Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 10
Glucose-lowering therapies, adequacy of metabolic control, and their relationship with comorbid depression in outpatients with type 2 diabetes in a tertiary hospital in Kenya
Authors Otieno CFF , Kanu JE, Karari EM, Okech-Helu V, Joshi MD, Mutai K
Received 12 October 2016
Accepted for publication 14 March 2017
Published 28 April 2017 Volume 2017:10 Pages 141—149
DOI https://doi.org/10.2147/DMSO.S124473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
CF Frederick Otieno,1 Joseph E Kanu,1 Emma M Karari,1 Violet Okech-Helu,2 Mark D Joshi,1 Kenn Mutai2
1Department of Clinical Medicine and Therapeutics, University of Nairobi, 2Kenyatta National Hospital, Nairobi, Kenya
Background: Depression and diabetes mellitus are important comorbid conditions with serious health consequences. When depression and diabetes are comorbid, depression negatively affects self-management activities of diabetes with serious consequences. Relationship between treatment regimens of diabetes, the adequacy of glycemic control, and occurrence of comorbid depression is not known among our patients.
Patients and methods: This was a cross-sectional descriptive study at the outpatient diabetes clinic of the Kenyatta National Hospital where 220 ambulatory patients with type 2 diabetes on follow-up were systematically sampled. Sociodemographic data and clinical information were documented. The Patient Health Questionnaire-9 (PHQ-9) was used to assess depression. Ethylenediaminetetraacetic acid-anticoagulated blood was used for glycated hemoglobin (HbA1C) assay on automated system, COBAS INTEGRA machine.
Results: Two hundred twenty patients with type 2 diabetes were enrolled. The prevalence of comorbid depression by PHQ-9 was 32.3% (95% confidence interval: 26.4%–38.6%). The majority, 69.5%, had poor glycemic control, HbA1C >7.0%, mean HbA1C was 8.9%±2.4%. Half, 50.4%, of the study subjects were on insulin-containing regimens. Over 8% (84.5%) of the participants with comorbid depression had poor glycemic control, which worsened with increasing severity of depression. There was significant correlation between comorbid depression and poor glycemic control, which is more consistent in the insulin-treated patients. However, patients on oral agents only, both with and without comorbid depression, were similar in their glycemic control.
Conclusion: Among our type 2 diabetic population with comorbid depression, a large proportion had poor glycemic control, which worsened with increasing severity of depression. The insulin treatment increased the odds of comorbid depression and poor glycemic control in patients. It is justifiable to screen for comorbid depression in patients with type 2 diabetes who are in poor glycemic control, especially the insulin-treated, and then provide specific and appropriate interventions that are necessary to optimize their metabolic outcomes.
Keywords: type 2 diabetes, comorbid depression, insulin therapy and poor glycemic control
Introduction
Depressive illness that occurs in patients with type 2 diabetes (comorbid depression) has been demonstrated to be associated with or a cause of poor self-care,1,2 poor glycemic control,3,4 and poor quality of life.5 Therefore, when depression and type 2 diabetes are comorbid, depression deters achievement of treatment goals.
The association of comorbid depression with poor glycemic control in patients with type 2 diabetes is common although this has not been a consistent finding in studies. It is thought to be bidirectional, where depression in diabetes leads to poor control and vice versa, but the causal pathways are not yet fully known. Fisher et al noted that there was lack of association between depression and glycemic control, which was probably due to diabetes-associated distress rather than presence or absence of depression, or by the increasing scores of the PHQ-9 depression tool.6
The other factors that contribute to poor glycemic control in patients with type 2 diabetes mellitus are multiple, including but not limited to poor adherence to medication,7,8 deteriorating disease,9,10 lack of treatment intensification,11,12 and poor self-care13,14 even in the absence of comorbid depression. However, the additional occurrence of depression would only compound the clinical care.
Our previous studies on ambulatory patients with diabetes, especially type 2 mellitus, demonstrated persistently poor (suboptimal) metabolic control15,16 in a large proportion of them. Although these surveys did not set out, a priori, to establish the specific causative factors, most patients on insulin-containing treatment were consistently not achieving their treatment goals.
