Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
[Gly14]-Humanin Ameliorates High Glucose-Induced Apoptosis by Inhibiting the Expression of MicroRNA-155 in Endothelial Microparticles
Authors Shen MY, Wang M, Liu Z, Wang S, Xie Y
Received 10 February 2021
Accepted for publication 20 April 2021
Published 24 May 2021 Volume 2021:14 Pages 2335—2347
DOI https://doi.org/10.2147/DMSO.S306026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Meng-Yuan Shen,1,2,* Miao Wang,1,* Zhihua Liu,1 Shurong Wang,1 Ying Xie1
1Department of Endocrinology, The Second Affiliated Hospital, Soochow University, Suzhou, Jiangsu, 215000, People’ s Republic of China; 2Department of Endocrinology, The First People’s Hospital of Fuyang District of Hangzhou City, Hangzhou, Zhejiang, 310000, People’ s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Xie
Department of Endocrinology, The Second Affiliated Hospital, Soochow University, Suzhou, Jiangsu, 215000, People’ s Republic of China
Email [email protected]
Background: Humanin, a newly emerging endogenously expressed cytoprotective peptide, has been shown to have anti-apoptotic properties effects by protecting neuronal cells injury. Endothelial microparticles (EMPs) are considered as vital mediators in intercellular communication. EMPs may regulate various physiological and pathological processes by transferring mRNAs and microRNAs (miRNAs) to recipient cells.
Methods: EMPs were isolated from human umbilical vein endothelial cells (HUVECs) by ultracentrifugation. EMPs were characterized by transmission electron microscopy and nanoparticle tracking analyses. Observation of EMPs uptake into HUVECs and the number of EMPs were realized by confocal microscopy. The expression of miR-155 was examined using real-time PCR. Cell apoptosis was examined by flow cytometry assay.
Results: We found that high glucose (HG) increased the number of EMPs and upregulated the expression of miR-155 contained within EMPs, which was mitigated by HNG pretreatment. miR-155 overexpression in EMPs reversed the effects of HNG pretreatment and increased apoptosis of target cells. Effects of HNG pretreatment on HG-treated endothelial cells (ECs) were mitigated after miR-155 mimic transfection into HUVECs while were augmented after miR-155 inhibitor transfection into HUVECs.
Conclusion: HNG inhibited HG-induced apoptosis of ECs and the effect of HNG may be mediated by inhibiting the transfer of EMPs miR-155 from HG-induced HUVECs to normal cells. This study provides a new direction for biological products related to humanin to treat vascular complications associated with all forms of diabetes mellitus.
Keywords: humanin, apoptosis, microRNAs, endothelial microparticles, endothelial cells
Introduction
Vascular lesions caused by diabetes are the leading cause of death and disability in diabetic patients.1,2 Injury and dysfunction of ECs are initial events in the development of diabetic vascular complications (micro- and macrovascular complications).3 Thus, it is of utmost importance to protect ECs against hyperglycemia in the treatment of vascular complications associated with diabetes mellitus.
MPs are small vesicles (0.1–1 μm) that are shed from various cells (eg leukocytes, endothelial cells, platelets) under both normal and pathological conditions.4,5 MPs containing cytoplasm and surface markers from their cells of origin can perform specific functions by exporting bioactive molecules, such as proteins, mRNA or miRNAs.6,7 MPs of various cellular origins are found in the plasma of healthy subjects, and their number increases under pathological conditions. Increasing evidences have revealed that EMPs containing miRNAs contribute to cellular communication and vascular homeostasis.8
miRNAs are small, endogenous, non-coding RNAs, which play a vital role in a wide range of biological processes, including cell apoptosis.9 However, an unstable miRNA molecular structure would be rapidly degraded after entering the systemic circulation.10 Recent studies have shown that EMPs have been suggested to serve as stable carriers for the transfer of miRNA cargo and miRNAs have been explored as novel biomarkers in various diseases, including diabetic vascular complications.11,12 EMPs carry various contents and types of miRNAs under the stimulation of the different factors.13,14 A new research area has recently emerged, focusing on the role of EMPs carrying different miRNAs in cell biology. Research has found that miR-155 may be a therapeutic target for preventing cardiac fibrosis induced by diabetes.15 However, miR155, which produced conflicting results on atherosclerosis, is of particular interest. Therefore, further study is needed to elucidate the role of EMP-mediated miR-155 delivery during the development of atherosclerosis.
