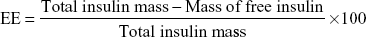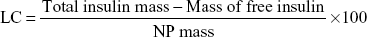Back to Journals » International Journal of Nanomedicine » Volume 12
Glucose- and temperature-sensitive nanoparticles for insulin delivery
Authors Wu JZ , Williams GR, Li HY, Wang D, Wu H, Li SD, Zhu LM
Received 22 January 2017
Accepted for publication 1 May 2017
Published 29 May 2017 Volume 2017:12 Pages 4037—4057
DOI https://doi.org/10.2147/IJN.S132984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Jun-Zi Wu,1 Gareth R Williams,2 He-Yu Li,1 Dongxiu Wang,3 Huanling Wu,1 Shu-De Li,4 Li-Min Zhu1
1College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai, People’s Republic of China; 2UCL School of Pharmacy, University College London, London, UK; 3Central Laboratory, Environmental Monitoring Center of Kunming, 4School of Basic Medical Sciences, Kunming Medical University, Kunming, People’s Republic of China
Abstract: Glucose- and temperature-sensitive polymers of a phenylboronic acid derivative and diethylene glycol dimethacrylate (poly(3-acrylamidophenyl boronic acid-b-diethylene glycol methyl ether methacrylate); p(AAPBA-b-DEGMA)) were prepared by reversible addition–fragmentation chain transfer polymerization. Successful polymerization was evidenced by 1H nuclear magnetic resonance and infrared spectroscopy, and the polymers were further explored in terms of their glass transition temperatures and by gel permeation chromatography (GPC). The materials were found to be temperature sensitive, with lower critical solution temperatures in the region of 12°C–47°C depending on the monomer ratio used for reaction. The polymers could be self-assembled into nanoparticles (NPs), and the zeta potential and size of these particles were determined as a function of temperature and glucose concentration. Subsequently, the optimum NP formulation was loaded with insulin, and the drug release was studied. We found that insulin was easily encapsulated into the p(AAPBA-b-DEGMA) NPs, with a loading capacity of ~15% and encapsulation efficiency of ~70%. Insulin release could be regulated by changes in temperature and glucose concentration. Furthermore, the NPs were non-toxic both in vitro and in vivo. Finally, the efficacy of the formulations at managing blood glucose levels in a murine hyperglycemic diabetes model was studied. The insulin-loaded NPs could reduce blood glucose levels over an extended period of 48 h. Since they are both temperature and glucose sensitive and offer a sustained-release profile, these systems may comprise potent new formulations for insulin delivery.
Keywords: diethylene glycol methyl ether methacrylate, 3-acrylamidophenylboronic acid, nanoparticle, thermosensitive, glucose sensitive, insulin delivery
Introduction
Diabetes is a highly prevalent chronic metabolic disease in which the body is unable to regulate effectively the concentration of glucose in the blood.1,2 It is at present one of the three major conditions that endanger human health (the others being cancer and cardiovascular disease).3 Over the past 20 years, the incidence of diabetes has increased sharply.4 The development of new and more effective treatment regimens is hence urgent.
In the clinic, insulin is very widely used as an efficient and low-cost intervention for diabetes.5,6 Unfortunately, being a biologic drug it is liable to undergo degradation and/or aggregation during formulation, which markedly reduces efficacy owing to the importance of tertiary structure. Although insulin can be administered through both injection and non-injection routes, the latter generally suffer from low bioavailability.7,8 However, daily administration of insulin by injection is uncomfortable for patients and can even be dangerous: the injection of excess insulin can result in serious shock responses such as hypoglycemia, syncope, or even death.9,10 To ameliorate these issues, much effort has been invested in the development of smart drug delivery systems that are able to release insulin in response to changes in the blood glucose level.
A significant number of reports of glucose-sensitive systems have emerged in the literature over the last decade or so.11–13 There are three main classes of glucose-responsive materials: concanavalin, glucose oxidase, and phenylboronic acid (PBA). Concanavalin and glucose oxidase are proteins and thus have some shortcomings in terms of high cost, instability, and antigenicity.14 In contrast, PBA has none of those disadvantages and has been widely studied.15,16 A diagram showing the reaction between PBA and glucose can be seen in the Supplementary materials, Scheme S1. Polymers based on PBA retain this glucose sensitivity; further, they generally possess high stability and can be structurally very versatile, and thus can be used to develop a broad range of systems.17,18 Unfortunately, glucose-sensitive PBA polymers face a number of challenges, including having shown some cytotoxicity. The pKa of PBA (~9.0) is much higher than the physiological pH, and thus, in the body, a PBA homopolymer is largely unionized.18 This renders it insoluble, and therefore it has only a minimal interaction with glucose in solution, limiting its ability to respond to changes in the concentration of the latter.
Researchers have adopted a range of approaches to overcome these drawbacks of PBA.19,20 One is to prepare polymers combining PBA with other monomers: this can result in polymers with more suitable pKa values (from 7.4 to 9.0) and better able to respond to glucose under physiological conditions.21 Polymers based on PBA can be used to make microparticles and hydrogels that can be loaded with insulin. For instance, Zhao et al22 prepared a new PBA-based glucose-responsive nanogel. They found that insulin could effectively be loaded into the gel, and insulin release was triggered by the presence of glucose. In addition, Sun et al23 reported poly(N-isopropylacrylamide-co-3-acrylamidophenylboronic acid-co-dextran-maleic acid)-coated silica nanoparticles (NPs) loaded with insulin, and found the cumulative release of insulin in vitro to be dependent on glucose concentration.
