Back to Journals » OncoTargets and Therapy » Volume 13
GPR30 Promotes the Phenotypic Switching of Vascular Smooth Muscle Cells via Activating the AKT and ERK Pathways
Authors Zha B, Qiu P, Zhang C, Li X, Chen Z
Received 30 December 2019
Accepted for publication 14 April 2020
Published 6 May 2020 Volume 2020:13 Pages 3801—3808
DOI https://doi.org/10.2147/OTT.S244128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Binshan Zha,1,* Peng Qiu,2,* Chenxin Zhang,3 Xinyuan Li,4 Zhiyong Chen1
1Department of Vascular and Thyroid Surgery, Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Department of Vascular Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, and Vascular Center of Shanghai Jiaotong University, Shanghai, People’s Republic of China; 3Department of Cardiovascular Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 4Reproductive Medicine Center, Department of Obstetrics and Gynecology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui 230022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiyong Chen
Department of Vascular and Thyroid Surgery, Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, No. 218 Jixi Road, Hefei 230022, People’s Republic of China
Tel +86 13856962362
Email [email protected]
Background: Lower extremity varicose veins (LEVVs) are a common venous disorder of venous dilation and tortuosity. The functional integrity of vascular smooth muscle cells (VSMCs), the majority of the cells in venous tissues, and their phenotypic differences play important roles in the occurrence and development of LEVV. However, the underlying mechanism remains unclear.
Methods: The expression of estrogen receptors ERα and ERβ and G-protein-coupled receptor 30 (GPR30) in LEVV tissues and the role of GPR30 in VSMC phenotypic switching were examined by Western blotting and quantitative real-time PCR. Finally, the related mechanisms underlying LEVVs were explored by Western blotting.
Results: The serum estradiol content was increased in LEVV patients compared with normal control patients, but the mRNA levels of ERα and ERβ were not significantly different. GPR30 was overexpressed in LEVVs, and high expression of GPR30 promoted the maintenance of a synthetic phenotype in which OPN, MMP-1 and MMP-9 were highly expressed and α-SMA was poorly expressed in VSMCs. Finally, the mechanism by which GPR30 promotes the phenotypic switching of VSMCs is dependent on the ERK1/2 and AKT pathways.
Conclusion: GPR30 may contribute to the pathogenesis of LEVVs by promoting the maintenance of a synthetic phenotype in VSMCs by activating the ERK1/2 and AKT pathways, and GPR30 might be a novel therapeutic target for clinical LEVV treatment.
Keywords: GPR30, vascular smooth muscle cell, varicose veins, phenotypic switch, AKT and ERK pathways
Introduction
Lower extremity varicose veins (LEVVs), a common venous disorder characterized by degenerative vein valves and excessive venous dilation and tortuosity, affect 10% to 40% of the adult population in China.1 The spectrum of LEVVs ranges from varicose veins to leg edema and serious skin changes such as hyperpigmentation, eczema, lipodermatosclerosis, and venous ulceration.2 Venous dilation is often thought to result from an inability of the venous smooth muscle to constrict in response to venous pressure or circulating vasoconstrictors.3 However, the pathophysiological mechanisms underlying LEVVs are not clearly understood.
Vascular smooth muscle cells (VSMCs) are highly specialized cells that can retain their plasticity and modulate their phenotype (contractile and synthetic phenotypes) in response to changes in the local environment. Phenotypic and functional abnormalities in VSMCs may be associated with the pathogenesis of LEVVs.4 An increase in secretory VSMCs leads to increased immature extracellular matrix (ECM) and decreased mature ECM, which makes maintaining cell stability and membrane integrity difficult.5,6 Therefore, exploring the factors that play a critical role in controlling the phenotypic transformation and migration of VSMCs is helpful and necessary for the development of novel therapeutic strategies to treat LEVVs.
