Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Improves Lung Function versus Monotherapies in GOLD Category A Patients with COPD: Pooled Data from the Phase III PINNACLE Studies
Authors Martinez FJ , Rabe KF , Lipworth BJ , Arora S, Jenkins M, Martin UJ , Reisner C
Received 6 September 2019
Accepted for publication 2 December 2019
Published 9 January 2020 Volume 2020:15 Pages 99—106
DOI https://doi.org/10.2147/COPD.S229794
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Fernando J Martinez,1 Klaus F Rabe,2 Brian J Lipworth,3 Samir Arora,4 Martin Jenkins,5 Ubaldo J Martin,6 Colin Reisner6,7
1Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, USA; 2Department of Pneumonology, LungenClinic Grosshansdorf and Christian-Albrechts University Kiel, Airway Research Center North, Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 3Division of Molecular and Clinical Medicine, Scottish Centre for Respiratory Research, Ninewells Hospital, University of Dundee, Dundee, Scotland, UK; 4Aventiv Research Inc., Columbus, OH, USA; 5Global Medicines Development, AstraZeneca, Cambridge, UK; 6Research and Development, AstraZeneca, Gaithersburg, MD, USA; 7R&D Biopharmaceuticals, AstraZeneca, Morristown, NJ, USA
Correspondence: Fernando J Martinez
Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York-Presbyterian Hospital/Weill Cornell Medical Center, 525 E 68th Street, Room M-522, Box 130, New York, NY 10065, USA
Tel +1646 962 2748
Email [email protected]
Background: The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends a short-acting bronchodilator or single long-acting bronchodilator as an initial pharmacological treatment for GOLD category A patients with COPD. We pooled data from the PINNACLE-1, -2, and -4 studies to evaluate the efficacy and safety of the dual bronchodilator fixed-dose combination glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI), formulated using co-suspension delivery technology, in GOLD category A patients with moderate-to-very severe COPD.
Materials and Methods: PINNACLE-1, -2, and -4 were Phase III, randomized, double-blind, parallel-group, multicenter studies (NCT01854645, NCT01854658, and NCT02343458). Patients received 24 weeks’ treatment with GFF MDI 18/9.6 μg, glycopyrrolate (GP) MDI 18 μg, formoterol fumarate (FF) MDI 9.6 μg, or placebo MDI twice daily. GOLD category A patients were identified based on a COPD Assessment Test score of 1), peak change from baseline in FEV1 within 2 hrs post-dose, and adverse events (AEs).
Results: The pooled intent-to-treat population comprised 729 GOLD category A patients. GFF MDI significantly improved morning pre-dose trough FEV1 at Week 24 versus GP MDI, FF MDI, and placebo MDI (least squares mean [LSM] differences 54 mL, 62 mL, and 188 mL, respectively; all p≤0.0053), and peak FEV1 at Week 24 versus GP MDI, FF MDI, and placebo MDI (LSM differences 124 mL, 104 mL, and 307 mL, respectively; all p<0.0001). Improvements over 24 weeks were comparable to at Week 24. The AE profile of GFF MDI in GOLD category A patients was similar to monocomponents and placebo MDI.
Conclusion: GFF MDI significantly improved lung function versus monocomponents and placebo MDI in GOLD category A patients with moderate-to-very severe COPD, with no unexpected safety findings.