The administration of insulin can cause problems in that the body temperature tends to rise upon its administration, because it triggers the burning of “brown fat” cells.24 Diabetic patients are more likely to have a fever than those not suffering from this condition,25 and this increase in body temperature can be an issue because it triggers a loss of appetite.26 To counteract this, patients must reduce the amount of insulin injected or there is a risk of hypoglycemia. Controlling the insulin levels in the body through injection is thus challenging, and a dynamic drug delivery system able to respond to temperature and glucose would be highly desirable. There are some studies23 that report this in the literature (~10 articles), but there remains much more to be done given the potential of such materials to improve patient health and well-being. To impart thermoresponsive behavior, several authors have combined PBA with thermosensitive blocks in copolymers.27,28
Thermoresponsive polymers undergo a temperature-driven chain-to-globule transition and may be generally described by two threshold temperatures. They are water soluble either above an upper critical solution temperature (UCST) or below a lower critical solution temperature (LCST).29 LCST polymers can self-assemble into micelles above the LCST. These micelles will then dissociate into aqueous solution when the temperature is lowered beneath the LCST. There are a range of moieties that can be used to provide such behavior. One commonly explored material is diethylene glycol methyl ether methacrylate (DEGMA), homopolymers of which are both thermoresponsive and biocompatible. A range of drug delivery systems based on DEGMA have been reported, including micelles, hydrogels, NPs, and hybrid particles.30,31 Poly(DEGMA) exhibits an LCST between 22 and 27°C,32 but this can be tuned by copolymerization.
In this work, a series of novel glucose- and temperature-sensitive copolymers of DEGMA and the PBA derivative 3-acrylamidophenylboronic acid (AAPBA) were prepared for the first time. These were generated by reversible addition–fragmentation chain transfer polymerization. NPs of this polymer were prepared following the method reported by Chai et al33 and fully characterized. The particles were loaded with insulin, the loading efficiency was quantified, and the insulin release profile was determined in vitro. The toxicology of the NP systems was examined both in vitro and in vivo. Finally, the therapeutic effects of the formulations were assessed using a murine model of diabetes.
Materials and methods
Materials
AAPBA was purchased from the Beijing Pure Chem. Co., Ltd. (Beijing, China) and DEGMA (98%) and S-1-dodecyl-S′-(α,α′,-dimethyl-α″-acetic acid) trithiocarbonate (DDATC) from Sigma-Aldrich (Shanghai, China). Dimethyl formamide (DMF) and 2,2-azo-bis-isobutyronitrile (AIBN; 97%) were procured from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China); the latter was purified by recrystallization from a 5% w/v solution in water before use. Dimethyl sulfoxide (DMSO) was sourced from the China National Medicines Corporation Ltd. (Beijing, China). Insulin with a nominal activity of 28 IU/mg was obtained from the Xuzhou Wanbang Biochemical Co., Ltd. (Jiangsu, China). All solvents used in this work were of analytical grade and were dried by storing over activated 4 Å molecular sieves for 24 h prior to use. Water was triple distilled before use.
Polymer synthesis
Poly(3-acrylamidophenylboronic acid) (p(AAPBA))
p(AAPBA) was synthesized by reversible addition–fragmentation chain transfer (RAFT) polymerization using a method previously reported.34 This used AAPBA (1,000 mg) as the monomer, DDATC (10 mg) as the RAFT agent, AIBN (1 mg) as the initiator, and DMF (1 mL) as the solvent. This mixture was placed into a 25 mL reaction tube, sealed, and purged with nitrogen for 30 min. The tube was transferred to an oil bath preheated to 70°C, where it was left for 12 h. The product was then recovered by rapid cooling in ice water for 5 min. p(AAPBA) was obtained by precipitating into diethyl ether, washing with acetone, and drying under vacuum for 75 h.
Poly(3-acrylamidophenylboronic acid-block-diethylene glycol methyl ether methacrylate) (p(AAPBA-b-DEGMA))
RAFT copolymerization was performed using p(AAPBA) as a macro RAFT agent, together with DEGMA as an additional monomer and AIBN (1 mg) as the initiator. These were dissolved in DMF (1 mL). As before, the mixture was sealed in a 25 mL reaction tube, purged with nitrogen for 30 min, and heated at 70°C for 12 h in an oil bath. The reaction was then quenched by immediate cooling in ice water for 5 min, and the product polymers obtained after precipitation into diethyl ether, washing with acetone, and drying under vacuum. By changing the ratio of p(AAPBA) to DEGMA, five polymers were prepared, as detailed in Table 1.
Polymer characterization
1H nuclear magnetic resonance (1H NMR) spectra were recorded at 24°C using a Unity-plus 400 NMR spectrometer (Varian, Palo Alto, CA, USA). Infrared (IR) spectra were collected on an FTS-6000 instrument (Bio-Rad Co., Hercules, CA, USA) using the KBr tablet method (sample:KBr mass ratio 1:100). Measurements were taken over the range of 500–4,000 cm−1 at a resolution of 2 cm−1. Thermogravimetric (TG) analysis was conducted under a flow of nitrogen using an STA 409PC analyzer (Netzsch, Selb, Germany), at a heating rate of 10°C·min−1.
The molecular weights (Mw and Mn) and molecular weight distributions of the polymers were determined by gel permeation chromatography (GPC) measurements on a Waters LS system (Waters LLC, Milford, MA, USA). Tetrahydrofuran (THF) was used as the eluent, at a flow rate of 1.0 mL·min−1, with a column temperature of 35°C.
The LCSTs of the polymers were determined using a UV-vis spectrophotometer (Lambda 35; Perkin Elmer, Waltham, MA, USA) fitted with a thermostatically controlled cuvette. Measurements were undertaken at a heating rate of 0.5°C·min−1. The absorbance at 500 nm of aqueous solutions of each polymer (1.0 mg·mL−1) was recorded at different temperatures. The LCST was defined as the temperature corresponding to 50% of the maximum absorbance.