Risk factors for LEVVs include a family history, older age, female gender, standing occupations, or a history of deep venous thrombosis. Estrogen plays a crucial role in the development of LEVVs. Previous studies reported that the levels of estrogen were increased in the sera of LEVV patients.7–10 Alternations in hormonal levels can induce hypertrophy and ther growth of the SMC layer in LEVV11–13 and its effects are mediated by the activation of three different receptors: the classical estrogen receptors ERα and ERβ and G-protein-coupled receptor 30 (GPR30). GPR30, also named G-protein-coupled estrogen receptor 1 (GPER1), can bind estrogen and acts as an estrogen receptor within the cell membrane. GPR30 leads to rapid nongenomic signalling events and transcriptional regulation.14 Studies have shown that GPR30 is widely overexpressed in various cancers and contributes to tumour proliferation and migration.15–17 GPR30 also provides neuroprotection against ischaemic stroke.18 In addition, GPR30 plays a key role in the cardiovascular system.19 However, there have been few reports about the role of GPR30 in the venous system. Furthermore, some studies have reported no changes in the expression of ERα and ERβ in LEVVs,20,21 or that expression levels of both receptors are upregulated in LEVVs.22,23 This inconsistency suggests that GPR30 may be involved in the progression of LEVVs. Therefore, in this study, the expression of three ERs in LEVV and normal great saphenous vein (GSV) tissues was evaluated, and the mechanism by which GPR30 regulates SMC phenotypic transformation was explored.
Materials and Methods
The First Affiliated Hospital of Anhui Medical University (Hefei, China) Human Research Ethics Committee approved the study protocol. All individuals provided written informed consent to be involved in the study.
Reagents
Recombinant human 17-β-estradiol (E2; ab120657) was purchased from Abcam (Cambridge, USA). Antibodies against GPR30, AKT, p-AKT, ERK, p-ERK, OPN and α-SMA were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against MMP-1 and MMP-9 were obtained from Zenbio (Chengdu, China). Antibodies against GPR30 and GAPDH were purchased from Proteintech (Wuhan, China).
Cell Culture and Human Tissues
Primary VSMCs were obtained from the GSV of a healthy organ donor with the consent of the donor and approval of the Ethics Committee of the First Affiliated Hospital of Anhui Medical University. VSMCs were cultured in phenol red-free DMEM (Gibco, Grand Island, NY) supplemented with 20% FBS (Gibco), 1% penicillin/streptomycin (Gibco) and 4 mM L-glutamine and then maintained at 37°C in a humidified incubator containing 5% CO2.
Twelve tissue samples were collected from patients at the First Affiliated Hospital of Anhui Medical University from June 2017 to August 2018. The varicose group consisted of 12 patients (6 men and 6 postmenopausal women; mean age: 53 years; range: 48–67 years) with LEVVs who had received high ligation and stripping. All 12 patients were confirmed to have primary LEVVs using colour Doppler ultrasound imaging before the surgery and showed different degrees of reflux of the saphenofemoral venous valve. Patients were classified as C3 to C5 according to the CEAP classification, with a disease duration of 2 to 10 years.
A total of 8 normal GSVs were obtained from 8 patients (3 men and 5 postmenopausal women; mean age: 63 years; range: 50–77 years) who were undergoing distal arterial bypass grafting surgery. The exclusion criteria were severe lower limb oedema, thrombophlebitis, phlebitis without thrombus, arterial disease, and diabetes. None of the female patients received any hormone replacement therapy, and all female patients had experienced menstrual flow for a minimum of 12 months.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from samples and cells utilizing TRIzol reagent (Beyotime, Shanghai, China) following the manufacturer’s instructions. After testing the RNA concentration and quantity using an ultraviolet spectrophotometer (Eppendorf, Hamburg, Germany), cDNA was synthesized with the PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time, Takara), and real-time qPCR was performed utilizing SYBR Green I (Q511, Vazyme) and an ABI 7300 software system (Applied Biosystems, Foster, CA, USA) according to the manufacturer’s protocol. Relative mRNA expression was normalized to the relative expression of the control gene, GAPDH. Each experiment was repeated in triplicate. The sequences of the primers used for qRT-PCR are shown in Table 1.
 |
Table 1 Primers Used for qRT-PCR (Quantitative Real-Time PCR) |
Measurement of Serum Estradiol (E2)
All blood samples were obtained in the morning between 8:00 am and 12:00 pm. The samples were stored for a maximum of 8 hours at 2°C to 8°C before centrifugation and separation of the serum. Serum was stored at −20°C. The E2 level was determined with a Cobas E601 fully automated immunoassay system (Roche, Basel, Switzerland).