Keywords: β2-agonist, bronchodilator, COPD, co-suspension delivery technology, lung function, muscarinic antagonist
Introduction
In their 2011 report, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) introduced categorizing patients with COPD based on their symptom burden and exacerbation risk.1 In this schema, GOLD category A patients have a low symptom burden and a low risk of exacerbations.2 A low symptom burden is defined as a COPD Assessment Test (CAT)3 score of <10 or a modified Medical Research Council (mMRC)4 score of <2,2 and a low risk of exacerbations is defined as no exacerbations or one moderate exacerbation (i.e., not leading to hospitalization) in the previous year.2
The GOLD 2020 report suggests that the GOLD categories may be used to guide initial pharmacological treatments for patients, with the most recent strategy proposing that GOLD category A patients are treated with a single short-acting bronchodilator or a single long-acting bronchodilator when starting pharmacological treatment.2 Dual bronchodilator therapy with a long-acting muscarinic antagonist (LAMA) plus a long-acting β2-agonist (LABA) as an initial therapy is recommended for highly symptomatic GOLD category D patients who have a high risk of exacerbations, and may be considered as an initial therapy for GOLD category B patients who have a low risk of exacerbations and severe breathlessness.2 Dual LAMA/LABA therapy is also recommended as a step-up treatment from long-acting bronchodilator monotherapy for patients with persistent breathlessness or exercise limitation, and for patients with persistent exacerbations, particularly those with low blood eosinophil levels.2
Many Phase III studies investigating LAMA/LABA fixed-dose combinations (FDCs) have excluded patients with a low symptom burden, for example, those defined by an mMRC score of <2,5–8 thereby excluding GOLD category A patients.2 The PINNACLE Phase III clinical trial program has demonstrated the efficacy and safety of the dual LAMA/LABA FDC glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI; Bevespi Aerosphere®), formulated using co-suspension delivery technology, in patients with moderate-to-very severe airflow obstruction who were not required to have a high symptom burden at study entry.9–11 Therefore, this allowed a pooled analysis of PINNACLE-1, -2, and -4 to evaluate the effects of GFF MDI on lung function and safety in GOLD 201712 category A patients with COPD who had moderate-to-very severe airflow obstruction. We hypothesized that this patient population would benefit from dual LAMA/LABA therapy. To our knowledge, the efficacy and safety of a LAMA/LABA FDC has rarely been studied in GOLD category A patients.
Materials and Methods
Study Design
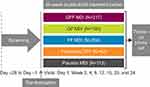
PINNACLE-1, -2, and -4 (NCT01854645, NCT01854658, and NCT02343458) were Phase III randomized, double-blind, placebo-controlled, parallel-group, multicenter studies (Figure 1).9,11 PINNACLE-1 was conducted in the USA, Australia, and New Zealand; PINNACLE-2 was conducted in the USA; and PINNACLE-4 was conducted in Asia, Europe, and the USA.
Patients received 24 weeks of treatment with GFF MDI 18/9.6 µg, glycopyrrolate (GP) MDI 18 µg, formoterol fumarate (FF) MDI 9.6 µg, or placebo MDI twice daily in all studies. PINNACLE-1 included an additional treatment arm in which patients received open-label tiotropium bromide 18 µg dry-powder inhaler (DPI) once daily (Spiriva® HandiHaler®). Patients who were receiving a stable dose of inhaled corticosteroid (ICS) at screening were permitted to continue its use throughout the studies.
Patients provided written informed consent prior to screening, and the studies were conducted in accordance with Good Clinical Practice, including the Declaration of Helsinki and the International Council for Harmonisation. The protocols were approved by local institutional review boards, as previously reported for each study (Table S1).9,11
Patient Population
Key inclusion and exclusion criteria have been previously described.9,11 Briefly, patients were current or former smokers (≥10 pack-years) who were 40–80 years of age, with an established clinical history of COPD as defined by the American Thoracic Society/European Respiratory Society.13 Patients were required to have a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio of <0.70, and an FEV1 of <80% predicted normal (FEV1 ≥750 mL if <30% predicted normal).
There were no inclusion criteria related to baseline symptom burden or exacerbation history. Patients were categorized as GOLD A if they had a CAT score of <10 at screening, and either no exacerbations or one exacerbation that did not lead to hospitalization in the previous year.12
Lung Function Outcomes
Subgroup analysis by GOLD category was performed in the pooled intent-to-treat (ITT) population for the lung function endpoints of change from baseline in morning pre-dose trough FEV1 (primary endpoint in all studies), and peak change from baseline in FEV1 within 2 hrs post-dose (secondary endpoint in all studies), which were the only endpoints that were pre-specified in the integrated analyses. Baseline FEV1 was defined as the mean of all evaluable 60- and 30-min pre-dose values on Day 1 of treatment.
Safety
Treatment-emergent adverse events (TEAEs) were monitored throughout the studies, and a subgroup analysis of TEAEs by GOLD category was performed for the pooled studies.
Statistical Analyses
The pooled ITT population included all patients who were randomized and received any amount of study treatment, analyzed according to treatment assigned. The pooled safety population included patients who were randomized and received any amount of study treatment, analyzed according to treatment received. The tiotropium DPI treatment arm from the PINNACLE-1 study was not included in these pooled analyses since this treatment arm was only present in one of the three studies, tiotropium was administered open-label, and only a small number of GOLD category A patients were included. However, lung function data for GOLD category A patients in the PINNACLE-1 study, including the open-label tiotropium DPI arm, are shown in Table S2.
