Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Gray Matter Density of the Dorsomedial Prefrontal Cortex Mediates the Relationship Between Catastrophizing and Anxiety in Somatic Symptom Disorder
Authors Pan X, Ding W, Sun X, Ji C, Zhou Q, Yan C, Zhou Y, Luo Y
Received 14 December 2020
Accepted for publication 18 February 2021
Published 9 March 2021 Volume 2021:17 Pages 757—764
DOI https://doi.org/10.2147/NDT.S296462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Yuping Ning
Xiandi Pan,1,* Weina Ding,2,* Xia Sun,3,* Chenfeng Ji,3 Qian Zhou,3 Chao Yan,4 Yan Zhou,2 Yanli Luo3
1Pudong New Area Mental Health Center, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Radiology, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Psychological Medicine, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, People’s Republic of China; 4Key Laboratory of Brain Functional Genomics (MOE&STCSM), Shanghai Changning-ECNU Mental Health Center, School of Psychology and Cognitive Science, East China Normal University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanli Luo
Department of Psychological Medicine, Renji Hospital, Shanghai Jiaotong University School of Medicine, Pujian Road 160, Shanghai, 200127, People’s Republic of China
Tel +86-21-68382998
Email [email protected]
Yan Zhou
Department of Radiology, Renji Hospital, Shanghai Jiaotong University School of Medicine, Pujian Road 160, Shanghai, 200127, People’s Republic of China
Tel +86-21-68385105
Email [email protected]
Objective: Catastrophizing is commonly co-occurrence with anxiety in somatic symptom disorder (SSD). However, the quantitative relationship between catastrophizing and anxiety in SSD and its underlying neuropsychopathology remains unclear.
Methods: To address the issue, twenty-eight SSD patients and twenty-nine healthy controls (HCs) completed the Hamilton anxiety scale and the catastrophizing subscale of the cognitive emotion regulation questionnaire. Then they underwent structural magnetic resonance imaging and voxel-based morphometry analysis was performed to obtain gray matter density (GMD) of the dorsomedial prefrontal cortex (dmPFC).
Results: Independent samples t-tests showed no significance between SSD patients and HCs in the scores on the catastrophizing subscale and GMD of the dmPFC. However, correlation analysis found that catastrophizing was significantly positively associated with anxiety in SSD. Further, mediation analyses revealed that GMD of the dmPFC (bilateral medial Brodmann area 8) mediated the relationship between catastrophizing and anxiety in SSD.
Conclusion: These findings support Kirmayer’s disease model of SSD that catastrophic interpretations of somatic symptoms resulted in increased anxiety and demonstrate that the dmPFC may be a potential neural site linking catastrophizing and anxiety in SSD.
Keywords: somatic symptom disorder, dorsomedial prefrontal cortex, catastrophizing, anxiety, mediating effect
Introduction
Somatic symptom disorder (SSD) is a novel disease definition in the 5th Diagnostic and Statistical Manual of Mental Disorders (DSM-5) based on a reorganization of somatoform disorder (SFD) diagnoses in DSM-IV. Individuals with SSD are characterized by disproportionate thoughts about the seriousness of somatic symptoms which may medically unexplained, and have persistently high level of anxiety about health or somatic symptoms. Even though their somatic symptoms may be improved or disappeared, their state of being symptomatic is persistent.1 Unlike SFD overemphasized the centrality of medically unexplained symptoms, the diagnostic criteria of SSD incorporated affective, cognitive, and behavioral components. This change provides a more comprehensive and accurate reflection of the clinical condition, but meanwhile, brings research gaps about the relationships among different kinds of symptoms.
Recently, researchers found SSD patients have multiple cognitive dysfunctioning including information processing, attention, memory, planning and flexibility.2,3 SSD patients possibly regard normal bodily sensations as physical illness because of catastrophic interpretations. Due to the impaired cognitive flexibility, they stubbornly evaluate their somatic symptoms as excessively threatening, harmful, or troublesome and frequently think the worst about their health. In such case, catastrophizing is reckoned as a possible cause that leading to increased emotional arousal and anxiety in SSD.4 However, the relationship between catastrophizing and anxiety in SSD has been little quantitatively studied because previous relationship studies were somatization-centred.