Preparation of NPs
p(AAPBA-b-DEGMA) NPs were prepared according to a previously reported method.33–35 A total of 10 mg of p(AAPBA-b-DEGMA) was dissolved in 2 mL of a mixed solvent system comprising DMSO and water (1:1 v/v), and 20 mL of water was added to the resultant solution under stirring (600 rpm). After 3 h, the suspension obtained was centrifuged at 12,000 rpm for 10 min and dispersed into water (10 mL), followed by transfer to a dialysis tube (molecular weight cutoff [MWCO] 6000 Da) and dialysis against water for 72 h (at room temperature). The organic solvent was removed by replacing the water every 4 h during the dialysis process. Subsequently, the materials were placed in a −80°C freezer for 12 h and then freeze-dried at −45°C (SJIA-10N instrument; Ningbo Dual Ka Instrument Co., Ltd. Ningbo, China) to yield blank polymer NPs.
Insulin-loaded p(AAPBA-b-DEGMA) NPs were prepared in a manner very similar to that used for blank NPs. As before, p(AAPBA-b-DEGMA) was dissolved in 2 mL of DMSO/water (1:1 v/v) to which was added 20 mL of an aqueous insulin solution under stirring (600 rpm). NP recovery and dialysis were then performed as for the blank NPs. The amount of free insulin in the supernatant was quantified by the Bradford method using a UV spectrometer (UV-1800; SHJH Company, Shanghai, China) at 595 nm.
The insulin encapsulation efficiency (EE) and loading capacity (LC) were calculated using the following equations:
|
|
NP characterization
Transmission electron microscopy (TEM) images were obtained using a JEM-2100 microscope (JEOL, Tokyo, Japan). Samples were prepared by casting an aqueous suspension of p(AAPBA-b-DEGMA) NPs (1 mg·mL−1) onto a copper grid covered with a thin film of formvar and carbon at 38°C. Zeta potential values were determined with a submicron particle size analyzer (ZetaPALS/90plus; Brookhaven Instruments Corporation, Holtsville, NY, USA).
NP responsiveness to stimuli
The blank p(AAPBA-b-DEGMA) NPs were evaluated for their pH, temperature, and glucose sensitivity following the methods used in a previous report.33 The pH sensitivity was assessed by placing the NPs (4 mg) in phosphate-buffered saline (PBS; 0.1 M, 10 mL) at pH 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, or 8.5 and determining their size by dynamic light scattering (DLS). Measurements were undertaken at 37°C using a Zetasizer Nano S Instrument (Malvern Instruments, Malvern, UK). Temperature sensitivity was studied in PBS (0.1 M, pH 7.4; NP concentration 0.4 mg·mL−1) by DLS quantification of the NP sizes at 12, 17, 22, 27, 32, 37, 42, and 47°C. Glucose sensitivity was explored by treating the blank NPs (4 mg) with glucose solutions (0, 1, 2, and 3 mg·mL−1) in PBS (pH 7.4, 0.1 M, 10 mL) for 0, 10, 20, 30, 40, 50, and 60 min at 22°C. The sizes of the NPs were subsequently measured by DLS.
In vitro release behavior
The in vitro release of the different insulin-loaded NPs was investigated according to a previously reported method.36 Insulin-loaded NPs (5 mg) were placed in 20 mL of a glucose solution in PBS (0.1 M, pH 7.4). Four different solutions with varied glucose concentrations (0, 1, 2, and 3 mg·mL−1) were employed. Release was measured under shaking (100 rpm) at 37°C. At predetermined times, 1 mL of supernatant was withdrawn, and the same volume of fresh pre-heated solution was added. The amount of free insulin was monitored using UV spectroscopy. Experiments were performed in triplicate, and the results are reported as mean ± standard deviation.
Toxicity
Cell viability assays
Cell viability was undertaken following a method similar to that used in the literature37 using NIH 3T3 cells (a non-cancerous mouse embryonic fibroblast cell line). The cell line was maintained in an incubator at 37°C with a 5% CO2 atmosphere. Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, New York, NY, USA) supplemented with 10% v/v fetal calf serum, 1% v/v non-essential amino acid solution, and 1% v/v penicillin/streptomycin solution (“complete DMEM”). Cells were seeded into 96-well plates at 10,000 cells per well (200 μL) and incubated for 24 h. One hundred microliters of a copolymer NP suspension (with concentrations ranging from 25 to 125 mg·mL−1) was then added to each well of the plates. After 24 h, the culture medium was removed and 20 μL of a solution of the MTT reagent in DMEM and 180 μL of complete DMEM were added to each well before the cells were incubated for a further 4 h. Subsequently, all the liquid medium was removed from each well and 100 μL of DMSO added to dissolve the formazan crystals that had formed. The plates were shaken at low speed for 10 min, after which the absorbance was quantified on a microplate reader (Multiskan Spectrum; Thermo Fisher Scientific, Waltham, MA, USA) at 570 nm. The cell viability was determined relative to a negative control of untreated cells (set to have 100% viability).
In vivo toxicity studies
All animal experimental procedures were undertaken in accordance with the Guidelines for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No 8523, revised 1985). Animals were procured from the Center of Laboratory Animal Science of Kunming Medical University. Ethical approval for all experiments was obtained from the Animal Care and Use Committee of Kunming Medical University (certificate number: KMMU 2014002) prior to in vivo work beginning.
