-centre, double-blind, three-arm randomized trial with a 4-week follow-up, which the participants and raters will be blind to treatment allocation. The high-dose group received 0.6mg i.m. scopolamine per day, the low-dose group received 0.3mg i.m. scopolamine at 9 a.m. and saline at 3 p.m. Finally, the saline control group received two injections of saline at 9 a.m. and 3 p.m. for 3 days in each group.
The study started on March 15, 2017 and completed on February 8, 2018. Scales were assessed at baseline and on days 1, 2, 3, 4, 7, 14, and 28 after intramuscular injection of scopolamine (i.m.). There were no significant differences in baseline HRSD scores, age, sex, body mass index (BMI) and duration of illness among the three groups. The missing data from the data set can be estimated using the group-based trajectory model (GBTM), integrate all available information using maximum likelihood, and provide asymptotically unbiased parameter estimates.19
Procedure
Group-based trajectory modeling analysis of the data of 66 subjects was performed following the i.m. injection of scopolamine added to antidepressants. Ethical approval and consent to participate: The trial protocol has been approved by the ethical review committee of Beijing Anding Hospital (No.2016–106, Beijing, China).
Subjects who met the criteria were randomly assigned to one of three treatment groups with 22 participants in each group, for a total of 66 participants. All subjects who received i.m. injections scopolamine was kept in the emergency ward for observation for the first 3 days, followed by clinical follow-up at 4, 7, 14, and 28 days after treatment. All participants received oral escitalopram (10 mg/day) on all 28 days.
Measures
Depression
Baseline Quick Inventory of Depressive Symptomatology and Self-Report (QIDS-SR16) score and HRSD-17 total score were comparable between the groups. HRSD-17 is a semi-structured interview in which patients are scored by independent raters who masked to the treatment assignments. QIDS-SR16 is a 16-entry self-filling scale that measures the severity of depressive symptoms over the past 7 days.
Statistical Analyses
The patient demographic characteristics (mean and standard deviation for continuous variables [age, BMI, total HRSD-17 score, QIDS-SR16 score at baseline], percentages for categorical variables [gender, race, education, and duration of illness]) were descriptive.
We used GBTM, a relatively new statistical method for finding clusters of individuals that exhibit similar trajectories over time for a given variable, such as mood symptoms.19 GBTM aims to distinguish subgroups based on trajectories over time in a way that is easy to summarize and understand. Individuals were assigned to the trajectory subgroup to which the probability of membership was highest according to the GBTM. This method consists of a finite number of distinct groups, assuming that the population is not homogeneous. The software used to construct the GBTM was SAS PROC TRAJ, developed by Jones et al.25
GBTM is used to determine the optimal number of categories through the combination of model fitting indicators, such as Akaike information criterion (AIC), Bayesian information criterion (BIC), sample size adjustment Bayesian information criterion, relative entropy, Lo – Mendell-Rubin – adjusted likelihood ratio test (LMR-LRT) and parameter push-over likelihood ratio test.26 It is considered to have a good relative fit based on low information standard values, significant likelihood ratio test results and high entropy.27 Entropy is an index of classification accuracy based on posterior probabilities, the higher Entropy values and smaller AIC, BIC, aBIC values denote better models. Explanatory power, theoretical coherence, and parsimony are also considered to determine the optimal number of classes.28 In this study, we examined the posterior probability and average group membership probability of each trajectory to help evaluate each model and determine the trajectory of depression. After determining the trajectory model, using the estimated class membership discussed above, logistic regression was used to examine the influence of factors on the identified depression trajectory group.
Results
Sociodemographic Characteristics
Table 1 shows the baseline demographic and disease severity data. Of the 66 subjects, 23 were male (34.8%) and 43 were female (65.2%); 40 participants had experienced one episode (60.6%) and 26 showed disease recurrence (39.4%). Most recurrence cases involved 1 (57.7%) or 2–3 (34.6%) episodes. The average age was 26.4 years (SD = 6.0), the average onset age was 24.4 years (SD = 6.7), and the average BMI was 21.8 kg/m2 (SD = 3.0). In total, 83.3% (55/66) of the subjects reported the duration of their current episode; in 72.7% of cases, it was ≥8 weeks. The education level for 59.1% of the subjects was graduate school, while for 28.8% it was college graduate, and for 12.1% it was less than high school. The average duration of illness was 2 years. The total HRSD-17 and QIDS-SR16 scores at baseline were 25.2 (SD = 4.6) and 17.0 (SD = 4.5), respectively.