Humanin is both an intracellular protein and a secreted protein. It is the first newly discovered peptide encoded in the mitochondrial genome, which exerts robust protective effects against a myriad of cytotoxic stimuli in many cell types.16 Humanin signals in cells or are released to act as autocrine/paracrine/endocrine cytoprotective factors playing a pivotal role in the cellular stress response.17 Previous studies have indicated that Humanin is a novel regulator of Hedgehog signal, which can prevent glucocorticoid-induced bone growth disorder.18 Combined treatment of HNG and dexamethasone can protect against dexamethasone-induced chondrocyte apoptosis in both articular and growth plate cartilage.19 Replacement of serine at position 14 of the HN peptide chain (Ser14) with glycine (Gly) leads to the formation of HNG, which has cytoprotective activity enhanced by 1000-fold when compared with that of Humanin.20 We previously demonstrated that HNG produced a dramatic reduction in the ratio of bax/bcl-2 induced by HG treatment in HUVECs, suggesting that HNG is anti-apoptotic.21 However, the specific mechanisms by which HNG protects against HG-induced ECs apoptosis are not clear.
In the current study, we attempted to clarify a function of the EMP-mediated delivery of miR-155 in the mechanism of HNG against HG-induced vascular endothelial cell apoptosis.
Materials and Methods
Reagents and Antibodies
Primary HUVECs were purchased from ScienCell (CA, USA). HNG was purchased from Sigma (St. Louis, MO, USA, cat.no.H6161). miR-155 mimic was obtained from GenePharma (Shanghai, China, cat.no.B02001), negative control (NC) mimic was obtained from GenePharma (Shanghai, China, cat.no.B04002), miR-155 inhibitor was obtained from GenePharma (Shanghai, China, cat.no. B03001), NC inhibitor was obtained from GenePharma (Shanghai, China, cat.no.B04003). Recombinant annexin-V was obtained from Abcam (CA, USA, cat.no.ab89493). Carboxyfluorescein succinimidyl ester (CFSE) was obtained from Sigma (St. Louis, MO, USA, cat.no.21888). EC growth media was obtained from ScienCell (San Diego, California, USA, cat.no.1001) and Lonza (Swiss, cat.no. CC-3162). Annexin V-FITC apoptosis assay kit was obtained from Absin (Shanghai, China, cat.no. abs50001). Lipofectamine™ 2000 Transfection Reagent was purchased from Invitrogen (Carlsbad, CA, USA, cat.no.11668). BCA protein assay kit was obtained from G-CLONE (Beijing, China, cat.no.PN0120).
Cell Culture and EMP Generation
Primary HUVECs were cultured in EC growth media with endothelial growth media Supplement Mix (ScienCell) under standard cell culture conditions (37 °C, 5% CO2). Cells were used from passages 3 to 6 when they reached 70–80% confluency.22 Cells were starved in a serum-free medium for 24 h to induce apoptosis. The cell culture supernatant was collected and followed with centrifuged at 4500×g for 15 minutes to remove cell debris. The supernatant was then ultracentrifuged (100,000×g, 90 minutes) to pellet EMPs at 4 °C using ultracentrifuge (OPTIMA L-90K0306170303, Beckman Instruments Inc. Fullerton, USA).23 The obtained EMPs were washed in sterile PBS (pH 7.4) and stored at −80 °C. To generate EMPs from ECs under HG conditions, confluent HUVECs were stimulated with 30 mM glucose for 72 h and then subjected to a serum-free medium for 24 h to generate EMPs. For the cells receiving HNG, cells were pretreated with HNG for 3 h. EMPs derived from HG-treated ECs were defined as HG-EMPs, while the EMPs extracted from supernatants of cells that had not undergone glucose stimulation were defined as control-EMPs. The concentration of EMPs was determined using a BCA protein assay kit. Pelleted EMPs were resuspended and used freshly.
Nanoparticle-Tracking Analysis
ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) was used to analyze suspensions containing vesicles. The diluted samples were illuminated by a monochromatic laser beam at 488 nm. We used NTA software (ZetaView 8.04.02 SP2) to analyze EMP samples. After optimization, each particle was first identified, and then tracked frame by frame, as well as its Brownian motion. We used a two-dimensional Stokes-Einstein equation based on the velocity of particle movement to determine particle size. The average, mode, and median EMP size were used to calculate the concentration of the samples, expressed in nanoparticles/mL.
Electron Microscopy
Pelleted EMPs were fixed in PBS with 2% paraformaldehyde, overnight at 4°C. The samples were embedded in Formvar-carbon, pelleted EMPs were washed with PBS, fixed with 1% glutaraldehyde, and then washed with phosphate-buffered saline (PBS) and finally treated with oxalate dioxyuranium (pH=7.0) droplet for 5 minutes and methylcellulose for 10 minutes. Experiments were performed on ice. Samples were then visualized on the electronic microscope.
EMP Immobilization, Immunofluorescence
As previously described,24 micro-wells (µ-Slides, Ibidi) were coated overnight with 50 µg/mL recombinant annexin-V. Purified EMPs, diluted in NaCl/Hepes buffer (with CaCl2, 10mM), were incubated at 37 °C for 30 minutes in the presence of CFSE (0.2 mM). Then, CFSE-labeled EMPs were then seeded in coated micro-wells and were immobilized in the dark at room temperature. When appropriate, experiments were also performed to record the change in the number of EMPs after fixation for 1 h.