Western Blotting
Tissues and cells were harvested and lysed with RIPA lysis buffer (Beyotime) containing 0.1% PMSF. After centrifugation at 120,000 rpm for 30 min at 4°C, protein concentrations were quantified using a BCA protein assay kit (Beyotime), and proteins (50 μg) were separated by 10% SDS-PAGE (SDS-polyacrylamide gel electrophoresis) and then transferred to PVDF membranes (Millipore, Boston, MA, USA). The membranes were blocked with 5% nonfat milk in PBS-0.1% Tween-20 (PBST) for 2 h at room temperature and subsequently incubated with the indicated antibodies at 4 °C overnight. After washing 3 times using PBST, the membranes were incubated with secondary antibody and then visualized using an ECL Plus kit. The grey values of the bands were analysed with ImageJ (NIH, Bethesda, MD, USA). GAPDH was a loading control on the same membrane to normalize the protein levels.
Plasmid Construction and Cell Transfection
PcDNA3.1 (+)-GPR30 and GPR30 siRNAs (sense: 5ʹ-GCACCUUCAUGUCGCUCUU −3ʹ and antisense: 5ʹ-AAGAGCGACAUGAAGGUGC-3ʹ) were purchased from General Biosystems (Hefei, Anhui). Transfection was accomplished using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions.
Statistical Analysis
All data are expressed as the mean±SEM (standard error of the mean) of at least three independent experiments. Differences between groups were analysed using a two-tailed unpaired Student’s t-test or one-way ANOVA following Dunnett’s t-test. All statistical analyses were performed using GraphPad 7 (GraphPad Software, Inc., La Jolla, CA, USA). When P<0.05, the difference was considered statistically significant.
Results
GPR30 Was Overexpressed in LEVV Tissues
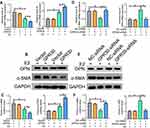
First, we found that the serum estradiol content was increased in LEVV patients (Supplementary Figure 1A). We also tested the expression of ERα and ERβ in LEVV patients and found that the mRNA levels of ERα and ERβ in LEVV tissues were not significantly different from those in normal GSV tissues (Supplementary Figure 1B). To investigate the functions of GPR30 in LEVVs, we examined the expression of GPR30 in LEVV tissues and normal GSV tissues by RT-qPCR and Western blotting. As shown in Figure 1A-C, both the mRNA and protein levels of GPR30 in LEVV tissues were markedly upregulated compared to those of normal GSV tissues. Together, these results indicated that GPR30 may play a role in LEVV development.
GPR30 Promoted SMC Switching from a Contractile to a Synthetic Phenotype
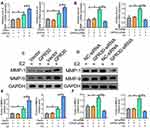
The major event in the development of LEVVs is the switching of SMCs from a contractile phenotype (a-SMAhigh phenotype) to a synthetic phenotype (OPNhigh phenotype). Therefore, we examined whether GPR30 regulates SMC phenotype switching. We treated VSMCs cells with E2 to mimic a microenvironment of high estrogen in vivo, and the results demonstrated that the overexpression of GPR30 did not affect a-SMA or OPN mRNA or protein levels. However, upon estrogen (a ligand of GPR30) stimulation, GPR30 decreased the expression of a-SMA and increased OPN expression (Figure 2A–C). Furthermore, after transfection with GPR30 siRNA and treatment with estrogen, GPR30 depletion significantly reversed the phenotypic transformation of SMCs, inhibited OPN expression and restored a-SMA mRNA and protein levels (Figure 2D–F). In addition, estrogen inhibited a-SMA expression and enhanced OPN production (Figure 2A–F). Finally, successful overexpression and knockdown of GPR30 were confirmed by RT-qPCR and Western blotting (Supplementary Figure 2A–D). Taken together, these results suggest that GPR30 promotes SMC phenotype switching and contributes to LEVV progression.