A total of 24 Kunming mice (12 males and 12 females) weighing 19–23 g were randomly divided into four groups of six mice each: three treatment groups and an untreated control group. The treatment groups were given 10, 20, or 40 mg·kg−1·d−1 of p(AAPBA-b-DEGMA) by intraperitoneal (IP) injection (1 mL·kg−1·d−1). The control mice were given 1 mL·kg−1·d−1 of saline solution by IP injection. After 60 days, the mice were sacrificed and their blood was collected. Their red blood cell counts (RBCs), white blood cell counts (WBCs), serum protein, hematocrit, platelet, hemoglobin, serum creatinine, serum glutathione, total cholesterol, glucose, uric acid, alanine aminotransferase (ALT), and aspartate transaminase (AST) levels were quantified using an automated Olympus AU5400 biochemistry analyzer (Olympus, Tokyo, Japan). The livers, kidneys, spleens, hearts, and lungs of the sacrificed mice were collected for hematoxylin–eosin (HE) staining.
In vivo hypoglycemic experiments
Again, all animal experimental procedures were undertaken in accordance with the Guidelines for the Care and Use of Laboratory Animals of the US National Institutes of Health. Ethical approval for all experiments was obtained from the Animal Care and Use Committee of Kunming Medical University (certificate number: KMMU 2014002) prior to in vivo work beginning.
A total of 24 diabetes mellitus Kunming mice were kindly provided by Kunming Medical University. The fasting blood glucose level of each mouse was >15.6 mmol·L−1 at the start of the experiment, and they were randomly divided into a diabetes mellitus group, insulin injection group, and a p(AAPBA-b-DEGMA) NP treatment group. In addition, six healthy mice were used as a control group. The p(AAPBA-b-DEGMA) group received insulin-loaded PAD-5-1 NPs by a single IP injection (2.14 mg·kg−1; 0.4 mg insulin·kg−1). The insulin injection group was given a total of 0.4 mg·kg−1 of insulin in the 60 h experimental period. This was divided into two doses of 0.16 mg·kg−1 and a third dose of 0.08 mg·kg−1, given on days 1, 2, and 3, respectively. Each insulin dose was dissolved into 1 mL of normal saline for injection. The diabetes mellitus group was injected with 1 mL·kg−1 of saline solution IP. The control group animals were untreated. Blood was collected from the tail vein (50 μL) periodically, and a glucose meter (Accu-Chek Performa; Roche, Basel, Switzerland) was used to quantify blood glucose.
Results
Polymer synthesis and characterization
p(AAPBA-b-DEGMA) (PAD) glycopolymers were synthesized via a sequential RAFT polymerization method (Scheme S2, Supplementary materials) with good yields (Table 1). Figure 1 shows the IR spectra of AAPBA, DEGMA, p(AAPBA), and PAD-5-1. AAPBA shows a number of characteristic absorption bands: a C=O stretch (1,660 cm−1), C=C stretch (1,640 cm−1), O–B–O bend (1,352 cm−1), and B–O stretch (1,014 cm−1). Vibrations from the benzene ring are present at 1,555 to 1,610 cm−1 and the acrylamide group at 698 and 791 cm−1. DEGMA shows a C=O stretch at 1,700 cm−1 and a C=C stretch at 1,650 cm−1. In the spectra of p(AAPBA) and PAD-5-1 (shown as an exemplar copolymer), the absorptions due to C=C groups disappear, indicating that successful polymerization had occurred. Analogous observations were made for the other PAD systems. The Fourier transform infrared spectroscopy (FTIR) spectra of all the copolymers contain the B–O stretch at 996 cm−1, which proved that AAPBA was incorporated into the polymer. The complete set of IR spectra can be found in the Supplementary materials, Figure S1. These findings are fully consistent with other reports.37,38
Figure 2 depicts the 1H NMR spectra of DEGMA, AAPBA, p(AAPBA), and PAD-5-1. The spectrum of DEGMA (Figure 2A) can be assigned as follows (D2O): δ 6.05 (1H, H1), 5.65 (1H, H2), 4.25 (2H, H3), 3.71 (2H, H4), 3.62 (2H, H5), 3.46 (2H, H6), 3.24 (3H, H7), and 1.79 (3H, H8). That of AAPBA (Figure 2B) contains the following resonances (DMSO-d6): δ 10.09 (1H, H4), 8.10–7.25 (phenyl H), 6.47 (1H, H2), 6.20 (1H, H3), and 5.75 (2H, H1). The spectrum of p(AAPBA) (Figure 2C; NaOD/D2O, pH 9.5) has peaks at δ of 8.40–6.33 (phenyl H), 2.95 (2H, H10), 2.14 (1H, H4), 1.75 (1H, H9), 0.50–1.25 and 2.33 (C12H25 group, H1, H2, and H3). The copolymers, exemplified by PAD-5-1 in Figure 2D, all show analogous features. The signals in the PAD-5-1 spectrum (NaOD/D2O, pH 9.5) are assigned as follows: δ 8.10–7.15 (phenyl H), 3.60 (1H, H7), 3.42 (2H, H6), 2.90 (1H, H5), 2.75 (1H, H4), and 1.78 (2H, H3).
Comparing the spectra of the monomers AAPBA and DEGMA with those of the polymers, it is clear that peaks corresponding to the ethylene groups have disappeared in p(AAPBA) and PAD-5-1, confirming successful polymerization. There are some small differences in these spectra from those in other reports.34–37 This manifests mainly in the protons of the benzene ring in p(AAPBA) and PAD-5-1, which are in slightly different positions. This can likely be attributed to the solvent systems used for NMR: p(AAPBA) and p(AAPBA-b-DEGMA) are very insoluble in water at neutral pH, and thus, an NaOD solution in NaOH was used as the solvent, resulting in some peak shifts. Nevertheless, it is clear that polymerization was successful. Similar results are found for all the PAD polymers generated; the additional NMR spectra are given in Figure S2.