ECs uptake experiments were performed according to the following method. In brief, pelleted EMPs diluted in PBS were incubated with 0.2 mM CFSE at 37 °C for 30 minutes, washed and centrifuged twice at 20,000 ×g, and resuspended in sterile PBS. HUVECs were incubated with CFSE-labeled EMPs for different time frames. After three washing steps, HUVECs were fixed in 4% paraformaldehyde (PFA), blocked with 5% bovine serum albumin (BSA) and stained with anti-CD31 (mouse monoclonal antibody, 1:10, Thermo Fisher Scientific, USA, cat.no. MA513188) followed by incubation with Cy3-conjugated goat anti-mouse antibody (1:500, Thermo Fisher Scientific, USA, cat.no. A10521). After washing three times, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1:2000, Thermo Fisher Scientific, USA, cat.no. D3571). The spinning disc confocal microscopy was used to visualize the cellular uptake of EMPs.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was isolated using Trizol LS reagent (Invitrogen, USA, cat.no. 10296010) according to the manufacturer’s instructions. To increase the yield of small RNAs, the RNA is precipitated in ethanol at −20 °C overnight with glycogen (Thermo Fisher Scientific, cat. no. R0551). RNA quality was measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Approximately 500 ng of the total RNA was reversely transcribed using a miRNA First Strand cDNA Synthesis kit (Sango Biotech, cat. no B532453) according to the manufacturer’s protocol. The single-stranded cDNA was amplified by real-time quantitative PCR using SYBR Green for detection [ABI‐7500 (Life Technologies, Darmstadt, Germany) PCR System]. Primers used: hsa-mir-155-5p forward, 5ʹ-CGC GTT AAT GCT AAT CGT GAT A-3ʹ, reverse, 5ʹ-AGT GCA GGG TCC GAG GTA TT −3ʹ, RT Primer, 5ʹ- GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA A-3ʹ; U6 was used as an endogenous control and was purchased from GenePharma (Shanghai, China, cat.no. E17002). Delta Ct method was used to quantify relative miRNA expression. CT values above 35 were defined as undetectable. For all PCR experiments, samples were run in triplicates.
Transfection of HUVECs and Co-Incubation of EMPs
HUVECs were seeded in 6-well plates and transfected using lipofectamine 2000 at a final concentration of 3 mg/mL according to the manufacturer’s recommendation. 100 nM miR-155 mimic, 100 nM negative control (NC) mimic, 200 nM miR-155 inhibitor, 200 nM NC inhibitor were transfected into HUVECs (70–80% confluence) for 24 h. The transfected cells were washed with new culture media for subsequent experiments.
For incubating transfected EMPs with cells, cells were seeded in culture bottle and transfected as above, cultured for 24 h in a serum-free medium to harvest EMP, EMPLipo, EMPNC mimic, and EMPmiR−155 mimic. The obtained EMPs were then incubated with target cells at a concentration of 50 μg/mL for 24 h.
Flow Cytometry
To identify the apoptotic rate, the percentage of cells undergoing apoptosis was determined by an annexin V-FITC apoptosis detection kit. HUVECs were cultured and transfected. Cells were harvested and washed three times with PBS at the indicated times. The cells were centrifuged at 500 g for 5 minutes and then suspended in 500 μL of binding buffer containing 5 μL FITC-conjugated annexin V antibody and 5 μL propidium iodide (PI). The mixture was incubated at room temperature for 15 minutes in the dark. The cells were detected by a flow cytometer (BD Biosciences, San Jose, CA, USA) within an hour.
Image Analysis
To quantify the number of CFSE-EMPs, CFSE staining was first used to identify EMPs, the diameter of the fluorescence was calculated by Fiji software (NIH) using the “Analyze Particles” function. All 8-bit images are set to automatic thresholds.
Statistical Analysis
Data are expressed as mean ± SEM. Means between the two categories were compared with the use of a two-tailed, unpaired Student’s t-test. Statistical significance was assumed when a null hypothesis could be rejected at P < 0.05. Statistical analysis was performed using GraphPad Prism 8. Error bars represent the SEM of three independent experiments.
Results
Characterization of EMP
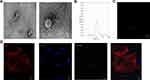
EMPs were generated from HUVECs in vitro as described. To further investigate the characteristic of EMP isolated according to our protocol, TEM revealed that EMPs appeared as a cluster of cup-shaped vesicles (Figure 1A). To further investigate the size distribution profile of EMPs, we performed a size detection using the Nanosight, revealing a size peak of 134 nm (Figure 1B). Most of the HUVEC-derived EMPs had a size of 0.1 to 1 μm in diameter. Confocal microscopy was performed to characterize EMPs and to study its uptake (Figure 1C). EMPs were labeled with CFSE, cell membranes were labeled with CD31, and the nuclei were labeled with DAPI. After the addition of the isolated CFSE-EMPs to HUVECs and incubation for 15 h, the uptake of the CFSE-EMPs by recipient cells was visualized under confocal microscope (Figure 1D).
