Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Glucocorticoid Alleviates Mechanical Stress-Induced Airway Inflammation and Remodeling in COPD via Transient Receptor Potential Canonical 1 Channel
Authors Wu X, Jia B, Luo X, Wang J, Li M
Received 4 May 2023
Accepted for publication 6 August 2023
Published 25 August 2023 Volume 2023:18 Pages 1837—1851
DOI https://doi.org/10.2147/COPD.S419828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Xiaojuan Wu,1 Baolin Jia,2 Xiaobin Luo,1 Jing Wang,3 Minchao Li3
1Department of Respiratory and Critical Care Medicine, Suining Central Hospital, Suining, Sichuan, 629000, People’ s Republic of China; 2Department of Oral and Maxillofacial Surgery, Suining Central Hospital, Suining, Sichuan, 629000, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, 400010, People’s Republic of China
Correspondence: Jing Wang; Minchao Li, Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Chongqing Medical University, 76 Linjiang Road, Chongqing, 400010, People’s Republic of China, Tel +86 023 62887761 ; +86 023 62887749, Email [email protected]; [email protected]
Background: Increased airway resistance and hyperinflation in chronic obstructive pulmonary disease (COPD) are associated with increased mechanical stress that modulate many essential pathophysiological functions including airway remodeling and inflammation. Our present study aimed to investigate the role of transient receptor potential canonical 1 (TRPC1), a mechanosensitive cation channel in airway remodeling and inflammation in COPD and the effect of glucocorticoid on this process.
Methods: In patients, we investigated the effect of pathological high mechanical stress on the expression of airway remodeling-related cytokines transforming growth factor β 1 (TGF-β 1), matrix metalloproteinase-9 (MMP9) and the count of inflammatory cells in endotracheal aspirates (ETAs) by means of different levels of peak inspiratory pressure (PIP) under mechanical ventilation, and analyzed their correlation with TRPC1. Based on whether patients regularly used inhaled corticosteroid (ICS), COPD patients were further divided into ICS group (n = 12) and non-ICS group (n=15). The ICS effect on the expression of TRPC1 was detected by Western blot. In vitro, we imitated the mechanical stress using cyclic stretch and examined the levels of TGF-β 1 and MMP-9. The role of TRPC1 was further explored by siRNA transfection and dexamethasone administration.
Results: Our results revealed that the TRPC1 level and the inflammatory cells counts were significantly higher in COPD group. After mechanical ventilation, the expression of TGF-β 1 and MMP-9 in all COPD subgroups was significantly increased, while in the control group, only high PIP subgroup increased. Meanwhile, TRPC1 expression was positively correlated with the counts of inflammatory cells and the levels of TGF-β1 and MMP-9. In vitro, mechanical stretch significantly increased TGF-β 1 and MMP-9 levels and such increase was greatly attenuated by TRPC1 siRNA transfection and dexamethasone administration.
Conclusion: Our results suggest that the increased TRPC1 may play a role in the airway inflammation and airway remodeling in COPD under high airway pressure. Glucocorticoid could in some degree alleviate airway remodeling via inhibition of TRPC1.
Keywords: chronic obstructive pulmonary disease, COPD, transient receptor potential canonical 1, TRPC1, airway inflammation, airway remodeling, mechanical stress
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex disease of the lung that is characterized by progressive airflow limitation caused by chronic inflammation and airway narrowing. Evidence has suggested that chronic airway inflammation was characterized by infiltration of the various inflammatory cells including neutrophils, macrophages, and lymphocytes into the airway and lung tissue, which was involved in the pathogenesis and progression of COPD.1–3 Airway remodeling is another critical feature of COPD, characterized by epithelial injury, thickening of the basement membrane or subepithelial fibrosis, mucous gland and goblet cells hyperplasia, increased smooth muscle mass and angiogenesis.4 Many cytokines such as transforming growth factor β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) play a major role in airway remodeling by induce the epithelial mesenchymal transition (EMT) and extracellular matrix deposition.5
Following epithelial injury, epithelial cells transformed into mesenchymal ones which was known as the airway epithelial–mesenchymal transition.6 EMT directly participates in the thickening of wall of airways which results in the airway remodeling in COPD.7 Researches have confirmed that MMP-9 paly an important role in mediating EMT in COPD through tissue remodeling by the degrading basement membrane collagen and extracellular matrix protein.8 Recent study demonstrated that MMP-9 and the progression of EMT were increased following exposure to cigarette smoke both in vivo and in vitro models.9 Another study also shown that TGF-β1 was a key factor in the EMT in the pathogenesis of COPD.10
The non-reversible narrowing of airways in COPD and the accompanied bronchoconstriction as well as the excessive mucus secretion result in the pathological upregulation of mechanical stress in the airway.11 High mechanical stress influences a variety of essential pathophysiological functions in the lung via mechanotransduction.12 Recent studies have shown that mechanical stress caused by the pathological high airway pressure could contribute to airway remodeling and induce airway inflammatory response.11–13 However, the underlying specific molecular mechanism between airway pressure and airway remodeling remains not fully deciphered.