The analyses of lung function by GOLD category were specified in an integrated statistical analysis plan that was developed after the reporting of data from PINNACLE-1 and -2, but prior to the unblinding of PINNACLE-4. All analyses were interpreted according to a nominal significance level of 0.05. No adjustment for multiplicity was applied, as type I error was controlled within individual studies. The lung function endpoints were analyzed using a linear repeated measures model with an unstructured covariance matrix, which included the following covariates: baseline FEV1, percent reversibility to albuterol sulfate, study (PINNACLE-1/PINNACLE-2/PINNACLE-4), treatment, visit, and treatment-by-visit interaction.
Results
Baseline Demographics and Clinical Characteristics
Of the 4983 patients who were included in the pooled ITT population, 729 (14.6%) were GOLD category A, 3693 (74.1%) were GOLD category B, 73 (1.5%) were GOLD category C, and 473 (9.5%) were GOLD category D (15 patients had missing data). The pooled GOLD category A safety population included 730 patients (one additional patient in the FF MDI treatment arm; this patient had entered multiple studies and was included only for the first of these in the ITT population, but was included for both instances in the safety population, as these periods were not overlapping).
The mean age (standard deviation [SD]) of GOLD category A patients in the pooled ITT population was 65.5 (7.6) years. The majority of patients were male (72.7%), White (59.4%), and 65.4% had moderate airflow obstruction (post-bronchodilator FEV1 50–<80% predicted). Approximately one third (36.1%) of patients were Asian or were current smokers (32.8%; Table 1). The mean total CAT score (SD) was 6.5 (2.2), and the mean COPD duration (SD) was 6.0 (5.7) years. Despite being GOLD category A, 30.2% of patients were using an ICS at baseline and were allowed to continue its use throughout the study. Baseline demographics and clinical characteristics were generally similar across treatment groups (Table 1).
 |
Table 1 Baseline Demographic and Clinical Characteristics for the Pooled PINNACLE-1, -2, and -4 Studies in GOLD 2017 Category A Patients (ITT Population) |
Change from Baseline in Morning Pre-Dose Trough FEV1
In GOLD category A patients, GFF MDI significantly improved change from baseline in morning pre-dose trough FEV1 at Week 24 versus GP MDI (least squares mean [LSM] difference 54 mL; p=0.0053), versus FF MDI (LSM difference 62 mL; p=0.0013), and versus placebo MDI (LSM difference 188 mL; p<0.0001). The findings over 24 weeks were consistent with those at Week 24, with significant improvements for GFF MDI versus GP MDI (LSM difference 62 mL), FF MDI (LSM difference 59 mL), and placebo MDI (LSM difference 183 mL; all p<0.0001; Table 2). The benefits of GFF MDI over monocomponents and placebo MDI were observed at all time points (Figure 2).
 |
Table 2 Lung Function Endpoints for the Pooled PINNACLE-1, -2, and -4 Studies in GOLD 2017 Category A Patients (ITT Population) |
Peak Change from Baseline in FEV1
GFF MDI improved peak change from baseline in FEV1 within 2 hrs post-dose at Week 24 versus GP MDI (LSM difference 124 mL), versus FF MDI (LSM difference 104 mL), and versus placebo MDI (LSM difference 307 mL; all p<0.0001). The findings over 24 weeks were consistent with those at Week 24, with significant improvements reported for GFF MDI versus GP MDI (LSM difference 136 mL), versus FF MDI (LSM difference 83 mL), and versus placebo MDI (LSM difference 300 mL; p<0.0001; Table 2). Furthermore, the benefits of GFF MDI over monocomponents and placebo MDI were observed at all timepoints (Figure 3).
Safety
In the GFF MDI group, 56.7% of GOLD category A patients experienced a TEAE throughout the studies, and 11.1% experienced a TEAE related to study treatment, in the opinion of the investigator. The most common TEAEs in the GFF MDI group were upper respiratory tract infection (6.9%) and viral upper respiratory tract infection (6.0%). Sixteen patients (7.4%) had serious TEAEs with GFF MDI, and four patients (1.8%) experienced a serious TEAE related to study treatment, in the opinion of the investigator. The most common serious TEAE was worsening of COPD, which occurred in seven patients (3.2%) in the GFF MDI treatment arm.