Pretreatment with HNG Reverses the Release of EMPs Induced by HG Treatment
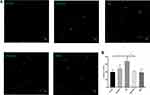
To explore the effect of HNG on EMPs release, we observed the changes in the number of EMPs by confocal microscopy (Figure 2A). A significant increase in the number of EMPs was observed after treating HUVECs with 30 mM glucose, while cells pretreated with 1 μM HNG for 3 h before glucose treatment showed a decrease when compared with those in the group of HUVECs that did not receive pretreatment (Figure 2B). The experimental conditions contained 30 mM D-mannitol to control for osmolality.
Pretreatment with HNG Down-Regulates HG-Induced miR-155 Expression in EMPs
To determine whether miR-155 in EMPs was involved in the mechanism of HNG preventing apoptosis induced by HG, quantitative real-time PCR was used to measure the expression of miR-155 in EMPs. As shown in Figure 3, the treatment of HUVECs with 30 mM HG for 72 h significantly increased the expression of miR-155 in EMPs and cell apoptosis increased, while pretreatment with 1 μM HNG for 3 h markedly decreased apoptosis. D-Mannitol, used here for osmolality control, did not show any significant effects on the rate of apoptosis.
Pretreatment with HNG Attenuated HG-Induced HUVECs Injury by Suppressing the Expression of miR-155 in EMPs to Exert an Anti-Apoptotic Effect
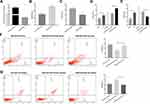
We further investigated whether HNG could affect HUVEC apoptosis by regulating the miR-155 content of EMPs. First, we established a co-culture system and detected HNG’s effects on apoptotic secretion in HUVECs. HUVECs were cultured for 72 h under HG conditions, and the extracted HG-EMPs from cell supernatant were co-incubated with normal ECs. HG-EMPs induced apoptosis in normal HUVECs. However, the treatment of HNG decreases apoptotic response of co-cultured HUVECs compared to HG-stimulated group (Figure 4A), suggesting that HNG could reduce the apoptotic response of HUVECs by weakening the release of EMPs. Then, to observe the role of miR-155 in the crosstalk between HUVECs, we performed a transfection of miR-155 mimic to HUVECs. RT-PCR results showed that, compared with NC, transfection with miR-155 mimic significantly increased the level of miR-155 in HUVECs, by almost 150 times (Figure 4B). Moreover, EMPs derived from HUVECs overexpressing miR-155 also had higher levels of miR-155 compared with NC mimic group, by more than 40 times (Figure 4C). Subsequently, HUVECs were treated with EMPs (EMPNC mimic, and EMPmiR−155 mimic) for 24 h. We found that compared with EMPNC mimic, EMPmiR−155 mimic could be more than double the levels of miR-155 in HUVECs (Figure 4D). These results illustrated that miR-155 was overexpressed successfully after transfection.
To further determine whether the effect of EMPs on endothelial cells under HG conditions was related to miR-155 and whether HNG played an anti-apoptotic role in endothelial cells through miR-155 in EMPs, we collected EMPs produced by endothelial cells that overexpressed miR-155 and found that EMPmiR−155 mimic significantly increased apoptosis of HUVECs in comparison with EMPNC mimic (Figure 4E). We transfected HUVECs with miR-155 mimic before HG treatment, collected the EMPs from these cells, and co-cultured the EMPs with target cells; flow cytometry also showed a similar trend in apoptosis rates (Figure 4F). These results indicated that miR-155 was involved in the process of HG-EMPs promoting apoptosis of endothelial cells. Moreover, the effects of HNG pretreatment were significantly reversed by miR-155 overexpression, presenting a significant increase in the target cell apoptosis rate (Figure 4G). Results indicated that HNG pretreatment might affect HG-treated HUVECs through down-regulating miR-155 in EMPs.
Pretreatment with HNG Improves the HG-Induced Apoptosis of Endothelial Cells Through Down-Regulating miR-155 in Cells
To further verify whether miR-155 is an important regulatory factor in the process of HNG exerting anti-HG-induced endothelial cell apoptosis, we first tested the effect of miR-155 overexpression and downregulation on cell apoptosis. Using Q-PCR, we found that the expression of miR-155 significantly increased in the cells transfected with miR-155 mimic (Figure 4B) and transfection of miR-155 inhibitor down-regulated miR-155 expression in the miR-155 inhibitor group compared to the NC group (Figure 5A). These results illustrated that miR-155 was overexpressed or silenced successfully after transfection. Thus, the HUVECs that were transfected with miR-155 mimic had a significantly higher apoptosis rate than NC (Figure 5B). The HUVECs that were transfected with the miR-155 inhibitor had significantly lower apoptosis than NC (Figure 5C). Similar results were found in HG environments (Figure 5D and E). These results suggested that miR-155 is involved in the regulation of HUVEC apoptosis induced by HG. However, the overexpression of miR-155 in cells mitigated the anti-apoptotic effect of HNG, inhibition of miR-155 in cells augmented with the anti-apoptotic effect of HNG on endothelial cells (Figure 5F and G), suggesting that the protective effect of HNG was realized by regulating the down-regulation of miR-155.