GPR30 Enhanced the Production of MMP-1 and MMP-9
VSMCs with the synthetic phenotype secrete many matrix metalloproteinases (MMPs), which play a significant role in SMC migration, leading to vascular tissue remodelling. Thus, we aimed to determine whether GPR30 regulates MMP secretion by VSMCs with a synthetic phenotype through a series of GPR30 overexpression and knockdown experiments. As shown in Figure 3A, C and E, the estrogen-induced production of MMP-1 and MMP-9 was enhanced after overexpression of GPR30. Moreover, as depicted in Figure 3, the results of RT-qPCR and Western blotting showed that knockdown of GPR30 markedly downregulated the estrogen-induced expression of MMP-1 and MMP-9 at both the mRNA and protein levels (Figure 3B, D and F). Taken together, these data demonstrate the important role of GPR30 in MMP secretion in SMCs with a synthetic phenotype.
The Ability of GPR30 to Activate VSMC Switching Is Mediated Through the ERK and AKT Pathways
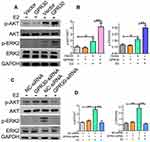
Activation of the ERK and AKT pathways has been shown to play a major role in VSMC phenotypic switching. Thus, we investigated the effects of GPR30 on the phosphorylation of ERK and AKT. As shown in Figure 4A and B, upon estrogen stimulation, GPR30 overexpression significantly enhanced the levels of phosphorylated ERK and AKT. The levels of phosphorylated ERK were increased by approximately two-fold, and the levels of phosphorylated AKT were enhanced by nearly 2-fold. Furthermore, we determined whether the function of GPR30 in VSMC phenotype switching is mediated via the ERK and AKT pathways by GPR30 expression knockdown. The results demonstrated that silencing GPR30 reduced the phosphorylation of these factors in response to estrogen treatment (Figure 4C and D). Thus, the effects of GPR30 are mediated through the ERK and AKT pathways.
Discussion
LEVV is a common vascular disease. The dysfunction of VSMCs is the main pathologic change in LEVV. This dysfunction manifests as phenotypic transformation and subsequent venous dilation and remodelling.4 Sex differences in the prevalence of varicose veins have been reported.24 In women, an association between pregnancy and high serum levels of oestradiol with the clinical manifestation of varicose veins has been suggested.7,8 Several lines of evidence also support a role for sex steroid hormones in the pathogenesis of varicose veins in men.9,10
Although changes in estrogen levels in a pathological state may have an important effect on the intravenous wall structure and extracellular matrix, their influence and that of their receptors on the pathogenesis of varicose disease are not fully understood. In addition, there have been few reports about the role of GPR30 in the venous system. Previous studies reported that changes in the expression of ERα and ERβ in LEVVs were not observed,20,21 or that the expression levels of both receptors were upregulated.22,23 Serra et al evaluated the gene expression of three ERs in chronic venous disease (CVD) and demonstrated that the expression of ERs is correlated with the severity of CVD, and their expression has been shown to be correlated with clinical stage.22 In our study, we found that the serum estradiol content was increased in LEVV patients compared with normal control patients, but the mRNA levels of ERα and ERβ were not significantly different. Similarly, we found that GPR30 was highly expressed in LEVV tissues, which may be due to the following several reasons: 1) epigenetic modification, such as histone ubiquitination, methylation, and acetylation; 2) the regulation of transcription factors; and 3) estrogen stimulation.
An increasing number of studies have demonstrated that hormonal changes play a key role in varicose vein development. High estrogen levels result in varicose vein progression and alternations in hormonal levels may promote VSMC proliferation and migration.11–13 In this study, we found that E2 enhanced VSMC phenotype switching and increased MMP secretion. Increasing evidence has shown that alterations in VSMC features represent a critical event in the pathobiology of the arterial wall by contributing to vascular remodelling. VSMCs in LEVV patients mainly exhibit the synthetic phenotype, which is characterized by a loss of contractility, enhanced proliferation and migration, and extracellular matrix-degrading enzyme secretion, leading VSMCs to migrate into the intimal layer of the blood vessel and ultimately promoting the development of intimal hyperplasia and restenosis. Thus, VSMC phenotype switching (the switch to a synthetic phenotype) is the most important pathological change in varicose vein patients. Our results suggest that high expression of GPR30 reduced the expression of a contractile phenotype marker (a-SMA), enhanced the expression of a synthetic phenotype marker (OPN) in VSMCs and promoted LEVV development.