A total of 36 patients (4.9%) discontinued from the study early due to TEAEs, with the highest incidence of discontinuations due to TEAEs in the placebo MDI group (7.1%). One death occurred in GOLD category A patients (FF MDI group; probable cardiovascular cause). The TEAE profile of GFF MDI in GOLD category A patients was generally similar to that of the monocomponents and placebo MDI (Table 3), and was consistent with the overall patient population in the PINNACLE studies.9,11
 |
Table 3 Summary of TEAEs for the Pooled PINNACLE-1, -2, and -4 Studies in GOLD 2017 Category A Patients (Safety Population) |
Discussion
This pooled analysis of the PINNACLE-1, -2, and -4 studies evaluated the lung function efficacy and safety of the LAMA/LABA FDC GFF MDI in GOLD category A patients. Consistent with the findings in the overall population, the majority of whom were GOLD category B (74.1%), GFF MDI improved lung function outcomes versus monocomponents and placebo in GOLD category A patients, with a magnitude of improvement that was comparable with the overall population.9,11 Furthermore, improvements with GFF MDI versus placebo for morning pre-dose trough FEV1 at Week 24 and over 24 weeks exceeded the suggested minimal clinically important difference of 100 mL (≥183 mL).14 Findings were consistent with a previous pooled analysis of PINNACLE-1 and -2, which showed that lung function benefits with GFF MDI were independent of baseline symptom burden.15 Our findings with GFF MDI are also generally consistent with a pooled post hoc analysis of tiotropium + olodaterol soft mist inhaler versus placebo and tiotropium monotherapy in GOLD category A patients with moderate-to-severe COPD, which also found lung function improvements were similar between GOLD groups.16 The lung function benefits from LAMA/LABA therapy need to be weighed against the known class effects of these bronchodilators.2
GOLD category A patients have a low symptom burden and low exacerbation risk and, therefore, there is limited capacity for improvements in symptoms, health-related quality of life, and exacerbation outcomes in these patients. Corresponding with this, a previous pooled analysis of the PINNACLE-1 and -2 studies showed that improvements in St George’s Respiratory Questionnaire score, rescue medication use, and the rate of moderate/severe COPD exacerbations with GFF MDI versus monocomponents and placebo MDI increased with higher baseline CAT scores.15
GFF MDI was well tolerated with no new or unexpected safety findings in GOLD category A patients compared with the overall population.9,11 The TEAE profile of GFF MDI was generally comparable to that of the LAMA and LABA monocomponents and placebo MDI.
Conclusion
GFF MDI showed significant improvements in lung function compared with the monocomponent MDIs and placebo MDI and was well tolerated in GOLD category A patients. Improving lung function is a key treatment goal in these patients and, therefore, dual long-acting bronchodilator therapy may be an appropriate alternative treatment option for GOLD category A patients.
Abbreviations
AE, adverse event; CAT, COPD Assessment Test; DPI, dry-powder inhaler; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate; GFF, glycopyrrolate/formoterol fumarate; GOLD, Global Initiative for Chronic Obstructive Lung Disease; GP, glycopyrrolate; ICS, inhaled corticosteroid; ITT, intent-to-treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LSM, least squares mean; MDI, metered dose inhaler; mMRC, modified Medical Research Council; SD, standard deviation; SE, standard error; TEAE, treatment-emergent adverse event.