EMPs Produced by HG-Treated Endothelial Cells Enter Normal Endothelial Cells at a Faster Rate
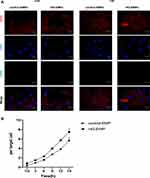
To further explore the causes of HG-EMPs promoting apoptosis, we examined the uptake of HG-EMPs and control-EMPs into normal ECs by immunofluorescence. We found that ECs accumulated higher levels of HG-EMPs compared to the uptake of control-EMPs at 15 h of incubation, and both are time-dependent. Moreover, the cellular uptake of HG-EMPs was faster than that of control-EMPs at 12 h of incubation. When co-incubated for 15 h, the uptake rate of the EMPs tended to be consistent (Figure 6A and B).
Discussion
Hyperglycemia is the main symptom of diabetes. Prolonged high blood glucose can induce endothelial dysfunction as an independent risk factor for poor prognosis associated with cardiovascular diseases, such as atherosclerosis and myocardial infarction.25 Improving endothelial function helps to maintain vascular stability and slowdown the development of diabetic vascular complications.26 An in-depth understanding of the pathophysiology and a search for new therapeutic targets are crucial to improve the clinical treatment of chronic complication of diabetes. Humanin, first identified in Alzheimer’s disease (AD) patients, has protective effects on neuronal cells.27 However, the specific mechanisms of HNG against apoptosis remain unclear. Our experimental results indicated that HNG could attenuate HG-induced apoptosis in HUVECs by reducing the expression of miR-155 in EMPs.
EMPs, serving as a class of extracellular vesicles, are important vectors of intercellular substance exchange and signal transduction and participate in disease progression.28–30 It has been reported in the studies on diabetes and its complications that EMPs carrying surface antigens, proteins and various bioactive molecules (cytokines, signaling proteins, miRNAs, etc.) play a critical role in vascular cellular communication.31 EMPs can be used as a marker of disease diagnosis and are expected to become a potential target or carrier for clinical treatment. However, few specific studies have been conducted. Our data showed that HG-induced ECs produced more EMPs and carry more miR-155, thereby leading to cell apoptosis, which were significantly reversed by HNG treatment. The experimental results indicated that miR-155 in EMPs was involved in the mechanism of HNG preventing cell apoptosis induced by HG.
MPs can transmit information to acceptor cells, thereby triggering phenotypic changes in the latter. MPs are targeted to the acceptor cell through specific molecular interactions involving membrane-exposed proteins, sugars or lipids (inset), or through unspecific macropinocytosis or micropinocytosis. The differences in the entry of MPs from different sources into recipient cells are not known. In this study, we co-incubated EMPs with ECs and found that the cellular uptake of HG-EMPs was higher than that of control-EMPs within 15 h. Moreover, the cellular uptake of HG-EMPs seemed to be faster than that of control-EMPs at 12 h of incubation. Therefore, these observations collectively demonstrated that HG-EMPs can transmit more damage signals generated by the hyperglycemic state among cells and are absorbed by target cells at a high rate, which may be a novel discovery to explore the mechanism of ECs injury induced by EMPs released from HUVECs treated with HG.
Unlike classical means of information transmission by way of direct contact with cells or secretion of cytokines, EMPs can carry complex molecular signal clusters to remotely modify and reprogram target cells.32 Zhang et al reported that EMPs delivering miR-155 into T lymphocytes are involved in the initiation of acute graft-versus-host disease.33 We attempted to investigate whether HNG could reduce the ability of injured ECs to transport damage signals to normal cells. First, EMPs were extracted from HUVECs transfected with miR-155 mimic or NC mimic, and then co-incubated with normal endothelial cells. The results showed that EMPmiR−155 mimic significantly increased cellular apoptosis. EMPmiR−155 mimic derived from HG-treated HUVECs induced cellular apoptosis in normal HUVECs. While EMPmiR−155 mimic derived from HUVECs treated with HNG and HG resulted in increased levels of apoptotic rate compared with the NC group, leading us to conclude that HNG could reduce the expression of miR-155 in EMPs, thus weakening the transmission of injury signals to normal ECs and protecting ECs.