On the other side, some researchers have shown interest in the mechanism of the catastrophizing-anxiety relationship. Hofmann found that perception of control over anxiety mediated the relation between catastrophizing and social anxiety.5 Fergus and Valentiner discovered that catastrophizing was only associated with health anxiety at high levels of intolerance of uncertainty.6 Bailey and Wells revealed that the effect of catastrophizing on health anxiety was explained by the proposed interaction with metacognition.7,8 However, these explores just considered the mediating role of sociopsychological factors. The underlying neuropsychopathology of the catastrophizing-anxiety relationship in SSD remains unknown.
Recent research demonstrated that the dmPFC has a role in cognitive regulation of emotion.9–11 The dorsomedial prefrontal cortex (dmPFC) is a section of the prefrontal cortex which mainly including the medial part of Brodmann area (BA) 8 and 9.12–14 It is a cerebral territory that acts as a conduit between cognitive control regions and emotional arousal provoking areas.15 Particularly, using a classical fear-conditioning paradigm, Raczka et al found that healthy subjects carried the NPSR1 T allele exhibited stronger conditioned stimuli-evoked brain activity in the dmPFC and the dmPFC activation was correlated with participants’ fear evaluations.16 Utilizing a guided hyperventilation task, Holtz et al revealed that participants showed an increased activation in the dmPFC during anticipation of hyperventilation and the dmPFC activation were positively correlated with the trait-like tendency to catastrophize about somatic symptoms.17 These results suggested that the dmPFC was likely the key neural substrate for catastrophizing of fear reactions16 and played a vital role in the catastrophizing-related process of anxiety generation. However, these studies only involved the dmPFC’s functional activities and based on healthy subjects. Whether the dmPFC’s structural factors engaged in the catastrophizing-anxiety relationship in SSD patients is still unclear.
Collectively, the catastrophizing-anxiety relationship in SSD have not been quantitatively identified and the neuropsychopathology underlie this relationship still remains unclear. To solve these problems, patients with SSD and age-, gender-, education-matched healthy controls (HCs) were recruited. Catastrophizing and anxiety data were collected from scale evaluation, and gray matter density (GMD) of subregions in bilateral dmPFC were calculated from MRI image. Accordingly, we hypothesized that: 1) SSD patients adopted more strategies of catastrophizing than HCs; 2) lower GMD of the dmPFC would be observed in SSD patients as compared with HCs; 3) catastrophizing was related to anxiety in SSD; and 4) the catastrophizing-anxiety relationship in SSD was mediated by GMD of the dmPFC.
Participants and Methods
Participants
This cross-sectional study enrolled 34 SSD patients who visited the Department of Psychological Medicine at Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine from March 2017 to June 2020, and 33 HCs were enrolled via advertisements posted in nearby communities. All patients were treatment-naive while met the diagnostic criteria for SSD in DSM-5. The diagnosis was validated by two qualified psychiatrists. Besides, all patients complied with the following inclusion criteria: a) ages between 18 and 65 years; b) right-handed. Participants with a diagnosis of uncontrolled somatic diseases, drug or substance abuse, presence of other mental diseases, current pregnancy, stroke within the past twelve months, cancer within the last three years, and contraindications for MRI were exempted. Of these participants, 28 SSD patients and 29 HCs had obtained complete clinical and MRI data. This study was approved by the local Ethics Committee of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine (KY2020-152) and following the stated guidelines. All participants read and signed informed consent documents prior to the experiment and all procedures were conducted in accordance with principles expressed in the Declaration of Helsinki.