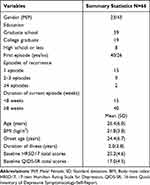 |
Table 1 Patients’ Baseline Characteristics |
Selection of the Trajectory Model Based on the HRSD-17
Table 2 provides the AIC, BIC, ABIC, LMR, VLMR, BLRT and entropy values for the 1-, 2- and 3-class linear and quadratic solutions. To model convergence, the intercept and quadratic parameters were fixed, while the slope could be freely estimated across classes. The AIC, BIC and ABIC decreased from the 2- to 3-class linear and quadratic models. The overall classification accuracy (entropy) of the 3-class linear model (0.828) was slightly higher than that of the 2-class model (0.750). The LMR and BLRT values for the 2-class models were significant, but not for the 3-class model. The entropy value for the 3-class quadratic model (0.913) was higher than that for the 2-class model (0.872), but the LMR, VLMR and BLRT values for the 3-class model were not significant. The 2-class quadratic model had a higher value than the linear model (0.750). Finally, we choose the 2‐class non-linear mixed model because of their high entropy and low BIC and ABIC values. The Lo–Mendell–Rubin–adjusted likelihood ratio test p-value was significant for this solution. Compared to the other models, the two-class solution has significant likelihood ratio tests, with better information criterion values. Thus, the 2-class non-linear quadratic parameter models can be considered optimal.
 |
Table 2 Fit Indices for 1- to 3-Class Trajectory Models of Depressive Symptoms |
Using trajectory analysis, we identified two distinct depression trajectories from baseline to the 28-day follow-up according to the total HRDS-17 score. The best fitting model, displayed in Figure 1, distinguished two subpopulations according to the scopolamine response that are heterogeneous in terms of the longitudinal trajectory of their depressive symptoms. This model had the best log-likelihood value, indicating that it converged successfully. The decision to distinguish two trajectories was based on the goodness of fit, trajectory modelling parameters and statistically significant results. The two depression trajectories were high/rapid declining and moderate/gradually declining.
 |
Figure 1 Two trajectories of depressive symptoms after the i.m. injection of scopolamine. |
Table 3 shows the parameter estimates of the growth factors for the classes in the 2-class non-linear quadratic model. Figure 1 shows the depressive symptom scores overtime, following the initiation of i.m. injection of scopolamine. Figure 1 shows the trajectories for the classes in the 2-class nonlinear quadratic model.
 |
Table 3 Summary of Group-Based Trajectory Analysis for Depressive Symptoms |
Turning to the parameter estimates for class 1, approximately 60.6% of the sample (n = 40) was assigned to class 1. A moderate/gradually declining depression trajectory was characterized by moderate initial depression followed by decreases over follow-up (significant moderate intercept, b = 13.85, standard error [SE] = 14.09, p ≤ 0.001; flat but significant negative slope, b=−0.39, SE = 0.14, p ≤ 0.01), and a significant quadratic parameter (est. = 0.01, SE = 0.00, p ≤ 0.01). Group 1 (“moderate/gradually declining”) experienced moderate depression and a mild decline over 4 weeks, and represented 60.6%of the weighted sample. Class 1 had an initial HRSD score of 13.85, lower than that of class 2 (20.61), while class 1 showed a rate of change of-0.39 over the 4-week period. In other words, it represents moderate levels depression, with a mild decline over time.
As shown in Figure 1, group 2 (“high/rapid declining”) included patients who has severe depression that declined toward the end of the study period, representing 39.4% of the weighted sample (significantly high intercept: Est.=20.61, SE = 7.16, p ≤ 0.001; significantly decreasing slope: Est.=−1.21, SE = 0.15, p ≤ 0.001; significant quadratic parameter: Est. = 0.03, SE = 0.00, p ≤ 0.001). The rapidly decreasing group had a depressive symptom score of 20.61 at baseline, which significantly decreased by approximately 1.21 points over 4 weeks. Class 2 had severe depression with a sharp decline seen over time.