miRNAs are mostly intracellular but are also present in body fluids.34 miRNAs are largely known to base pair with the 3′-UTR of target mRNAs, downregulating their stability and translation to regulate gene expression, playing an important role in the development of many cardiovascular diseases, especially atherosclerosis.35 Studies show that miR-155 is associated with inflammatory channels of atherosclerosis. However, the role of miR-155 in the pathogenesis of atherosclerosis is debated. miR-155 exerted anti-atherogenic roles by repressing calcium-regulated heat-stable protein 1.36 However, another study showed that downregulated miR-155 contributed to inhibit inflammatory response, thus producing an anti-atherogenic effect.37 In animal experiments, Apo-E knockout mice exhibited a significantly reduced degree of atherosclerosis when miR-155 was simultaneously knocked out.38 Consistent with those results, our study showed that miR-155 promoted apoptosis of ECs. After transfecting HUVECs with miR-155 mimics, inhibitors or NC, we found that the apoptosis rate of the miR-155 mimic group was significantly higher than the NC group, and inhibiting miR-155 promoted a significant reduction in cell apoptosis. Furthermore, similar results were found under HG conditions, indicating that miR-155 is a positive regulator of cell apoptosis.
In recent years, Humanin and its analogues have become the focus of research for the treatment of various chronic diseases, including neurodegenerative diseases, cardiovascular diseases, diabetes, etc.39 Humanin could significantly inhibit the formation of amyloid fibers and regulate pancreatic β-cell amyloids, which is a pathological feature of type 2 diabetes.40 In addition, Humanin is also a key regulator of peripheral insulin action. Injection of Humanin into the third ventricle of rats and peripheral infusion of the potent Humanin analogue HNGF6A can improve the sensitivity of liver and peripheral insulin.41 Humanin delays and improves the development of diabetes by activating transcription activation factor 3 to inhibit beta-cell apoptosis and increase insulin sensitivity.42 Our previous study found that HNG inhibits HG-induced apoptosis. In order to further verify whether miR-155 is an important regulatory factor in the process of HNG exerting anti-HG-induced endothelial cell apoptosis, miR-155 mimic or inhibitor was transfected into HUVECs. As expected, the overexpression of miR-155 in cells reversed the anti-apoptotic effect of HNG, while inhibition of miR-155 in cells augmented the anti-apoptotic effect of HNG on endothelial cells, confirming the outstanding role of miR-155 in HNG-treated HUVECs. Taken together, these results strongly suggest that HNG inhibits HG-induced EC apoptosis and maintains vascular homeostasis, possibly by regulating the expression levels of miR-155 in EMPs.
At present, there is a lack of effective drugs for the prevention and treatment of vascular complications of diabetes. There have been no reports of Humanin’s protective role by regulating the miRNAs encapsulated in EMPs. Our results may have potential clinical implications. Our data indicated that HNG promotes HG-induced endothelial apoptosis of endothelial cells by regulating the expression of miR-155 in EMPs, which could improve vascular endothelial dysfunction, maintains vascular homeostasis, and thereby delays the progression of vascular complications of diabetes. In addition, our bioinformatics analyses showed that PARP1 could be the potential target genes of miR-155. PARP1 is a member of the PARP protein family. In previous studies, we found that HNG diminishes the HG-induced increase in the expression of PARP.21 In future studies, we will further explore the specific mechanisms by which miR-155 functions. We have to admit that our study has some limitations in methods and techniques regarding the source of MPs, and we try to avoid potential deviations by setting up control groups to cope with the defects of the study. In conclusion, EMPs and its cargo microRNA may be a promising diagnostic biomarker and drug target for patients with diabetes. Considering the beneficial effects of Humanin on the improvement of insulin sensitivity, and protection of islet donor cells, it may be used as a novel therapeutic strategy for the clinical treatment of diabetes.
Conclusion
In summary, our results indicate that HNG pretreatment inhibits HG-induced apoptosis of vascular endothelial cells by down-regulating miR-155, which was transferred to adjacent vascular endothelial cells via cell-derived EMPs. Our studies highlighted a novel mechanism demonstrating that Humanin can protect damaged blood vessels by decreasing the release of EMPs carriers from HG-induced endothelial cells via anti-apoptosis. EMPs could serve as a potential tool for the specific delivery of miRNAs, contributing to a stronger therapeutic effect of HNG in anti-diabetic vascular complications therapy.
Abbreviations
miRNAs, microRNAs; MPs, microparticles; EMPs, endothelial microparticles; EC, endothelial cell; HNG, [Gly14]-Humanin; HUVECs, human umbilical vein endothelial cells; CFSE, carboxyfluorescein succinimidyl ester; PBS, phosphate-buffered saline; PFA, paraformaldehyde; BSA, bovine serum albumin; PI, propidium iodide; NC, negative control; AD, Alzheimer’s disease.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Thanks to Prof. Liang-yi Chen for his guidance and help in this study, and to Prof. Jin-cai Luo for his help in obtaining the sources of cells in this study. (Institute of Molecular Medicine, Peking University, China).