Good glycemic control remains a key focus of diabetes therapy throughout the lifetime of the patients. Thus, any factor that impacts negatively on this goal should be looked for and remedial intervention instituted. Comorbid depression in patients with type 2 diabetes mellitus is one such factor. We hypothesized that the demands of diabetes therapy and need to achieve optimal glycemic control in the patients would cause or enhance depression.
Patients and methods
Study setting
This study was a descriptive cross-sectional design, conducted at the diabetes outpatient clinics in Kenyatta National Hospital. A systematic sampling method was used to recruit the subjects, wherein every second patient on the minor clinic day and every fourth on the major clinic day who met the inclusion criteria were selected.
Study participants: inclusion criteria
The subjects of study were patients of age ≥30 years with a documented diagnosis of type 2 diabetes of, or more than, 1 year, attending the diabetes clinic and who gave informed consent to participate. Participants who were able to speak and understand Kiswahili and/or English were selected.
Exclusion criteria
Patients with prior psychiatric illness other than depression, stroke, heart failure, overt kidney disease, and visual impairment, and who failed to give consent to participate in the study were excluded. Twelve patients with variable degrees of cognitive impairment and multiple overt comorbidities were excluded.
Study variables
The dependent variable was comorbid depression. The independent variables were sociodemographic and clinical characteristics, glucose-lowering therapies, and glycemic control by glycated hemoglobin (HbA1C).
Data collection instruments and methods
The key instrument used to collect sociodemographic and clinical data was Patient Health Questionnaire-9 (PHQ-9).17 It is a nine-item, self-reported questionnaire that scores per item: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day), and total scores 0–27, used to assess symptoms of and screen for depression. A score of 10 or higher had a sensitivity of 88% and specificity of 88% for detecting major depressive disorder (MDD). The higher the total score, the more severe is the depression. PHQ-9 has been validated locally and Kiswahili (a local language spoken by a large population, even those without formal education) version made available. The data collection was done face-to-face by trained clinical officers with Diploma in Clinical Medicine, supervised by one of the authors, KJE.
The participant was seated comfortably in a chair with back support, both feet flat on the floor. Blood pressure (BP) was then taken after brachial artery pulse was identified on the antecubital fossa, using mercury sphygmomanometer, in mmHg. The cuff was placed snugly on the arm with the inflatable inner bladder centered over the brachial artery and the lower edge of the cuff ~5 cm above the natural crease of the elbow. The participant was instructed to sit quietly without activity for 5 minutes; BP was then measured by manual inflation of the cuff and the Korotkoff sounds auscultated: systolic BP was taken at the appearance, and diastolic BP at the disappearance of Korotkoff sounds. After 1-minute rest time, BP measurement was repeated. The average of the two readings constituted the final BP. Electronic weighing scale was used for weight (kilograms) and a stadiometer to measure height (meters) vertically against the wall of the clinic, with participant standing without shoes.
Blood sample was drawn from the antecubital fossa, placed in ethylenediaminetetraacetic acid (EDTA)-primed bottle, then the specimens were stored at a temperature of 2°C–8°C. Analysis of blood samples was done after 4 weeks interval. The anticoagulated whole blood specimen was hemolyzed automatically on COBAS INTEGRA system with HBA1C reagent in the predilution cuvette for automated analysis of HbA1C.
Sample size determinations
Using the Cochran formula,18 with 95% confidence interval (CI), within a precision of 6.2%, and p the prevalence of 33% from an Ethiopian study.19 A sample size of 220 study participants was obtained primarily to determine the prevalence of comorbid depression.
Sampling procedure
The hospital has diabetes clinic running every day, and the main clinic runs once weekly on Fridays, where ~150–200 patients with diabetes are seen. Mini clinic runs on the other days of the week, but the number of patients with diabetes is fewer. About 85%–95% have type 2 diabetes and they formed the sampling frame. Every fourth patient on the main clinic day and every second patient on a mini clinic day were selected on fitting the inclusion criteria. Figure 1 is a flow chart that depicts the recruitment process of the study participants.
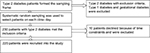  | Figure 1 A flow chart of subject recruitment into the study. |
Operational definitions
Depression was defined on a PHQ-9 score: above or equal to 10 was described as clinical depression.17 Depression was confirmed by a qualified psychiatrist.