Clinical and Psychological Assessment
The Hamilton Anxiety Scale (HAMA) (Hamilton, 1959) was used to measure the anxiety of participants. The 14 items HAMA have two subscales. The psychic subscale addresses mental agitation and psychological distress and the somatic subscale is focusing on somatic complaints related to anxiety. To exclude the impacts of somatic symptoms on the relationships between catastrophizing and anxiety. These two subscales were separately used as research target (dependent variable) and control variable (covariate) in the present study. Catastrophizing was assessed by the Cognitive Emotion Regulation Questionnaire (CERQ). There are four items in the CERQ (Item-29: I often think that what I have experienced is much worse than what others have experienced; Item-30: I keep thinking about how terrible it is what I have experienced; Item-31: I keep thinking about how terrible it is what I have experienced; Item-32: I continually think how horrible the situation has been), which are specifically designed to assess catastrophizing and this subscale is reliable (Cronbach’s α=0.79) in its Chinese version.18
MRI Data Acquisition and Processing
All MRI data were collected on a Signa HDxt 3T scanner (GE Healthcare, USA) in the Department of Radiology at Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine. An eight-channel standard head coil with foam padding was employed to control head motion. During scanning, the participants were instructed to remain motionless, stay awake, eyes closed, and thinking nothing. Structural MRI data were collected using an 3D T1-weighted fast spoiled gradient recalled sequence: repetition time = 5.6 ms, echo time = 1.8 ms, inversion time = 450 ms, flip angle = 15°, field of view = 256 mm x 256 mm, matrix = 256 x 256, slice thickness = 1.0 mm, gap = 0, number of slices = 156. Voxel-based morphometry (VBM)19,20 was utilized to calculate the gray matter density (GMD). VBM analyze finished in the Statistical Parametric Mapping (SPM12) toolbox including the following cardinal steps: segmented the images to get the gray matter, white matter, and cerebrospinal fluid;21 normalized (voxel size = 2mm) the images into Montreal Neurological Institute (MNI) space template brain22 by the Diffeomorphic Anatomical Registration Through Exponential Lie Algebra (DARTEL) method;23 smoothed the images using Gaussian kernel approach (FWHM = 8 mm). Then, the Brainnetome Atlas (BNA)24 was used to extract GMD value of 4 subregions in the dmPFC (bilateral medial part of BA 8 and 9).12,13
Statistical Analysis
Statistical analyses were performed using SPSS 24.0 and statistical level was set at 0.05. Independent samples t-test was utilized to investigate the difference in catastrophizing scores and GMD of 4 subregions in bilateral dmPFC between SSD patients and HCs. Correlation analysis partialled out gender, age, education, course, and somatic complaints was used to ascertain the relationship between catastrophizing scores and psychic anxiety scores. Mediation analyses were employed to investigate whether the dmPFC mediated this catastrophizing-anxiety relationship. Catastrophizing scores was taken as independent variable (X), psychic anxiety scores as dependent variable (Y), and GMD of each subregion in bilateral dmPFC as mediation variable (M), to build 4 single-mediator mediation models. Linear regression analyses were operated to investigate catastrophizing’s effect on the dmPFC (X→M), the dmPFC’s effect on anxiety (M→Y), and catastrophizing’s direct effect on anxiety (X→Y), respectively25 (Figure 1). X→M and M→Y simultaneously significant was considered M has a mediating effect between X and Y.25 Then, a resampling-based p-value adjustment procedure recommended by MacKinnon et al26,27 was conducted by the PROCESS v3.5 macro28 in SPSS. The PROCESS using a nonparametric bootstrapping method which calculated M’s indirect effect between X and Y (Figure 1), and a bootstrap estimate of 95% confidence intervals (CI) for the indirect effect. The indirect effect was considered statistically significant when CI did not include “0”. In this study, analyses were carried out using 5000 bootstrap samples. All mediation analyses procedures were adjusted for gender, age, education, course, and somatic complaints.