Association Between Conditions and Trajectory Classes
We next examined the associations between conditions and trajectory classes (Table 4). By chi-square test, we find that there is no significant difference in trajectory membership between conditions (χ² = 2.4 [df = 2], P = 0.30). The three conditions did not significantly differ in terms of membership of the two classes. It was further found that participants in the low-dose group were more likely to be placed in the high/rapid declining trajectory group than those in the high-dose group (42.3% vs 34.6%) and saline control group (42.3% vs 23.1%), but the difference was not significant. In addition, the high-dose group was more likely to be classified into the moderate/gradually declining trajectory compared to participants in the low-dose group (32.5% vs 27.5%), but the difference was again not significant.
 |
Table 4 Trajectory Class Membership by Condition |
Baseline Predictors of Trajectory Class
We further examined the correlation between baseline predicted values and trajectory categories, as shown in Table 5. We used multinomial regression models comprising binary and continuous predictors, with trial entered as a covariate. Multinomial regression involves comparisons to specific reference classes. The results showed no significant association between patients’ basic information, such as age, education, age of first onset, first episode or duration of illness with trajectory class (all p ≥ 0.05).
 |
Table 5 Univariate Logistic Regression Analysis of Factors Associated with Being Class 2 Trajectory |
Discussion
GBTM may be useful for identifying subgroups responsive to scopolamine was added to antidepressants. GBTM can be used to identify clusters of individuals showing similar disease progression over time, which is a form of finite mixture modeling of unobserved subpopulations.29 The method allows empirical identification of the typical symptom trajectories observed in RCTs in terms of number and prevalence, and may allow responsivity to interventions to be characterized.19 The clinical subgroups are mostly defined based on experience, and the trajectory classifications may be less arbitrary than the indicators typically used to define intervention responsivity.21 Trajectory analysis model can predict who will benefit from the intervention and who will not benefit from the intervention, which is critical to reduce adverse reactions of some patients, improve the intervention and determine which patients should be provided with which intervention.30 Trajectory modeling can provide a more comprehensive understanding of intervention effects. Group-based trajectory modeling is now commonly used, but this technique has not been applied to characterize distinct trajectories patterns of the response to i.m. injections scopolamine augmentation in depressive disorder.
To our knowledge, this study is the first to apply group-based trajectory modeling in the context of i.m. administration of scopolamine adds to the antidepressants in moderate to severe major depressive disorder. Using data from an RCT, we identified two trajectories of depression symptoms in participants who received i.m. injections of scopolamine added to antidepressants. The results indicated that there are two different trajectories of i.m. administration of scopolamine: high/rapid declining (39.4%) and moderate/gradually declining. These trajectories were characterized by a decline in depression symptoms within 28 days and were primarily distinguished based on baseline symptom severity. The high/rapid declining trajectory was characterized by severe symptoms at pretest that declined rapidly over the 28-day follow-up. The moderate/gradually declining trajectory was characterized by less severe initial in symptoms that showed a moderate reduction over time; most of the sample was assigned to this class.
The high/rapid declining trajectory showed that after i.m. injection of scopolamine, depressive symptoms declined more rapidly in patients with more severe depression. The rapid antidepressant effects of scopolamine are thought to be related to its faster entry into the brain than other antimuscarinic drugs.31 A RCT found a rapid and strong response to intravenous (i.v.) infusions scopolamine in depressed patients with generally poor prognosis.10 Although the efficacy of scopolamine on treatment-resistant depression (TRD) is controversial, most studies show that showed that i.v. administration of scopolamine provides rapid and powerful relief of symptoms in medication-free patients with TRD.17,32 Although previous studies did not find that the severity of depression was related to the efficacy of scopolamine, most studies have suggested that scopolamine is more effective for severe depression and depression patients with a poor treatment response. This also suggests that scopolamine may have a better and faster curative effect on severe depression patients when added to antidepressants, thus representing a new treatment option for this group of severe depression.