Severity of depression was categorized by scores: moderate (10–14), moderately severe (15–19), and severe (20–27).
Body mass index (BMI) was calculated from weight in kilograms divided by height (meters) squared and expressed in kg/m2, then classified as underweight (<19.5 kg/m2), normal (20–24.9 kg/m2), preobese/overweight (25–29.9 kg/m2), and obese (≥30 kg/m2) (The International Obesity Task Force of WHO 2000).20
Blood pressure: A subject was considered to have hypertension if he/she had known before and was on BP-lowering drugs. However, for subjects with no prior history of hypertension, BP ≥140/90 mmHg was considered hypertensive (JNC 8 Guidelines 2014).21
Diabetes control: HbA1C ≤7% was categorized as good control and HbA1C >7% as poor/suboptimal control (ADA 2015 Recommendations).22 HbA1C was assayed by an automated COBAS INTEGRA machine using blood that was EDTA-anticoagulated.
Data quality control
The instrument used, PHQ-9, has been validated locally and found to be culturally sensitive. For this study, a pilot run was done by the research assistants to minimize interobserver errors.
Data analysis
The prevalence of comorbid depression was calculated and presented as a percentage with 95% CI. Sociodemographic attributes (gender, marital status) and clinical attributes (duration of diabetes, categories of treatment groups, comorbidity) were analyzed as categorical variables. The HbA1C values were summarized as mean (standard deviation [SD]) and used as either a continuous or categorical variable (of good or poor control). We compared mean values (SD) using Student’s t-test, while differences of variables across groups were analyzed using analysis of variance. We used Chi-square test to determine any associations with categorical data. We used binary logistic regression to identify the predictors of comorbid depression. The statistical tests were done at 5% level of significance where P value less than or equal to 0.05 was interpreted as significant.
Ethical considerations
Approval to conduct the study was obtained from both the Department of Clinical Medicine and Therapeutics and the University of Nairobi/Kenyatta National Hospital Research, and approval was obtained from the University of Nairobi/Kenyatta National Hospital Ethics Review Board (UoN/KNH-ERB) before data collection.
Patients gave written informed consent. Any significant clinical and laboratory findings such as abnormal BP, BMI, and HbA1C results were communicated to patients and their primary physicians for clinical decision-making in their management. Those who were found to have depression were referred to the mental health clinic for management. Blood samples were used only for the purpose of this study and were discarded after the study.
Results
A total of 220 patients with type 2 diabetes were recruited into this study and most of the study participants were aged 45–64 years with a mean age of 57.1±8.6 years. As shown in Table 1, majority were females (n=131, 59.5%). Over 85% were married, and the remaining proportion were classified as single, separated, or widowed. More than half (n=126, 57.3%) had some employment whether self-employed or been employed. A large proportion of the study participants had some form of education with either primary (n=86, 39.1%) or secondary education (n=84, 38.2%).
About half of the subjects (n=12, 50.9%) were paying their outpatient bills themselves. The patients’ access to health care services in this public health facility is cost-shared. Almost half (n=99, 45%) of the study participants had been diagnosed with diabetes for <5 years. More than half of the study population (n=118, 53.6%) were on oral antidiabetic drugs alone, while 36.4% were on combined oral antidiabetic drugs and insulin treatment, and the rest were on insulin-only glucose-lowering treatment. Majority of the study subjects (n=157, 71.4%) had hypertension (n=135, 61.3%) and had BMI ≥25 kg/m² where 36.8% were overweight and 24.5% were obese.
Discussion
Our study population had female predominance, but they were more disadvantaged in formal education and ability to access medicines. They were more obese and their glycemic control was poorer (Table 1). Current guidelines on care of diabetes recommend individualization approach to index cases to optimize the desired outcomes.22,23 This, therefore, requires that clinicians establish more than the sociodemographic attributes and clinical laboratory information. It is imperative that comorbid conditions to diabetes are determined for a more complete care.
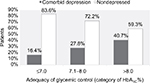
Comorbid depression is emerging as a frequent and important accompanying condition to type 2 diabetes. It affects self-care and the glycemic control of patients, yet it is rarely looked for in routine ambulatory care. Not many studies have explored the specific activities of self-care that are most affected by comorbid depression. Our study demonstrated rising proportion of patients with comorbid depression as the metabolic control worsened (Figure 2).