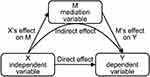 |
Figure 1 The schematic diagram of mediation analyses. |
Results
Demographic, Clinical and Psychological Characteristics, and GMD of the dmPFC of Participants
As shown in Table 1, there were no significant group differences in gender, age, and education. The level of psychic anxiety (t = 2.216, p = 0.031) and somatic complaints (t = 5.780, p ≤ 0.001) of SSD patients were significantly higher than HCs. However, no significant difference in scores of catastrophizing on the CERQ and GMD of 4 subregions in the dmPFC between SSD patients and HCs was found.
 |
Table 1 Demographic, Clinical and Psychological Characteristics, and GMD of the dmPFC of Participants |
Relationship Between Catastrophizing and Anxiety in SSD
Figure 2 shows the results of correlation analysis. After gender, age, education, course, and somatic symptoms were partialled out, catastrophizing scores of the CERQ was significantly and positively related to psychic scores of the HAMA (r = 0.416, p = 0.049).
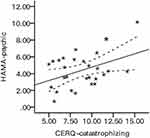 |
Figure 2 The relationship between catastrophizing and anxiety in SSD. Abbreviations: CERQ, cognitive emotion regulation questionnaire; HAMA, Hamilton anxiety scale; SSD, somatic symptom disorder. |
Mediation Effect of the dmPFC on the Relationship Between Catastrophizing and Anxiety
Table 2 illuminates the mediation analyses results of 4 models. Linear regression analyses detected no significant results of catastrophizing’s effect on the dmPFC and the dmPFC’s effect on anxiety simultaneously in any model. However, indirect effect analyses found that GMD of lBA8m (β = −0.126, 95% CI = [−0.322, −0.013], Figure 3A) and rBA8m (β = −0.100, 95% CI = [−0.272, −0.004], Figure 3B) in the dmPFC mediated the relationship between catastrophizing scores of the CERQ and psychic scores of the HAMA, even when gender, age, education, course, and somatic symptoms were adjusted.
 |
Table 2 GMD of the dmPFC Mediates the Catastrophizing-Anxiety Relationship in SSD |
Discussion
In the present study, we examined if SSD patients adopted more strategies of catastrophizing and have lower GMD of the dmPFC, then we investigated whether catastrophizing was related to anxiety in SSD and whether the dmPFC played as a mediator in this relationship. There were two main findings: 1) catastrophizing was significantly positively associated with anxiety in SSD patients; 2) GMD of bilateral medial BA 8 in the dmPFC mediated this relationship.
First, we repeated the catastrophizing-anxiety relationship in SSD patients which consistent with previous studies in healthy subjects and anxiety disorder patients.6–8,29,30 Catastrophizing was considered as a central characteristic of anxiety disorders31 and reckoned as a reason increased emotional arousal and anxiety in Kirmayer’s disease model of SSD.4 Studies have shown that catastrophizing and anxiety were co-existed in SSD patients32–34 and both improved by psychotherapy.34 Particularly, a cognitive-behavioral therapy study found that the pre-post changes in pain catastrophizing and the pre-post changes in state anxiety was positively correlated in SSD patients with predominant pain.35 As a supplement, the present study found that catastrophizing was significantly positively associated with anxiety in SSD patients, that is, SSD patients with more catastrophic interpretations of somatic symptoms have a higher level of anxiety. Accompanied by previous studies, this result provided an initial quantitative evidence about the psychological mechanism and supported Kirmayer’s theoretical model of SSD. And based on the present result, we suggested to pay more attention to the catastrophic thinking of SSD patients in the process of psychotherapy.