TG and derivative thermogravimetric (DTG) curves for p(AAPBA) and PAD-5-1 are given in Figure 3, and the remaining data are presented in Figure S3 (Supplementary materials). p(AAPBA) displays three mass losses at ~66°C, 360°C, and 412°C. PAD-5-1 has two mass losses at ~313°C and 386°C. For p(AAPBA), the first mass loss at 66°C is attributed to water adsorbed onto the polymer. The mass loss at 313–360°C corresponds to the thermal decomposition of pendent sugar residues.39,40 The last stage of mass loss is the thermal degradation of the backbone, which occurs at a lower temperature for PAD-5-1 than for p(AAPBA). These observations agree closely with the literature.34
The molecular weights of the polymers were determined by GPC and are summarized in Table 2. An increasing proportion of p(AAPBA) in the p(AAPBA-b-DEGMA) co-polymers leads to the Mw and Mn increasing, while the polydispersity index (PDI) is roughly constant. This agrees with a previous report for a vinylcaprolactam/AAPBA polymer.41
NP formation and characterization
Photographs of suspensions of the different p(AAPBA-b-DEGMA) NPs are provided in Figure 4, along with TEM images. The polymer NPs all form stable suspensions in water and comprise submicron-sized spherical particles. The particles appear to increase in size with increasing amounts of p(AAPBA) in the polymer.
The key physicochemical properties of the NPs are summarized in Table 3. The zeta potential of the NPs produced became increasingly negative as the p(AAPBA) content increased, in accordance with other work in the literature.42 As a result, the NP suspensions should become more stable. In contrast, a reduced proportion of DEGMA raised the LCST. All the NPs have low PDIs, ranging from 0.09 to 0.14, with no clear patterns in the data.
Figure 5 illustrates the changes in the NPs’ hydrodynamic diameters that arose following changes in temperature and pH values. AAPBA shows glucose sensitivity at high pH.13 However, the inclusion of DEGMA in the copolymer is expected to decrease the pKa of AAPBA,16 and it is necessary to examine whether the co-polymers are glucose sensitive under physiological conditions. The results obtained after exposing the NPs to PBS at different pHs (Figure 5A) show that the diameters of PAD-50-1 and PAD-20-1 decrease in size by 22 nm and 14 nm, respectively, as the pH is increased from 5 to 9. In contrast, the diameters of PAD-10-1, PAD-5-1, and PAD-2-1 increase by 12, 36, and 44 nm. The temperature data (Figure 5B) for all the NPs reveal that the diameter declines with an increase in temperature. This is most pronounced for PAD-50-1, as a result of it having the highest DEGMA content.
PBA derivatives can react with 1,2- or 1,3-diol compounds to form reversible five- or six-membered ring esters,43 and thus, in general, PBA-based polymers have good glucose sensitivity.44,45 This was explored for the PAD polymers prepared in this work. The diameters of the NPs in glucose solutions of various concentrations are illustrated in Figure 6. It is clear that the PAD-50-1 and PAD-20-1 NPs undergo minimal change in size as the glucose concentration rises from 0 to 3 mg·mL−1, even after immersion for an hour. This is likely to be because there is little of the glucose-sensitive AAPBA monomer in these polymers. The hydrodynamic diameters of the NPs of PAD-10-1, PAD-5-1, and PAD-2-1 increase gradually with the immersion time in glucose solution, however, showing them to have distinct glucose sensitivity. This increase in size becomes more profound as the glucose concentration is raised from 0 to 3 mg·mL−1. Concomitantly, the values of I/I0 (the light scattering intensity of the glucose-treated NP suspensions divided by the scattering intensity of the particles without glucose treatment) decrease gradually as the glucose concentration increases.
The increase in hydrodynamic diameter was greatest in the 3 mg·mL−1 glucose solution in all cases, reflecting the ability of the particles to take up glucose and expand. With the greater p(AAPBA) content going from PAD-50-1 to PAD-5-1, the hydrodynamic diameter increased more noticeably with the rising glucose concentration. PAD-2-1 behaves very similarly to PAD-5-1, which might be because steric hindrance means that not all the AAPBA groups in PAD-2-1 can react with the glucose present.
The stability of the NPs in aqueous solution was studied over 6 weeks (Figure S4A). No changes in size are seen, indicating that the p(AAPBA-b-DEGMA) NPs are stable in aqueous solution with no aggregation observed. The glucose sensitivity is found to be reversible (Figure S4B). All the NPs swelled after the first treatment with 3 mg·mL−1 glucose and then contracted back to their original size upon transfer to distilled water. When subsequently treated with a glucose solution, the NPs swell again.
Considering the earlier results, of the five different p(AAPBA-b-DEGMA) NPs, PAD-5-1 appeared to be the optimal system. PAD-5-1 and PAD-2-1 were the most glucose-sensitive materials, with both being very similar in this regard. PAD-5-1 was the more temperature sensitive of these two materials (ie, had a lower LCST) and further, since AAPBA is very expensive, is more cost-effective than PAD-2-1. PAD-5-1 was thus taken forward into further work.
Insulin loading and release
Insulin loading into a formulation is affected by hydrophobic and hydrophilic interactions, electrostatic interactions, and hydrogen bonding, inter alia.46 Since insulin contains amino acids, it can form stable complexes with the AAPBA sections of the PAD copolymers through these interactions,47 in accordance with other reports.48
PAD-5-1 was loaded with insulin using solutions of varied concentrations, and the results are summarized in Table 4. These data show that insulin was easily encapsulated into the PAD-5-1 NPs, with an LC of ~12%–19% and EE of ~67%–75%. Unsurprisingly, increasing the concentration of insulin in the solution used for loading leads to greater LCs but lower EEs. The loading observed in this work is higher than that in other reports: Guo et al34 reported an LC of ~10% and EE of ~60%, while Li et al39 obtained an LC of ~13%.