Gonzalez et al7 did a meta-analysis of studies on interactions of depression and diabetes self-care. They reported that the most affected activity was the missing of clinic appointments, but only minimal-to-moderate negative effect on medication adherence.
Our study found the prevalence of comorbid depression of 32.3%. The affected persons had overall poorer glycemic control, which worsened with severity of depression, and more so, in those on insulin therapy.
Gonzalez et al24 in their survey of 879 patients with type 2 diabetes in an outpatient setting explored the relationship of comorbid depression with self-care and impact on adherence to medication. They found a 2.3-fold increased odds of missing medications among those with major depression. They challenged the categorical diagnosis of depression as a composite of symptoms. Instead, they advocated for exploration of the specific symptoms of depression and their impact on nonadherence. Their study did not isolate the specific therapies for adherence evaluation, as either insulin-based or oral agents-only. However, we could infer that the pharmacokinetics of oral agents (long-acting) and insulin (relatively shorter-acting) make missing doses of insulin more unforgiving than oral agents, with poorer metabolic control as the end result.
Katon et al,25 in their cohort study of 4117 patients with type 2 diabetes, reported that depression was associated with poor adherence to medications, which explained the poor clinical and metabolic control that they observed, but not the lack of treatment intensification by clinical care providers.
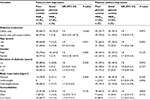
In our study, we noted that both groups of patients with and without comorbid depression, who were on oral antidiabetic agents, had similar mean HbA1C. But more importantly, we also found that the study patients with comorbid depression on insulin – either combined with oral agents or as sole therapy – had poorer glycemic control than those with and without comorbid depression on oral antidiabetic agents only (Table 2). Apparently, the sponsorship of treatment, either by self or other (a relative or insurance), was not significantly associated with comorbid depression, thus discounting financial constraints as a factor in comorbid depression in these study subjects (Table 3). Consequently, one would ask whether insulin therapy conferred undue burden to diabetes care on the patients who were using it.
Chao et al,26 Vijan et al,27 and Peyrot et al28 in the DAWN study did observe that need for insulin came with significant negative perceptions (and depressed mood) in those patients using it.
Others have recognized, as well, that higher levels of diabetes-related distress are significantly linked to elevated HbA1C.29,30 Our study patients on insulin therapy had quite high levels of HbA1C. Ascher-Svanum et al31 studied 985 patients with type 2 diabetes prospectively over 24 months, initiated them on insulin therapy, and examined them for depression, diabetes-related distress, and depressed mood. At the end of that study, glycemic control improved on insulin (as expected), the depressed mood declined, but the diabetes-related distress was unchanged, implying that insulin therapy worked well to control glycemia but did not reduce diabetes-related distress in patients who were using it. Rather, it may have enhanced it. Our study, however, did not look for specific distress signals in the subjects on insulin because the PHQ-9 tool used does not by design look for it.
Fisher et al32 did a longitudinal study on 506 patients with type 2 diabetes. They assessed them over 18 months for MDD, depressive symptoms, and diabetes-associated distress. They found no relationship between HbA1C as a measure of glycemic control and both MDD and depressive symptoms, but diabetes-associated distress was related to HbA1C both at cross-sectional and longitudinal levels. Fisher et al33 also noted that ~70% of patients with type 2 diabetes and high levels of diabetes-associated distress did not attain the criteria for depression on a Center for Epidemiological Studies Depression Scale. However, Niraula et al,34 in their cross-sectional study on 385 persons living with type 2 diabetes in Kathmandu, Nepal, reported that insulin use increased (by 2 points) the scores of depression on the Beck Depression Inventory 1978 version scale. These observations imply that an overlap occurs between diabetes-associated distress and depression. But, between diabetes-associated distress and depression, the main driver of poor metabolic control in such patients has not been determined.