Recent mechanism studies have shown that scopolamine, as a non-selective muscarinic acetylcholine receptor (M-AChRs) antagonist, scopolamine rapidly increases the mechanistic target of rapamycin complex 1 (mTORC1) signaling in the medial prefrontal cortex (mPFC).33 One hypothesis is that GABA interneurons mediate the rapid antidepressant effects of scopolamine by blocking muscarinic receptors on GABAergic interneurons, resulting in disinhibition of pyramidal neurons and increased glutamate transmission.34 Although the mechanism of action of scopolamine has not yet been verified, the above research provides ideas and prospects for our future mechanism research.
The study was divided into three treatment groups based on dose, a high-dose group of 0.6mg daily, a low-dose group of 0.3mg daily and a placebo control group, found no correlation between dose group and group-based trajectory (high/rapid declining vs moderate/gradually declining). In the preliminary study, the antidepressant effects of scopolamine were dose dependent.35 Previous studies established the antidepressant effect of scopolamine at an i.v. dose of 2–4 μg/kg (100kg, 0.2–0.4mg) in patients with MDD.10 Evidence suggests that scopolamine (4 ug/kg i.v.) has a rapid and powerful antidepressant effect.11 In an open-label study, a significant antidepressant effect was observed on the second day of i.m. injection of scopolamine (0.4 mg), although the magnitude of the effect was small36 due to the relatively low bioavailability during i.m. administration; this dose is equivalent to ~2 ug/kg i.v. in a 100-kg individual (0.2mg).35 These findings suggest that higher doses of scopolamine may be needed to obtain sufficient antidepressant effects. Although scopolamine is well absorbed via the transdermal route, it is unclear whether the maximum concentration obtained by the IV route could be obtained by a more slowly absorbed route (eg, the transdermal route).37 Accordingly, no correlation between dose and trajectory was found in this study, which may be related to the low dose of the drug in the trial (0.3mg) and the low bioavailability caused by factors during intravenous administration.
Some abnormalities in cholinergic receptor function in patients with depression exhibit gender effects. One study showed that women reacted more strongly to scopolamine than men, with both men and women showing a rapid antidepressant response to scopolamine, but the magnitude of the response was greater in women.18 Gender differences in plasma hormone levels following cholinergic stimulation suggest elevated cholinergic sensitivity in premenopausal depressed patients due to estrogenic effects.38,39 Previous studies40 found that genetic variation in the 2 muscarinic (M2) cholinergic receptor gene (A/T 1890) is specifically associated with MDD in female subjects. Elevated hormone levels associated with increased cholinergic sensitivity in premenopausal women with major depressive disorder,38 and estrogen can promote the release of acetylcholine and the activity of choline acetyltransferase; M2 receptor stimulation enhances the function of estrogen-induced N-methyl-D-aspartate receptor (NMDAR).41 These findings add to the current conclusion that women exhibit a greater antidepressant response to scopolamine compared to men.18,38,41 However, no correlation between gender and group-based trajectory was found in this present study; gender was not the most sensitive factor for distinguishing the high/rapid and moderate/gradually declining trajectories. The above differences were not found in our study, which may be related to factors such as the relatively small sample size, route of administration (i.m.) and inclusion of a placebo group, thereby limiting the generalizability of the results.
Our study had some limitations that should be acknowledged. First, the results were obtained following i.m. injections only; explore the efficacy of other routes of administration of scopolamine and the feasibility of continued administration. Second, the analyses were post hoc. Third, the small sample may have affected trajectory group characteristics; validation in studies with larger samples is needed.