The drug release behavior of PAD-5-1 NPs loaded with the different insulin amounts (Table 4) was tested at different temperatures. Data for insulin loading (I-L)-4 are given in Figure 7, and the remaining data are presented in Figure S5 (Supplementary materials). In general, insulin release is slower at higher temperatures, with the formulation with lowest LC (I-L-1) showing the least sensitivity to temperature. The NPs are hence able to regulate their glucose release in response to changes in temperature.
The results suggest that if the body heats up as a result of insulin production, then the NPs will slow their release rate to counteract this change. Similarly, a reduction in body temperature will cause an increase in insulin release from the NPs. It should be noted that the temperature range explored is somewhat wider than physiological conditions, even in illness, but in extreme cases, the body temperature can exceed 40°C, and so, these experiments provide insight into how the systems would behave in conditions of high fever (more common in those with diabetes).
Drug release from I-L-4 was also explored in solutions of different glucose concentrations (see Figure 7B for data at 37°C). In all cases at 37°C, a burst of release is observed in the first hour, followed by sustained insulin release over at least the next 24 h. A plateau is seen after 36 h when the glucose concentration was 3 mg·mL−1 and a greater amount of insulin release obtained at higher glucose concentrations. This effect arises because as the glucose concentration is increased the NPs will swell, and as they do so, embedded insulin can diffuse out into solution. The glucose sensitivity is greatest at 37°C (see Figure S6 for data recorded at other temperatures), and insulin release slows with increasing temperature. The percentage release values observed and the release rates are broadly in agreement with the literature.49–51
It is thus clear that the NPs prepared in this work are able to respond to both changes in glucose concentration and temperature. If used in the treatment of diabetes, they would therefore be able to regulate insulin levels in response to these stimuli, helping to ensure that patients do not run the risk of hypoglycemia. The mechanism proposed for the insulin loading and glucose-responsive nature of the materials is summarized in Scheme S3 in the Supplementary materials.
In vitro cytotoxicity and animal toxicity testing
The cytotoxicity of the different p(AAPBA-b-DEGMA) NPs on NIH 3T3 cells was determined using the MTT method. The cells were exposed to the NPs at final concentrations from 8.33 to 41.7 mg·mL−1, and the results are given in Figure 8. After incubating for 24 h, the relative cell viability was close to or higher than 100% of the negative control (untreated cells) in all cases, indicating that the presence of the glycopolymer did not negatively impact cell viability. There is perhaps a slight tendency for the cell viability to be higher with the polymers with the lowest AAPBA content, but this is not particularly marked. The photos of NIH 3T3 cells in culture (Figure S7) show no changes in cell morphology after exposure to the NPs.
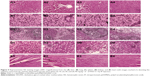
Although a helpful initial experiment, the MTT method cannot fully reflect the in vivo toxicity of the materials accurately, and hence following the promising results from MTT, it was necessary to conduct animal toxicity tests to determine whether the materials are safe in vivo. For animal toxicity, research has found that PBA has a certain level of toxicity: when given orally to rats, the LD50 is 740 mg·kg−1, and when applied to the abdominal cavity in mice, the LD50 is 320 mg·kg−1.52,53 This toxicity is likely to be affected by the incorporation of PBA into a copolymer; in vivo toxicity results for PAD-5-1 are presented in Figure 9 and Table 5. HE staining images of the livers, kidneys, hearts, spleens, and lungs of mice given the PAD-5-1 NPs by IP injection (Figure 9) show no obvious signs of damage after the 60-day treatment period. A quantification of blood biochemical markers (Table 5) also showed no obvious changes from the negative control group, demonstrating that the materials had no toxicity in vivo.
In vivo hypoglycemic studies
To quantify glucose levels, the blood of diabetes mellitus mice was periodically taken from the tail vein for analysis. Before treatment, the mice in the negative control group were lively and active, while the diabetic mice were listless. After treatment, the behavior of the control and treatment groups was much more similar.
The glucose levels recorded (Figure 10) show that there were no significant changes in glucose level over 60 h for the control and diabetic groups. In contrast, the glucose level decreases rapidly over the first hour after injection for the PAD-5-1 NP group and insulin injection group. Our results agree well with previously reported studies in this regard.47,49,54 The insulin-loaded NPs and injection-treated groups have very similar glucose levels from 0 to 48 h after treatment, but after 48 h, the former showed an increase in blood glucose levels. This suggests that the NPs induce a stable hypoglycemic effect in the body over 48 h, but after this time, their efficacy is decreased. In future studies, we will seek to optimize the formulations further to extend their duration of effectiveness.
A number of researchers have used insulin-loaded NPs for oral administration to mice or rats. Guo et al38 prepared a methacrylate and 3-acrylamidophenyl boronic acid-based amphiphilic glycopolymer and observed a significant decrease in blood glucose levels after oral administration. Other studies have also proven that orally administered NPs can effectively lower blood sugar levels in animals, and our results are consistent with those reported by Zhang et al21 and Sun et al.55 An AAPBA nanogel has additionally been explored for nasal administration.47 However, treatment of diabetes via oral or nasal administration is problematic because the durations of hypoglycemic efficacy are low, as Guo et al38 report – typically 12–24 h. Here, we find that we can at least double this time using our novel NPs and IP injection.