Indeed, our study found deteriorating glycemic control (rising HbA1C) with increasing severity of depression and across the types of treatment: from 8.4% in oral antidiabetic agents-only, through 9.2% in combined orals-plus-insulin, to 9.9% in the insulin-only treatment group (Tables 3 and 4). It is probable that there were interactions between diabetes, depression, and treatment that our study would not unravel by its cross-sectional design. Although more people with longer duration of diabetes used insulin, their treatment choices were not significantly associated with severity of depression. Not surprising because the choices of their treatment were made without any knowledge of presence of depression (Table 4). One study by Aikens et al35 looked at patients with type 2 diabetes (103 on insulin-only and 155 on oral agents-only treatment). They found treatment regimen–depression interaction, which had a significant influence on the level of HbA1C attained, and also demonstrated a much stronger association of depression with insulin-based treatment but none with use of oral antidiabetic agents only.
The multivariate analysis showed that insulin-based treatment, either single or combined with oral agents, and female gender were the only significant determinants of presence of comorbid depression and poor glycemic control in our study subjects (Table 5).
Our study did not determine adherence to therapies by the subjects; however, some studies have reported that patients with type 2 diabetes have relatively low levels of adherence to insulin therapy.36,37 These studies did not look for comorbid depression, but they offer further explanation on why insulin-treated patients tend to have poorer glycemic control.
Snoek et al38 have opined that depression is a heterogeneous construct defined by the presence of specific symptoms over a specified duration while diabetes-related distress reflects an emotional response to the demands of diabetes care. These constructs, defined on a scale of validated instruments such as PHQ-9, overlap in patients with diabetes, yet they have unique differences and, probably, interventional requirements. As Snoek et al38 suggested, comorbid depression is probably a composite of major depression, depressive symptoms, and diabetes-associated distress that should be screened for with appropriate tools. The study by Aikens et al39 used PHQ-9 and analyzed their data both as symptoms and dichotomy of depression being present or absent. They found a significant relationship between glycemic control and change in depressive symptoms that was more pronounced in the insulin-treated patients than those on oral agents. It may not be a surprise, therefore, that intervention studies on comorbid depression to improve glycemic control in type 2 diabetes have not yielded uniform results.40–42
For case finding of comorbid depression, it may be more compelling to screen type 2 diabetes patients with poor glycemic control who are using insulin-containing regimens and then stratify them for successful treatment.
Acknowledgments
We are indebted to the academic staff of the Department of Clinical Medicine and Therapeutics who made critical review of the proposal before the study was undertaken. The authors are truly grateful to the nursing staff at the diabetes outpatient clinic who assisted at all stages of the study, especially facilitating the triage and recruitment of the study subjects. We are also indebted to Dorcas, Secretary of the Department of Clinical Medicine and Therapeutics, who spared time to type the final manuscript. Finally, our gratitude goes to the subjects who participated in the study voluntarily. As the authors, we declare that we did not have any special or competing interest served by this research and publication. This was self-funded by the authors JEK and CFO.
Author contributions
KJE: design of study, data collection, read the drafts, and made input. OFCF: design of the study, wrote all the drafts, and the final manuscript. KEM: design of study, read the drafts, and made input. OHV: design of the study, read the drafts, and made input on the aspects of mental health. JMD: design of study, read all the drafts, and made input. MK: participated in the design, sample size calculation, and statistical analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Albright TL, Parchman M, Burge SK; RRNeST Investigators. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33(5):354–360. | ||
Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. | ||
Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventative care. Diabetes Care. 2004;27(9):2154–2160. | ||
Georgiades A, Zucker N, Friedman KE, et al. Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosom Med. 2007;69(9):235–241. | ||
Lee H, Chapa D, Kao CW, et al. Depression, quality of life, and glycemic control in individuals with type 2 diabetes. J Am Acad of Nurse Practitioners. 2009;21(4):214–224. | ||
Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034–1036. | ||
Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment non-adherence: a meta-analysis. Diabetes Care. 2008;31(12):2398–2403. | ||
DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk Factor for non-compliance with medical treatment meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. | ||
UK Prospective Diabetes Study 16: Overview of six years’ therapy of type 2 diabetes: a progressive disease. Diabetes. 1995;44(11):1249–1258. | ||
Levy JC, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10 year-follow up of Belfast Diet study. Diabetic Med. 1998;15(4):290–296. | ||
Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risk of cardiovascular events in patients with Type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. | ||
Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of type 2 diabetes mellitus. Primary Care Diabetes. 2010;4(4):203–207. | ||
D’Souza MS, Karkada SN, Venkatesaperumal R, Natarajan J. Self-care behaviors and glycemic control among adults with type 2 diabetes. GSTF J Nursing Health Care (JNHC). 2015;2(1):29–40. | ||
Tan MY, Magarey J. Self-care practices of Malaysian adults with diabetes and sub-optimal glycaemic control. Patient Educ Couns. 2008;72(2):252–267. | ||
Otieno CF, Kariuki M, Ng’ang’a L. Quality of glycaemic control in ambulatory diabetics at Kenyatta National Hospital. East Afr Med J. 2003;80(6):406–410. | ||
Otieno CF, Vaghela V, Mwendwa FW, Kayima JK, Ogola EN. Cardiovascular risk factors in patients with type 2 diabetes mellitus in Kenya: levels of control attained at the outpatient diabetic clinic of Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82(12 Suppl):S184–S190. | ||
Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with PHQ-9: a meta-analysis. CMAJ. 2012;184(3):E191–E196. | ||
Cochran WG. Sampling Techniques. 3rd ed. New York, NY: John Wiley & Sons; 1977. | ||
Teklay G, Hussien J, Tesfaye D. Non-adherence and associated factors among type 2 diabetic patients at Jimma University specialized hospital, Southwest Ethiopia. J Med Sci. 2013;13(7):578–584. | ||
International Obesity Task Force of WHO 2000. Proposed classification of weight by body mass index (BMI). Int J Obes. 2004;28:152–158. | ||
James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. | ||
American Diabetes Association Standards of Medical care in Diabetes—2015. Diabetes Care. 2015;38:S1–S2. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycaemia in type 2 diabetes: a patient-centered approach: position statement of ADA and EASD. Diabetes Care 2012;35(6):1364–1379. | ||
Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30(9):2222–2227. | ||
Katon WJ, Russo J, Lin EH, et al. Diabetes and poor disease control: is co-morbid depression associated with poor medication adherence or lack of treatment intensification? Psychosom Med. 2009;71(9):965–972. | ||
Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depression symptoms and medication adherence in persons with diabetes. Res Social Adm Pharm. 2005;1(4):508–525. | ||
Vijan S, Hayward RA, Ronis DL, Hofer TP. Brief Report: the burden of diabetes therapy, implications for design of effective patient-centered treatment regimens. J Gen Intern Med. 2005;20(5):479–482. | ||
Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. | ||
Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–264. | ||
Fisher L, Hessler DM, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36(9):2551–2558. | ||
Ascher-Svanum H, Zagar A, Jiang D, et al. Associations between glycaemic control, depressed mood, clinical depression and diabetes distress before and after Insulin initiation: an exploratory, post Hoc analysis. Diabetes Ther. 2015;6(3):303–316. | ||
Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycaemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–28. | ||
Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–548. | ||
Niraula K, Kohrt BA, Flora MS, et al. Prevalence of depression and associated risk factors among persons with type 2 diabetes mellitus without a prior psychiatric history: a cross-sectional study in clinical settings in urban Nepal. BMC Psychiatry. 2013;13:309. | ||
Aikens JE, Perkins DW, Piette JD, Lipton B. Association between depression and concurrent type 2 diabetes outcomes varies by diabetes regimens. Diabetic Med. 2008;25(11):1324–1329. | ||
Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245. | ||
Gramer JA, Pugh MJ. Influence of insulin use on glycaemic control: how well do adults follow prescriptions for insulin? Diabetes Care. 2005;28(1):78–83. | ||
Snoek FJ, Bremmer MA, Hermanns N. Depression and diabetes 1. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol. 2015;3(6):450–460. | ||
Aikens JE, Perkins DW, Lipton B, Piette JD. Longitudinal analysis of depressive symptoms and glycaemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1177–1181. | ||
Markowitz S, Gonzalez JS, Wilkinson JL, Safren SA. Treating depression in diabetes: emerging findings. Psychosomatics. 2011;52(1):1–18. | ||
Huang Y, Wei X, Wu T, Chen R, Guo A. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260. | ||
Bogner HR, Morales KH, de Vries HF, et al. Integrated management of type 2 diabetes mellitus and depression treatment to improve medication adherence: a randomized controlled trial. Ann Fam Med. 2012;10(1):15–22. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.