Author Contributions
M.S. and Y.X. conceived and proposed the idea. M.S., Y.X., and MW designed the work. M.S., X.Y., S.W., and MW contributed to the interpretation of data for the work. M.S., Y.X., and Z.L. drafted the work. Y.X., Z.L., S.W., and M.W. revised it critically for important intellectual content. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the National Natural Science Foundation of China (81670742) and the Suzhou Municipal Health Bureau (szxk201804).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. doi:10.1016/j.cjca.2017.12.005
2. Yaribeygi H, Butler AE, Barreto GE, et al. Antioxidative potential of antidiabetic agents: a possible protective mechanism against vascular complications in diabetic patients. J Cell Physiol. 2019;234(3):2436–2446. doi:10.1002/jcp.27278
3. Maruhashi T, Kihara Y, Higashi Y. Diabetes and endothelial dysfunction. Diabetes Aging Related Complicat. 2017;22(1):45–58. doi:10.1007/978-981-10-4376-5_4
4. El-Gamal H, Parray AS, Mir FA, et al. Circulating microparticles as biomarkers of stroke: a focus on the value of endothelial- and platelet-derived microparticles. J Cell Physiol. 2019;234(10):16739–16754. doi:10.1002/jcp.28499
5. Akbar N, Azzimato V, Choudhury RP, et al. Extracellular vesicles in metabolic disease. Diabetologia. 2019;62:2179–2187. doi:10.1007/s00125-019-05014-5
6. Jia Y, Zheng Z, Guan M, et al. Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med. 2018;50(5):1–13. doi:10.1038/s12276-018-0084-3
7. Wang Z, Zhu H, Shi H, et al. Exosomes derived from M1 macrophages aggravate neointimal hyperplasia following carotid artery injuries in mice through miR-222/CDKN1B/CDKN1C pathway. Cell Death Dis. 2019;10(6):422. doi:10.1038/s41419-019-1667-1
8. Nakaoka H, Hirono K, Yamamoto S, et al. MicroRNA-145-5p and microRNA-320a encapsulated in endothelial microparticles contribute to the progression of vasculitis in acute Kawasaki Disease. Sci Rep. 2018;8(1):1016. doi:10.1038/s41598-018-19310-4.
9. Michlewski G, Cáceres JF. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25(1):1–16. doi:10.1261/rna.068692.118
10. Aiso T, Takigami S, Yamaki A, et al. Degradation of serum microRNAs during transient storage of serum samples at 4 °C. Ann Clin Biochem. 2018;55(1):178–180. doi:10.1177/000456321770233
11. Dimassi S, Karkeni E, Laurant P, et al. Microparticle miRNAs as biomarkers of vascular function and inflammation response to aerobic exercise in obesity? Obesity. 2018;26(10):1584–1593. doi:10.1002/oby.22298
12. Kuhn S, Splith K, Ballschuh C, et al. Mononuclear-cell-derived microparticles attenuate endothelial inflammation by transfer of miR-142-3p in a CD39 dependent manner. Purinergic Signal. 2018;14(4):423–432. doi:10.1007/s11302-018-9624-5
13. Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59(11):2037–2046. doi:10.1194/jlr.R084640
14. da Silveira JC, de Ávila AC, Garrett HL, et al. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol. 2018;236(1):R15–27. doi:10.1530/JOE-17-0200
15. Li Y, Duan J, He Q, et al. miR-155 modulates high glucose induced cardiac fibrosis via the Nrf2/HO-1 signaling pathway. Mol Med Rep. 2020;22(5):4003–4016. doi:10.3892/mmr.2020.11495
16. Kim SJ, Mehta HH, Wan J, et al. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging (Albany NY). 2018;10(6):1239–1256. doi:10.18632/aging.101463
17. Zaman F, Zhao Y, Celvin B, et al. Humanin is a novel regulator of Hedgehog signaling and prevents glucocorticoid‐induced bone growth impairment. FASEB J. 2019;33(4):4962–4974. doi:10.1096/fj.201801741R
18. Kumfu S, Charununtakorn ST, Jaiwongkam T, et al. Humanin exerts neuroprotection during cardiac ischemia-reperfusion injury. J Alzheimers Dis. 2018;61(4):1343–1353. doi:10.3233/JAD-170708
19. Celvin B, Zaman F, Aulin C, et al. Humanin prevents undesired apoptosis of chondrocytes without interfering with the anti-inflammatory effect of dexamethasone in collagen-induced arthritis. Clin Exp Rheumatol. 2020;38(1):129–135.