MMPs also have a significant effect on vascular tissue remodelling and play a role in the pathogenesis and progression of vascular disease. In the progression of SMC phenotype switching from the contractile type to the synthetic type, the MMPs such as MMP-2 and MMP-9 are overexpressed and contribute to the migratory capacity of SMCs.5 Our results demonstrated that GPR30 promotes MMP secretion in SMCs with a synthetic phenotype.
The ERK and AKT pathways are essential for SMC migration and proliferation and MMP secretion.25,26 Moreover, GPR30 activation may cause the dissociation of Gα-GTPase from the heterotrimeric Gαβγ complex. The dissociated Gβγ subunit activates membrane-associated MMPs with subsequent transient activation of the mitogen-activated protein kinase ERK-1/2.27,28 In addition, the GPR30 pathway is associated with the activation of AKT.29,30 In this study, we found that GPR30 regulated the ERK and AKT signalling pathways in a high-estrogen environment, suggesting a function of GPR30 in VSMC phenotype switching by enhancing ERK and AKT activity. Taken together, these results suggest that GPR30 is a potential therapeutic target in LEVV treatment.
This study does have some limitations. First, we selected only postmenopausal females for inclusion because the estradiol level at postmenopausal status is more stable. The age distribution of the patients is consistent with that of other studies on the role of ERs in LEVV.7,22,23 This may lead to bias. Second, due to the limitations of this study design, based on our data, we could not conclude how sex differences and menopausal status affect the development of LEVV. In our further research, we could evaluate the GPR30 level in premenopausal women and compared GPR30 expression between premenopausal and postmenopausal women to obtain more accurate results.
In conclusion, our study demonstrated that GPR30 is overexpressed in LEVVs and that high expression of GPR30 promotes the maintenance of a synthetic phenotype in VSMCs by activating the ERK1/2 and AKT pathways, which increases our understanding of the function of GPR30 in LEVVs. Most importantly, our findings suggest that GPR30 is a novel and promising therapeutic target for the prevention of LEVV.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Culture Project of National Natural Science Foundation of the First Affiliated Hospital of Anhui Medical University (No. 2018kj28).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Xu HM, Zhao Y, Zhang XM, Zhu T, Fu WG. Polymorphisms in MMP-9 and TIMP-2 in Chinese patients with varicose veins. J Surg Res. 2011;168(1):e143–148. doi:10.1016/j.jss.2010.11.002
2. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248–1252. doi:10.1016/j.jvs.2004.09.027
3. Raffetto JD, Qiao X, Beauregard KG, et al. Functional adaptation of venous smooth muscle response to vasoconstriction in proximal, distal, and varix segments of varicose veins. J Vasc Surg. 2010;51(4):962–971. doi:10.1016/j.jvs.2009.11.037
4. Xu Y, Bei Y, Li Y, et al. Phenotypic and functional transformation in smooth muscle cells derived from varicose veins. J Vasc Surg Venous Lymphat Disord. 2017;5(5):723–733. doi:10.1016/j.jvsv.2017.04.009
5. Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330.
6. Naoum JJ, Hunter GC, Woodside KJ, Chen C. Current advances in the pathogenesis of varicose veins. J Surg Res. 2007;141(2):311–316. doi:10.1016/j.jss.2006.08.007
7. Ciardullo AV, Panico S, Bellati C, et al. High endogenous estradiol is associated with increased venous distensibility and clinical evidence of varicose veins in menopausal women. J Vasc Surg. 2000;32(3):544–549. doi:10.1067/mva.2000.107768
8. Vin F, Allaert FA, Levardon M. Influence of estrogens and progesterone on the venous system of the lower limbs in women. J Dermatol Surg Oncol. 1992;18(10):888–892. doi:10.1111/j.1524-4725.1992.tb02922.x
9. Özcan S, Tezcan O, Kurt T, et al. Serum estradiol/free testosterone ratio can be important predictor for varicose vein recurrence in men. Int Angiol. 2015;34(6):576–581.