Ethics Approval and Informed Consent
The studies were conducted in accordance with Good Clinical Practice, including the Declaration of Helsinki and the International Council for Harmonisation. The protocols were approved by local institutional review boards, as previously reported for each study.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Acknowledgments
The authors would like to thank Andrea Maes for her valuable contributions to this analysis, and all the patients and their families and the team of investigators, research nurses, and operations staff involved in these studies. Employees of the sponsor and AstraZeneca were involved in various aspects of the conception and design of the studies, acquisition of data, and analysis and interpretation of data, and input into manuscript development. The sponsor did not place any restriction on authors about the statements made in the final article. Medical writing support, under the direction of the authors, was provided by Pauline Craig, PhD, of CMC Connect, a division of McCann Health Medical Communications Ltd, Glasgow, UK, funded by AstraZeneca, Gaithersburg, USA, in accordance with Good Publication Practice (GPP3) guidelines.17 Data included in this manuscript have been presented in a poster at the American Thoracic Society International Conference 2019, May 17–22 2019, Dallas, TX, USA.18
Author Contributions
MJ, UJM, and CR made substantial contributions to the conception or design of the study. FJM, KFR, BJL, and SA made substantial contributions to the acquisition of reported data. MJ made substantial contributions to the analysis of the data. All authors made substantial contributions to the interpretation of the data. All authors reviewed or critically revised the manuscript, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
FJM reports personal fees and non-financial support from American College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, Chiesi, Concert, Continuing Education, Gala, Genentech, GlaxoSmithKline, Inova Fairfax Health System, Miller Communications, National Association for Continuing Education, Novartis, Pearl – a member of the AstraZeneca Group, PeerView Communications, Prime Communications, Puerto Rican Respiratory Society, Roche, Sunovion, and Theravance; non-financial support from ProterixBio; personal fees from American Thoracic Society, Columbia University, Haymarket Communications, Integritas, inThought Research, MD Magazine, Methodist Hospital Brooklyn, New York University, Teva, Unity, UpToDate, WebMD/MedScape, and Western Connecticut Health Network; and grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and National Institutes of Health. KFR reports personal fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi Pharmaceuticals, InterMune, Novartis, Sanofi, and Teva; and grants from the Ministry of Education and Science, Germany. BJL is one of a number of co-investigators on an AstraZeneca-sponsored grant received by the University of Dundee to support genomic studies in COPD. He has also received speaker fees from AstraZeneca; payment for consulting and speaking from Boehringer Ingelheim and Chiesi; grant support from Boehringer Ingelheim, Chiesi, and Janssen; advisory board and speaker fees from Teva; and consulting fees from Sandoz, Cipla, Dr Reddys, and Lupin. MJ, UJM, and CR are employees of AstraZeneca and hold stock and/or stock options in the company. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. 2011 report: Global Strategy for the Diagnosis, Management and Prevention of COPD; 2011. Available from: https://goldcopd.org.
2. Global Initiative for Chronic Obstructive Lung Disease. 2020 report: Global Strategy for the Diagnosis, Management and Prevention of COPD; 2020. Available from: https://goldcopd.org.
3. GlaxoSmithKline. COPD Assessment Test; 2009. Available from: http://www.catestonline.org/.
4. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.580
5. Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–1079. doi:10.1164/rccm.201505-1048OC
6. Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi:10.1016/j.rmed.2013.06.001
7. Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–486. doi:10.1016/S2213-2600(14)70065-7
8. Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg therapy in COPD: a randomized, controlled study. Chest. 2014;145(5):981–991. doi:10.1378/chest.13-1579
9. Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest. 2017;151(2):340–357. doi:10.1016/j.chest.2016.11.028
10. Hanania NA, Tashkin DP, Kerwin EM, et al. Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with chronic obstructive pulmonary disease. Respir Med. 2017;126:105–115. doi:10.1016/j.rmed.2017.03.015
11. Lipworth BJ, Collier DJ, Gon Y, et al. Improved lung function and patient-reported outcomes with co-suspension delivery technology glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a randomized Phase III study conducted in Asia, Europe, and the USA. Int J Chron Obstruct Pulmon Dis. 2018;13:2969–2984. doi:10.2147/COPD.S171835
12. Global Initiative for Chronic Obstructive Lung Disease. 2017 report: Global Strategy for the Diagnosis, Management and Prevention of COPD; 2017. Available from: https://goldcopd.org/archived-reports/.
13. Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi:10.1183/09031936.04.00014304
14. Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–124. doi:10.1081/COPD-200053377
15. Martinez FJ, Fabbri LM, Ferguson GT, et al. Baseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPD. Chest. 2017;152(6):1169–1178. doi:10.1016/j.chest.2017.07.007
16. Singh D, Gaga M, Schmidt O, et al. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO® studies. Respir Res. 2016;17(1):73. doi:10.1186/s12931-016-0387-7
17. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi:10.7326/M15-0288
18. Martinez FJ, Rabe KF, Lipworth BJ, et al. Glycopyrrolate/formoterol fumarate metered dose inhaler (GFF MDI) improves lung function in GOLD category A patients with COPD: pooled data from the Phase III PINNACLE studies. Am J Respir Crit Care Med. 2019;199:A3345.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.