Conclusion
In summary, the GBTM used in this study revealed two distinct trajectories of depressive symptoms: high/rapid and moderate/gradually declining. Scopolamine added to antidepressants can effectively relieve the symptoms of patients with severe depression, and it decreases faster than patients with moderate depression. Gender and scopolamine dose did not appear to influence the magnitude of the response. These findings have promoted our understanding of the heterogeneity of patients with depression, suggesting that we can give priority to following an i.m. injection of scopolamine for patients with major depressive disorder, to help them quickly relieve depression symptoms and further reduce the suicide risk caused by depression. However, imaging data and biological samples of sensitive populations still need to be collected to identify the brain regions and biomarkers sensitive to scopolamine, so as to further identify sensitive populations. The antidepressant efficacy of scopolamine, the most appropriate dose and route of administration, and the duration of the antidepressant effect still require further validation. Continued research on scopolamine is imperative because it can provide immediate relief for patients and is particularly useful for inpatients.
Data Sharing Statement
The final data for this study can be supplied on request. The data and materials are available from the corresponding author on reasonable request. The data analysed in this study have been previously reported in Zhou et al.23
Ethics Statement
We obtained a written informed consent from all the participants. This study was approved by the Ethics Committee of the Beijing Anding Hospital, Capital Medical University (approval number: 2016127FS-2) and followed the tenets of Helsinki Declaration.
Acknowledgments
The authors would like to express their gratitude to Edit Springs (https://www.editsprings.cn) for the expert linguistic services provided.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Training Plan for High Level Public Health Technical Talents Construction Project (Discipline Backbones−02-39).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Chiu M, Vigod S, Rahman F, et al. Mortality risk associated with psychological distress and major depression: a population-based cohort study. J Affect Disord. 2018;234:117–123. doi:10.1016/j.jad.2018.02.075
2. World Health Organisation. Depression; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/depression.
3. Conley AA, Norwood AEQ, Hatvany TC, et al. Efficacy of ketamine for major depressive episodes at 2, 4, and 6-weeks post-treatment: a meta-analysis. Psychopharmacology. 2021;238(7):1737–1752. doi:10.1007/s00213-021-05825-8
4. Rakesh G, Pae CU, Masand PS. Beyond serotonin: newer antidepressants in the future. Expert Rev Neurother. 2017;17(8):777–790. doi:10.1080/14737175.2017.1341310
5. Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015;20:35–39. doi:10.1016/j.coph.2014.11.005
6. Navarria A, Wohleb ES, Voleti B, et al. Rapid antidepressant actions of scopolamine: role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015;82:254–261. doi:10.1016/j.nbd.2015.06.012
7. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24–26. doi:10.1097/WNF.0b013e318278b703
8. Zarate C, Duman RS, Liu G, et al. New paradigms for treatment-resistant depression. Ann N Y Acad Sci. 2013;1292:21–31. doi:10.1111/nyas.12223
9. Taub N. Naltrexone and scopolamine rapidly reduce symptoms of Major Depressive Disorder (MDD): a double blinded randomized controlled pilot study. Open J Depress. 2019;8(1):1–4. doi:10.4236/ojd.2019.81001
10. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121–1129. doi:10.1001/archpsyc.63.10.1121
11. Drevets WC, Zarate CA
12. Khajavi D, Farokhnia M, Modabbernia A, et al. Oral scopolamine augmentation in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(11):1428–1433. doi:10.4088/JCP.12m07706
13. Park L, Furey M, Nugent AC, et al. Neurophysiological changes associated with antidepressant response to ketamine not observed in a negative trial of scopolamine in major depressive disorder. Int J Neuropsychopharmacol. 2019;22(1):10–18. doi:10.1093/ijnp/pyy051
14. Zarate CA
15. Furey ML, Drevets WC, Szczepanik J, et al. Pretreatment differences in BOLD response to emotional faces correlate with antidepressant response to scopolamine. Int J Neuropsychopharmacol. 2015;18(8):pyv028–pyv028. doi:10.1093/ijnp/pyv028