10. Kendler M, Makrantonaki E, Kratzsch J, et al. Elevated sex steroid hormones in great saphenous veins in men. J Vasc Surg. 2010;51(3):639–646. doi:10.1016/j.jvs.2009.07.128
11. Huang X, Jin Y, Zhou D, Xu G, Huang J, Shen L. IQGAP1 modulates the proliferation and migration of vascular smooth muscle cells in response to estrogen. Int J Mol Med. 2015;35(5):1460–1466. doi:10.3892/ijmm.2015.2134
12. Bastos AN, Alves MM, Monte-Alto-Costa A, et al. α-smooth muscle actin, fibrillin-1, apoptosis and proliferation detection in primary varicose lower limb veins of women. Int Angiol. 2011;30(3):262–271.
13. Montague CR, Hunter MG, Gavrilin MA, et al. Activation of estrogen receptor-alpha reduces aortic smooth muscle differentiation. Circ Res. 2006;99(5):477–484. doi:10.1161/01.RES.0000238376.72592.a2
14. Wilkenfeld SR, Lin C, Frigo DE. Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids. 2018;133:2–7. doi:10.1016/j.steroids.2017.11.005
15. Wang D, Hu L, Zhang G, Zhang L, Chen C. G protein-coupled receptor 30 in tumor development. Endocrine. 2010;38(1):29–37. doi:10.1007/s12020-010-9363-z
16. Petrie WK, Dennis MK, Hu C, et al. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int. 2013;2013:472720. doi:10.1155/2013/472720
17. Hua H, Zhang H, Kong Q, Jiang Y. Mechanisms for estrogen receptor expression in human cancer. Exp Hematol Oncol. 2018;7(1):24. doi:10.1186/s40164-018-0116-7
18. Kosaka Y, Quillinan N, Bond C, Traystman R, Hurn P, Herson P. GPER1/GPR30 activation improves neuronal survival following global cerebral ischemia induced by cardiac arrest in mice. Transl Stroke Res. 2012;3(4):500–507. doi:10.1007/s12975-012-0211-8
19. Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55(1–3):17–25. doi:10.1016/j.vph.2011.06.003
20. Bergqvist A, Bergqvist D, Fernö M. Estrogen and progesterone receptors in vessel walls Biochemical and Immunochemical Assays . Acta Obstet Gynecol Scand. 1993;72(1):10–16. doi:10.3109/00016349309013341
21. Perrot-Applanat M, Cohen-Solal K, Milgrom E, et al. Progesterone receptor expression in human saphenous veins. Circulation. 1992;2975–2983.
22. Serra R, Gallelli L, Perri P, et al. Estrogen receptors and chronic venous disease. Eur J Vasc Endovasc Surg. 2016;52(1):114–118. doi:10.1016/j.ejvs.2016.04.020
23. Garcia-Honduvilla N, Asunsolo A, Ortega MA, et al. Increase and redistribution of sex hormone receptors in premenopausal women are associated with varicose vein remodelling. Oxid Med Cell Longev. 2018;2018:3974026. doi:10.1155/2018/3974026
24. Robertson L, Evans C, Fowkes FG. Epidemiology of chronic venous disease. Phlebology. 2008;23(3):103–111. doi:10.1258/phleb.2007.007061
25. Muto A, Fitzgerald TN, Pimiento JM, et al. Smooth muscle cell signal transduction implications of vascular biology for vascular surgeons. J Vasc Surg. 2007;45(Suppl A):A15–24. doi:10.1016/j.jvs.2007.02.061
26. Zhang L, Xu Z, Wu Y, Liao J, Zeng F, Shi L. Akt/eNOS and MAPK signaling pathways mediated the phenotypic switching of thoracic aorta vascular smooth muscle cells in aging/hypertensive rats. Physiol Res. 2018;67(4):543–553. doi:10.33549/physiolres.933779
27. Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16(8):362–367. doi:10.1016/j.tem.2005.08.005
28. Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi:10.1038/47260
29. Yang WR, Zhu FW, Zhang JJ, et al. PI3K/Akt activated by GPR30 and Src regulates 17β-estradiol-induced cultured immature boar sertoli cells proliferation. Reprod Sci. 2017;24(1):57–66. doi:10.1177/1933719116649696
30. Fujiwara S, Terai Y, Kawaguchi H, et al. GPR30 regulates the EGFR-Akt cascade and predicts lower survival in patients with ovarian cancer. J Ovarian Res. 2012;5(1):35. doi:10.1186/1757-2215-5-35
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.