Conclusion
In this study, a series of new amphiphilic block glycopolymers based on PBA and diethylene glycol methyl ether methacrylate was synthesized. The Mw and Mn of the polymers increase with the proportion of boronic acid in the reaction mixture. The polymers could be assembled into stable spherical NPs, 50–250 nm in size, which exhibited glucose-sensitive behavior at physiological pH. When the NPs were immersed in glucose solutions, the particle size increased with the glucose concentration. They were also thermoresponsive, showing a distinct reduction in size when the temperature was increased through the LCST. Insulin could be encapsulated into the NPs, with an LC of ~12%–20%. The release of insulin was found to accelerate with an increase in the glucose concentration of the release milieu and to slow down with rising temperature. The biocompatibility of the polymers is very high, both in vitro and in vivo, indicating their potential for use in the biomedical fields. In addition, the polymer NPs were able to induce hypoglycemic effects in a murine model of diabetes, with efficacy maintained for 48 h. Overall, the data indicate that the glycopolymers developed in this work could be used as self-regulated insulin delivery systems in the future.
Acknowledgments
This investigation was supported by grant 16410723700 from the Science and Technology Commission of Shanghai Municipality, the National Natural Science Foundation of China (81360128), the Biomedical Textile Materials “111 Project” of the Ministry of Education of China (No B07024), and the UK-China Joint Laboratory for Therapeutic Textiles (based at Donghua University).
Disclosure
The authors report no conflicts of interest in this work.
References
Mukherjee A, Morales-Scheihing D, Butler PC, Soto C. Type 2 diabetes as a protein misfolding disease. Trends Mol Med. 2015;21(7):439–449. | ||
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. | ||
Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. | ||
Jovanovič L, Liang Y, Weng W, Hamilton M, Chen L, Wintfeld N. Trends in the incidence of diabetes, its clinical sequelae, and associated costs in pregnancy. Diabetes Metab Res Rev. 2015;31(7):707–716. | ||
Nolan CJ, Ruderman NB, Kahn SE, et al. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64(3):673–686. | ||
D’alessio D, Häring HU, Charbonnel B, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170–178. | ||
Ibraheem D, Elaissari A, Fessi H. Administration strategies for proteins and peptides. Int J Pharm. 2014;477(1):578–589. | ||
Zhao Y, Sun C, Shi F, et al. Preparation, characterization, and pharmacokinetics study of capsaicin via hydroxypropyl-beta-cyclodextrin encapsulation. Pharm Biol. 2016;54(1):130–138. | ||
Holger JS, Stellpflug SJ, Cole JB, Harris CR, Engebretsen KM. High-dose insulin: a consecutive case series in toxin-induced cardiogenic shock. Clin Toxicol. 2011;49(7):653–658. | ||
Barski L, Kezerle L, Zeller L, et al. New approaches to the use of insulin in patients with diabetic ketoacidosis. Eur J Intern Med. 2013;24(3):213–216. | ||
Karathanasis E, Bhavane R, Annapragada AV. Glucose-sensing pulmonary delivery of human insulin to the systemic circulation of rats. Int J Nanomedicine. 2007;2(3):501–513. | ||
Smith NB. Perspectives on transdermal ultrasound mediated drug delivery. Int J Nanomedicine. 2007;2(4):585–594. | ||
Song Y, Li Y, Xu Q, Liu Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomedicine. 2017;12:87–110. | ||
Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20(12):2555–2565. | ||
Wu J, Bremner DH, Li H, et al. Phenylboronic acid-diol crosslinked 6-O-vinylazeloyl-d-galactose nanocarriers for insulin delivery. Mater Sci Eng C. 2017;76:845–855. | ||
Zhang Y, Guan Y, Zhou S. Synthesis and volume phase transitions of glucose-sensitive microgels. Biomacromolecules. 2006;7(11):3196–3201. | ||
Ma R, Shi L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: synthesis and applications in drug delivery. Polym Chem. 2014;5(5):1503–1518. | ||
Ma R, Yang H, Li Z, et al. Phenylboronic acid-based complex micelles with enhanced glucose-responsiveness at physiological pH by complexation with glycopolymer. Biomacromolecules. 2012;13(10):3409–3417. | ||
Springsteen G, Wang B. A detailed examination of boronic acid–diol complexation. Tetrahedron. 2002;58(26):5291–5300. | ||
Jiang G, Jiang T, Chen H, et al. Preparation of multi-responsive micelles for controlled release of insulin. Colloid Polym Sci. 2015;293(1):209–215. | ||
Zhang Y, Liu K, Guan Y, et al. Assembling of gold nanorods on P (NIPAM–AAPBA) microgels: a large shift in the plasmon band and colorimetric glucose sensing. RSC Adv. 2012;2(11):4768–4776. | ||
Zhao L, Ding J, Xiao C, et al. Phenylboronic acid-functionalized polypeptide nanogel for glucose-responsive insulin release under physiological pH. J Control Release. 2015;213(1):e69. | ||
Sun L, Zhang X, Zheng C, et al. Glucose-and temperature-responsive core–shell microgels for controlled insulin release. RSC Adv. 2012;2(26):9904–9913. | ||
Jacobsson B, Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972;13(5):651–656. | ||
Pennachio DL, Rana-Mukkavilli G. Confusion and fever in a patient with diabetes. Patient Care. 2000;34(17):145. | ||
Baracos VE, Whitmore WT, Gale R. The metabolic cost of fever. Can J Physiol Pharmacol. 1987;65(6):1248–1254. | ||