Second, mediation analyses revealed that GMD of the dmPFC (bilateral medial BA 8) mediated the catastrophizing-anxiety relationship in SSD patients. On one side, numbers animal and human studies have demonstrated that the dmPFC participated in the process of anxiety. Cocaine withdrawal induced heightened anxiety in rats was accompanied by an altered reactivity of the dmPFC36 and neurotoxic lesion of the dmPFC could reverse rats’ high anxiety-like behavior.37 Artificial anxiety in healthy human participants was associated with the dmPFC-amygdala coupling38,39 and transcranial magnetic stimulation at the dmPFC enhanced the evoked anxiety responses.40 On the other side, several studies have found that the dmPFC were involved in the cognitive process of anxiety. Functional MRI study of healthy human subjects showed that threat cues signaling risk of future electrical shock activated the dmPFC.41 Moreover, the dmPFC activation was correlated with participants’ fear evaluations16 and the trait-like tendency to catastrophizing of somatic symptoms.17 By mediation analyses, the present study revealed that the dmPFC mediated the relationship between catastrophizing and anxiety in SSD patients for the first time. That is, catastrophizing of somatic symptoms was accompanied by structural variation of the dmPFC which involved in pathological anxiety in SSD patients. This result strengthened the assumption that the dmPFC acted as a conduit between cognitive control regions and emotional arousal provoking areas.15
However, contrary to our anticipation, no difference in catastrophizing and GMD of the dmPFC between SSD patients and HCs were found in the present study. It is found that SSD patients had more pain catastrophizing33 and SSD patients with predominant somatization had more catastrophizing.32 Unlike previous study focus on a specific symptom, SSD patients in this study had different kind of somatic symptoms including pain and somatization. We thought SSD symptoms variation plus relatively small sample might be a possible explanation to this result. No study associated GMD of the dmPFC with SSD before. In related disease, adolescents with medically unexplained functional neurological symptoms showed increased gray matter volume of the dmPFC;42 however, patients with generalized anxiety disorder had reduction of gray matter volume of the dmPFC.43 Because SSD included certain kind of medically unexplained somatic symptoms and anxiety symptoms, the two existing related research results were inconsistent and contradictory. So, more researches are needed to find out the truth.
Limitations
The present study has several limitations. First, this study involved a relatively small sample because individuals with SSD prefer other medical settings to psychiatric departments.1 There are inevitably some biases in the present study. Second, catastrophizing and GMD of the dmPFC in SSD patients showed no difference compared with HCs which might limit the clinical significance of this study. Third, as a preliminary study, we only investigated the mediating effect of structural factors of the dmPFC. Further research should include functional factors to gain a more comprehensive understanding of the dmPFC in the neural mechanism of SSD. Fourth, this study was cross-sectional, so directionality can only be inferred. Prospective longitudinal studies should be designed to confirm the causal relationships among the dmPFC structural alteration, catastrophizing, and anxiety in SSD.
Conclusion
The present study confirmed a significantly positive relationship between catastrophizing and anxiety in SSD. Further, we revealed that GMD of the dmPFC (bilateral medial Brodmann area 8) mediated this catastrophizing-anxiety relationship. These findings supported Kirmayer’s disease model of SSD about catastrophic interpretations of somatic symptoms resulted in increased anxiety and demonstrated that the dmPFC may be a potential neural site linking catastrophizing and anxiety in SSD.
Acknowledgments
The authors would like to thank all the patients and volunteers for their participation in this study. Thank Jialiang Mao, Kaiji Ni, Shengliang Chen and Wei Feng in helping to recruit participants. This research was supported by Health Industry Clinical Research Project of Shanghai Municipal Health Commission (201940029), Shanghai Clinical Research Center for Mental Health (19MC1911100), the grants from Shanghai Municipal Health Commission (2019ZB0201), and Innovative Research Team of High-Level Local Universities in Shanghai.
Author Contributions
Conceived and designed the experiments: XDP, YLL, YZ, WND. Acquisition of data: WND, YLL, CFJ, XS, XDP. Analysis and interpretation of data: XDP, CY, QZ. Took part in drafting the article or revising it critically for important intellectual content: XDP, CY, XS, YZ, QZ, WND, YLL, CFJ. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Somatic Symptom Disorder and related disorders. Diagnostic and Statistical Manual of Mental Disorders.