16. Szczepanik J, Nugent AC, Drevets WC, et al. Amygdala response to explicit sad face stimuli at baseline predicts antidepressant treatment response to scopolamine in major depressive disorder. Psychiatry Res Neuroimaging. 2016;254:67–73. doi:10.1016/j.pscychresns.2016.06.005
17. Ellis JS, Zarate CA
18. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479–2488. doi:10.1038/npp.2010.131
19. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi:10.1146/annurev.clinpsy.121208.131413
20. Brière FN, Janosz M, Fallu JS, et al. Adolescent trajectories of depressive symptoms: codevelopment of behavioral and academic problems. J Adolesc Health. 2015;57(3):313–319. doi:10.1016/j.jadohealth.2015.05.012
21. Thibodeau MA, Quilty LC, De Fruyt F, et al. Latent classes of nonresponders, rapid responders, and gradual responders in depressed outpatients receiving antidepressant medication and psychotherapy. Depress Anxiety. 2015;32(3):213–220. doi:10.1002/da.22293
22. Sunderland M, Wong N, Hilvert-Bruce Z, et al. Investigating trajectories of change in psychological distress amongst patients with depression and generalised anxiety disorder treated with internet cognitive behavioural therapy. Behav Res Ther. 2012;50(6):374–380. doi:10.1016/j.brat.2012.03.005
23. Zhou J, Yang J, Zhu X, et al. The effects of intramuscular administration of scopolamine augmentation in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled trial. Ther Adv Psychopharmacol. 2020;10:2045125320938556. doi:10.1177/2045125320938556
24. Zhou J, Wang W, Yang J, et al. Scopolamine augmentation of a newly initiated escitalopram treatment for major depressive disorder: study protocol for a randomized controlled trial. Trials. 2019;20(1):33. doi:10.1186/s13063-018-3132-3
25. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. doi:10.1177/0049124101029003005
26. Nylund KL, Asparouhov T, Muthén B. Deciding on the number of classes in latent class analysis and growth mixture modeling: a monte carlo simulation study. Struct Equ Modeling. 2007;14(4):535–569. doi:10.1080/10705510701575396
27. Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2010;2(1):302–317. doi:10.1111/j.1751-9004.2007.00054.x
28. Muthén B. Statistical and substantive checking in growth mixture modeling: comment on Bauer and Curran (2003). Psychol Methods. 2003;8(3):384–393. doi:10.1037/1082-989X.8.3.369
29. Cook A, Christine M. Group-based modeling of development. J Am Stat Assoc. 2006;101(473):405.
30. Kazdin AE. Developing a research agenda for child and adolescent psychotherapy. Arch Gen Psychiatry. 2000;57(9):829–835. doi:10.1001/archpsyc.57.9.829
31. Frey KA, Koeppe RA, Mulholland GK, et al. In vivo muscarinic cholinergic receptor imaging in human brain with [11C] scopolamine and positron emission tomography. J Cereb Blood Flow Metab. 1992;12(1):147–154. doi:10.1038/jcbfm.1992.18
32. Furey ML, Drevets WC, Hoffman EM, et al. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70(3):280–290. doi:10.1001/2013.jamapsychiatry.60
33. Voleti B, Navarria A, Liu RJ, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742–749. doi:10.1016/j.biopsych.2013.04.025
34. Wohleb ES, Wu M, Gerhard DM, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126(7):2482–2494. doi:10.1172/jci85033
35. Ebert U, Grossmann M, Oertel R, et al. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41(1):51–60. doi:10.1177/00912700122009836
36. Gillin JC, Sutton L, Ruiz C, et al. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry. 1991;30(2):157–169. doi:10.1016/0006-3223(91)90170-q
37. Nachum Z, Shahal B, Shupak A, et al. Scopolamine bioavailability in combined oral and transdermal delivery. J Pharmacol Exp Ther. 2001;296(1):121–123.
38. Rubin RT, Abbasi SA, Rhodes ME, et al. Growth hormone responses to low-dose physostigmine administration: functional sex differences (sexual diergism) between major depressives and matched controls. Psychol Med. 2003;33(4):655–665. doi:10.1017/s0033291703007426
39. Riemann D, Hohagen F, Bahro M, et al. Sleep in depression: the influence of age, gender and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. Eur Arch Psychiatry Clin Neurosci. 1994;243(5):279–290. doi:10.1007/bf02191586
40. Comings DE, Wu S, Rostamkhani M, et al. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114(5):527–529. doi:10.1002/ajmg.10406
41. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809–816. doi:10.1016/j.neuroscience.2004.01.013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