Sood N, Bhardwaj A, Mehta S, Mehta A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23(3):748–770. | ||
Zhou M, Xie J, Yan S, et al. Graphene@ poly(phenylboronic acid) microgels with selectively glucose-responsive volume phase transition behavior at a physiological pH. Macromolecules. 2014;47(17):6055–6066. | ||
Peng CL, Chen YI, Liu HJ, Lee PC, Luo TY, Shieh MJ. A novel temperature-responsive micelle for enhancing combination therapy. Int J Nanomedicine. 2016;11:3357–3369. | ||
Nistor MT, Chiriac AP, Nita LE, Vasile C. Characterization of the semi-interpenetrated network based on collagen and poly (N-isopropyl acrylamide-co-diethylene glycol diacrylate). Int J Pharm. 2013;452(1–2):92–101. | ||
Asadian-Birjand M, Bergueiro J, Rancan F, et al. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym Chem. 2015;6(32):5827–5831. | ||
Sun K, Xu M, Zhou K, Nie H, Quan J, Zhu L. Thermoresponsive diblock glycopolymer by RAFT polymerization for lectin recognition. Mater Sci Eng C. 2016;68(5):172–176. | ||
Chai Z, Ma L, Wang Y, Ren X. Phenylboronic acid as a glucose-responsive trigger to tune the insulin release of glycopolymer nanoparticles. J Biomater Sci Polym Ed. 2016;27(7):599–610. | ||
Guo Q, Zhang T, An J, et al. Block versus random amphiphilic glycopolymer nanopaticles as glucose-responsive vehicles. Biomacromolecules. 2015;16(10):3345–3356. | ||
Zhao L, Xiao C, Wang L, Gai G, Ding J. Glucose-sensitive polymer nanoparticles for self-regulated drug delivery. Chem Commun. 2016;52(49):7633–7652. | ||
Brooks WLA, Sumerlin BS. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem Rev. 2015;116(3):1375–1397. | ||
Guo Q, Wu Z, Zhang X, Sun L, Li C. Phenylboronate-diol crosslinked glycopolymeric nanocarriers for insulin delivery at physiological pH. Soft Matter. 2014;10(6):911–920. | ||
Guo H, Li H, Gao J, et al. Phenylboronic acid-based amphiphilic glycopolymeric nanocarriers for in vivo insulin delivery. Polym Chem. 2016;7(18):3189–3199. | ||
Li W, Liu M, Chen H, et al. Phenylboronate-diol crosslinked polymer/SWCNT hybrid gels with reversible sol-gel transition. Polym Adv Technol. 2014;25(2):233–239. | ||
Jin J, Wang J, Sun P, et al. Hydrophilic interface-crosslinked polymer micelles: a platform for nanoreactors and nanocarriers. Polym Chem. 2013;4(16):4499–4505. | ||
Wu JZ, Bremner DH, Li HY, Sun XZ, Zhu LM. Synthesis and evaluation of temperature-and glucose-sensitive nanoparticles based on phenylboronic acid and N-vinylcaprolactam for insulin delivery. Mater Sci Eng C. 2016;69(32):1026–1035. | ||
Hasegawa U, Inubushi R, Uyama H, Uematsu T, Kuwabata S, van der Vlies AJ. Mannose-displaying fluorescent framboidal nanoparticles containing phenylboronic acid groups as a potential drug carrier for macrophage targeting. Colloids Surf B Biointerfaces. 2015;136:1174–1181. | ||
Barba V, Cuahutle D, Ochoa ME, et al. Two new complexes of phenylboronic acid with N-salicylidene-4-aminobutanol. Inorganica Chim Acta. 2000;303(1):7–11. | ||
Cheng C, Zhang X, Wang Y, et al. Synthesis of glucose-sensitive block glycopolymers based on phenylboronic acid via RAFT polymerization. J Control Release. 2011;152:e267–e269. | ||
Wang D, Liu T, Yin J, et al. Stimuli-responsive fluorescent poly (N-isopropylacrylamide) microgels labeled with phenylboronic acid moieties as multifunctional ratiometric probes for glucose and temperatures. Macromolecules. 2011;44(7):2282–2290. | ||
Wang Y, Zhang X, Han Y, Cheng C, Li C. pH-and glucose-sensitive glycopolymer nanoparticles based on phenylboronic acid for triggered release of insulin. Carbohydr Polym. 2012;89(1):124–131. | ||
Zheng C, Guo Q, Wu Z, et al. Amphiphilic glycopolymer nanoparticles as vehicles for nasal delivery of peptides and proteins. Eur J Pharm Sci. 2013;49(4):474–482. | ||
Jin X, Zhang X, Wu Z, et al. Amphiphilic random glycopolymer based on phenylboronic acid: synthesis, characterization, and potential as glucose-sensitive matrix. Biomacromolecules. 2009;10(6):1337–1345. | ||
Wu Z, Zhang X, Guo H, et al. An injectable and glucose-sensitive nanogel for controlled insulin release. J Mater Chem. 2012;22(42):22788–22796. | ||
Yao Y, Zhao L, Yang J, Yang J. Glucose-responsive vehicles containing phenylborate ester for controlled insulin release at neutral pH. Biomacromolecules. 2012;13(6):1837–1844. | ||
Sinha A, Chakraborty A, Jana NR. Dextran-gated, multifunctional mesoporous nanoparticle for glucose-responsive and targeted drug delivery. ACS Appl Mater Interfaces. 2014;6(24):22183–22191. | ||
Lee IP, Sherins RJ, Dixon RL. Evidence for induction of germinal aplasia in male rats by environmental exposure to boron. Toxicol Appl Pharmacol. 1978;45(2):577–590. | ||
Heindel JJ, Price CJ, Field EA, et al. Developmental toxicity of boric acid in mice and rats. Fundam Appl Toxicol. 1992;18(2):266–277. | ||
Yao Y, Shen H, Zhang G, Yang J, Jin X. Synthesis of poly (N-isopropylacrylamide)-co-poly (phenylboronate ester) acrylate and study on their glucose-responsive behavior. J Colloid Interface Sci. 2014;431:216–222. | ||
Sun L, Zhang X, Wu Z, et al. Oral glucose-and pH-sensitive nanocarriers for simulating insulin release in vivo. Polym Chem. 2014;5(6):1999–2009. |
Supplementary materials
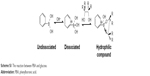  | Scheme S1 The reaction between PBA and glucose. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.