20. Hashimoto Y, Niikura T, Tajima H, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Aβ. Proc Natl Acad Sci U S A. 2001;98(11):6336–6341. doi:10.1073/pnas.101133498
21. Xie Y, Liu ZH, Li XY, et al. Protection effect of [Gly14]-Humanin from apoptosis induced by high glucose in human umbilical vein endothelial cells. Diabetes Res Clin Pract. 2014;106(3):560–566. doi:10.1016/j.diabres.2014.09.020
22. Lu Q, Meng Q, Qi M, et al. Shear-sensitive lncRNA AF131217.1 inhibits inflammation in HUVECs via regulation of KLF4. Hypertension. 2019;73(5):e25–34. doi:10.1161/HYPERTENSIONAHA.118.12476
23. Sekuła M, Janawa G, Stankiewicz E, et al. Endothelial microparticle formation in moderate concentrations of homocysteine and methionine in vitro. Cell Mol Biol Lett. 2011;16(1):69–78. doi:10.2478/s11658-010-0040-2
24. Briens A, Gauberti M, Parcq J, et al. Nano-zymography using laser-scanning confocal microscopy unmasks proteolytic activity of cell-derived microparticles. Theranostics. 2016;6(5):610–626. doi:10.7150/thno.13757
25. Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60(1):119–126. doi:10.1016/j.healun.2017.01.584
26. Beckman JA, Hurwitz S, Fisher NDL. Arginine impairs endothelial and executive function in older subjects with cardiovascular risk. J Am Soc Hypertens. 2018;19(1):290. doi:10.1016/j.jash.2018.07.002
27. Hashimoto Y, Niikura T, Ito Y, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci. 2001;21(23):9235–9245. doi:10.1523/jneurosci.21-23-09235.2001
28. Jaiswal R, Luk F, Gong J, et al. Microparticle conferred microRNA profiles - implications in the transfer and dominance of cancer traits. Mol Cancer. 2012;11(1):37. doi:10.1186/1476-4598-11-37
29. Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379(10):958–966. doi:10.1056/NEJMra1704286
30. Nieuwland R, Falcon-Perez JM, Soekmadji C, et al. Essentials of extracellular vesicles: posters on basic and clinical aspects of extracellular vesicles. J Extracell Vesicles. 2018;7(1):
31. Pfeifer P, Ackerschott A, Ebert S, et al. P6549Inflammasome-induced endothelial microparticles impair cellular function in arterial smooth muscle cells. Eur Heart J. 2018;39(suppl_1). doi:10.1093/eurheartj/ehy566.p6549
32. Zheng Z, Liu L, Zhan Y, et al. Adipose-derived stem cell-derived microvesicle-released miR-210 promoted proliferation, migration and invasion of endothelial cells by regulating RUNX3. Cell Cycle. 2018;17(8):1026–1033. doi:10.1080/15384101.2018.1480207
33. Zhang R, Wang X, Hong M, et al. Correction: endothelial microparticles delivering microRNA-155 into T lymphocytes are involved in the initiation of acute graft-versushost disease following allogeneic hematopoietic stem cell transplantation. Oncotarget. 2017;8(35):60000. doi:10.18632/oncotarget.15579
34. Kai K, Dittmar RL, Sen S. Secretory microRNAs as biomarkers of cancer. Semin Cell Dev Biol. 2018;78:22–36. doi:10.1016/j.semcdb.2017.12.011
35. McManus DD, Rong J, Huan T, et al. Messenger RNA and microRNA transcriptomic signatures of cardiometabolic risk factors. BMC Genom. 2017;18(1):139. doi:10.1186/s12864-017-3533-9
36. Li X, Kong D, Chen H, et al. MiR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci Rep. 2016;6(1):21789. doi:10.1038/srep21789
37. Huang J, Yang Q, He L, et al. Role of TLR4 and miR-155 in peripheral blood mononuclear cell-mediated inflammatory reaction in coronary slow flow and coronary arteriosclerosis patients. J Clin Lab Anal. 2018;32(2):
38. Chen Y, Liu Y, Pan Q, et al. MicroRNA-155 regulates ROS production, NO generation, apoptosis and multiple functions of human brain microvessel endothelial cells under physiological and pathological conditions. J Cell Biochem. 2015;116(12):2870–2881. doi:10.1002/jcb.25234
39. Wang X, Wu Z, He Y, et al. Humanin prevents high glucose-induced monocyte adhesion to endothelial cells by targeting KLF2. Mol Immunol. 2018;101:245–250. doi:10.1016/j.molimm.2018.07.008
40. Levine ZA, Teranishi K, Okada AK, et al. The mitochondrial peptide humanin targets but does not denature amyloid oligomers in type II diabetes. J Am Chem Soc. 2019;141(36):14168–14179. doi:10.1021/jacs.9b04995
41. Muzumdar RH, Huffman DM, Atzmon G, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4(7):e6334. doi:10.1371/journal.pone.0006334
42. Hoang PT, Park P, Cobb LJ, et al. The neurosurvival factor Humanin inhibits β-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59(3):343–349. doi:10.1016/j.metabol.2009.08.001
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.