2. de Vroege L, Timmermans A, Kop WJ, van der Feltz-cornelis CM. Neurocognitive dysfunctioning and the impact of comorbid depression and anxiety in patients with somatic symptom and related disorders: a cross-sectional clinical study. Psychol Med. 2018;48(11):1803–1813. doi:10.1017/S0033291717003300
3. de Vroege L, Koppenol I, Kop WJ, Riem MME, van der Feltz-cornelis CM. Neurocognitive functioning in patients with conversion disorder/functional neurological disorder. J Neuropsychol. 2020. doi:10.1111/jnp.12206
4. Kirmayer LJ, Taillefer S, Turner SM. Somatoform disorders. Contemp Psychiatry. 1997;76(9):1333–1338.
5. Hofmann SG. Perception of control over anxiety mediates the relation between catastrophic thinking and social anxiety in social phobia. Behav Res Ther. 2005;43(7):885–895. doi:10.1016/j.brat.2004.07.002
6. Fergus TA, Valentiner DP. Intolerance of uncertainty moderates the relationship between catastrophic health appraisals and health anxiety. Cognit Ther Res. 2011;35(6):560–565. doi:10.1007/s10608-011-9392-9
7. Bailey R, Wells A. Metacognitive beliefs moderate the relationship between catastrophic misinterpretation and health anxiety. J Anxiety Disord. 2015;34:8–14. doi:10.1016/j.janxdis.2015.05.005
8. Bailey R, Wells A. Is metacognition a causal moderator of the relationship between catastrophic misinterpretation and health anxiety? A prospective study. Behav Res Ther. 2016;78:43–50. doi:10.1016/j.brat.2016.01.002
9. Northoff G, Heinzel A, Bermpohl F, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Hum Brain Mapp. 2004;21(3):202–212. doi:10.1002/hbm.20002
10. Che X, Luo X, Tong D, Fitzgibbon BM, Yang J. Habitual suppression relates to difficulty in regulating emotion with cognitive reappraisal. Biol Psychol. 2015;112:20–26. doi:10.1016/j.biopsycho.2015.09.011
11. Wang HY, Xu GQ, Ni MF, et al. Neural mechanisms of implicit cognitive reappraisal: preceding descriptions alter emotional response to unpleasant images. Neuroscience. 2017;347:65–75. doi:10.1016/j.neuroscience.2017.01.047
12. Lieberman MD. Social: Why Our Brains are Wired to Connect. Oxford University Press; 2013:187.
13. Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94(1):35–79. doi:10.1152/physrev.00041.2012
14. Chapados C, Petrides M. Ventrolateral and dorsomedial frontal cortex lesions impair mnemonic context retrieval. Proc Biol Sci. 2015;282(1801):20142555. doi:10.1098/rspb.2014.2555
15. Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage. 2008;42(2):998–1031.
16. Raczka KA, Gartmann N, Mechias ML, et al. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: a potential neurogenetic basis for catastrophizing. Mol Psychiatry. 2010;15(11):1045, 1067–1074. doi:10.1038/mp.2010.79
17. Holtz K, Pane-Farre CA, Wendt J, Lotze M, Hamm AO. Brain activation during anticipation of interoceptive threat. NeuroImage. 2012;61(4):857–865. doi:10.1016/j.neuroimage.2012.03.019
18. Zhu XZ, Luo FS, Yao SQ, Pauerbach R. Reliability and validity of the cognitive emotion regulation questionnaire-chinese version. Chin J Clin Psychol. 2007;22(2):288–307.
19. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi:10.1006/nimg.2000.0582
20. Goto M, Yamashita F, Kawaguchi A, et al. The effect of single-scan and scan-pair intensity inhomogeneity correction methods on repeatability of voxel-based morphometry with multiple magnetic resonance scanners. J Comput Assist Tomogr. 2018;42(1):111–116.
21. Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi:10.1016/j.neuroimage.2005.02.018
22. Collins DL, Zijdenbos AP, Kollokian V, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17(3):463–468. doi:10.1109/42.712135
23. Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
24. Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508–3526.
25. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi:10.1037/0022-3514.51.6.1173
26. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Ann Rev Psychol. 2007;58(1):593–614. doi:10.1146/annurev.psych.58.110405.085542
27. Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10(2):87–99. doi:10.1007/s11121-008-0109-6
28. Bolin JH. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. J Educ Meas. 2014;51(3):335–337. doi:10.1111/jedm.12050
29. Chan SM, Chan SK, Kwok WW. Ruminative and catastrophizing cognitive styles mediate the association between daily hassles and high anxiety in Hong Kong adolescents. Child Psychiat Hum D. 2015;46(1):57–66. doi:10.1007/s10578-014-0451-9
30. Gautreau CM, Sherry SB, Sherry DL, Birnie KA, Mackinnon SP, Stewart SH. Does catastrophizing of bodily sensations maintain health-related anxiety? A 14-day daily diary study with longitudinal follow-up. Behav Cogn Psychother. 2015;43(4):502–512. doi:10.1017/S1352465814000150
31. Beck AT, Clark DA. An information processing model of anxiety: automatic and strategic processes. Behav Res Ther. 1997;35(1):49–58. doi:10.1016/S0005-7967(96)00069-1
32. Rief W, Hiller W, Margraf J. Cognitive aspects of hypochondriasis and the somatization syndrome. J Abnorm Psychol. 1998;107(4):587–595. doi:10.1037/0021-843X.107.4.587
33. Fayed N, Andres E, Rojas G, et al. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand. 2012;126(2):115–125. doi:10.1111/j.1600-0447.2011.01820.x
34. Yoshino A, Okamoto Y, Doi M, et al. Effectiveness of group cognitive behavioral therapy for somatoform pain disorder patients in Japan: a preliminary non-case-control study. Psychiatry Clin Neurosci. 2015;69(12):763–772. doi:10.1111/pcn.12330
35. Yoshino A, Okamoto Y, Jinnin R, Takagaki K, Mori A, Yamawaki S. Role of coping with negative emotions in cognitive behavioral therapy for persistent somatoform pain disorder: is it more important than pain catastrophizing? Psychiatry Clin Neurosci. 2019;73(9):560–565. doi:10.1111/pcn.12866
36. El Hage C, Rappeneau V, Etievant A, et al. Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex. PLoS One. 2012;7(8):8. doi:10.1371/journal.pone.0043535
37. Prinssen EP, Nicolas LB, Klein S, et al. Imaging trait anxiety in high anxiety F344 rats: focus on the dorsomedial prefrontal cortex. Eur Neuropsychopharmacol. 2012;22(6):441–451. doi:10.1016/j.euroneuro.2011.11.001
38. Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. NeuroImage. 2012;60(1):523–529. doi:10.1016/j.neuroimage.2011.11.096
39. Vytal KE, Overstreet C, Charney DR, Robinson OJ, Grillon C. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. J Psychiatry Neurosci. 2014;39(5):321–329. doi:10.1503/jpn.130145
40. Gonzalez-Escamilla G, Chirumamilla VC, Meyer B, et al. Excitability regulation in the dorsomedial prefrontal cortex during sustained instructed fear responses: a TMS-EEG study. Sci Rep. 2018;8. doi:10.1038/s41598-018-32781-9
41. Klumpers F, Kroes MC, Heitland I, et al. Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol Psychiatry. 2015;78(8):582–589. doi:10.1016/j.biopsych.2014.07.034
42. Kozlowska K, Griffiths KR, Foster SL, Linton J, Williams LM, Korgaonkar MS. Grey matter abnormalities in children and adolescents with functional neurological symptom disorder. Neuroimage Clin. 2017;15:306–314. doi:10.1016/j.nicl.2017.04.028
43. Schienle A, Ebner F, Schafer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(4):303–307. doi:10.1007/s00406-010-0147-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.