The mammalian transient receptor potential (TRP) superfamily of cation channels is widely distributed throughout the body and is activated by a diverse range of chemical and physical stimuli including mechanical stress, light, thermal stimulation and sound.14 Transient receptor potential canonical 1 (TRPC1), one of these superfamilies, is an important mechanosensitive cation (MscCa) in vertebrates and plays a critical role in converting the sensed mechanical stimuli into biological signals by transuding stretch into Ca2+ flux across the cell membrane.15 Calcium is an important intracellular second messenger involved in various cellular processes regulation including some cytokines release such as fibroblast growth factor (FGF-2), TGF-β1 and MMP-9.16–18 Our previous study has demonstrated that mechanical stretch activated TRPC1 channels in bronchial epithelial cells which played an important role in bronchial wall thickness, smooth muscle hyperplasia and hypertrophy, extracellular matrix (ECM) deposition.19 In addition, study reported that TRPC1 was abundantly expressed in airway epithelial cells of patients with COPD,20 and we speculated that TRPC1 channel might play an important role in the airway remodeling and the airway inflammation in COPD induced by increased mechanical stress. Our present study aimed to investigate whether the increased mechanical stretch upregulated the expression of TGF-β1 and MMP-9, the airway remodeling associated cytokine, and the counts of inflammatory cells, and explore the role of TRPC1 in these processes, which could provide a novel therapeutic strategy for COPD. We further analysed the effects of glucocorticoid on airway remodeling induced by stretch.
Materials and Methods
Subjects
We recruited 60 subjects to participate in our experiment: 27 subjects with stable COPD and 33 control subjects. These participants who were with gallstone or gallbladder polyp and required elective abdominal surgery were presented to the Second Clinical Hospital of Chongqing Medical University from December 2016 to December 2017 and to the Suining Central Hospital from June 2018 to June 2020. The diagnosis of COPD was based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.21 These patients did not experience any exacerbation and were not with respiratory tract infection at least one month before admission. Based on whether patients regularly used inhaled corticosteroid (ICS), budesonide (0.5mg twice daily) or fluticasone (100μg twice daily), COPD patients were further divided into ICS group (n = 12) and non-ICS group (n = 15). Patients in ICS group received ICS treatment for more than 4 weeks. Exclusion criteria included: 1) a history of coexisting pulmonary diseases, such as bronchiectasis, cystic fibrosis, interstitial lung disease, or lung cancer; 2) α-1-antitrypsin deficiency, 3) immunodeficiency disease. The control group consisted of patients who had not any pulmonary diseases.
All the patients were intubated and ventilated invasively in a pressure-controlled mode, with an inspiratory oxygen fraction (FiO2) concentration of 50–60%, inspiratory: expiratory ratio of 1:2 and a frequency of 12–20 min−1. According to the level of peak inspiratory pressure (PIP), COPD and control group were, randomly and respectively, divided into high PIP (>25 cmH2O, n = 8 and n = 10) subgroup, moderate PIP (20–25 cmH2O, n = 9 and n = 12) subgroup, and low PIP (<20 cmH2O, n = 10 and n = 11) subgroup. Positive end expiratory pressure (PEEP) in all the groups was set at 5 cmH2O. In total, 72 patients were included in this study. Twelve patients did not finish the study protocol because of unforeseeable hypoxemia and further adjustment of ventilator settings. Once tidal volume or oxyhemoglobin saturation were unable to maintain normality under our ventilator parameters setting, the patients would terminate the experiment. This study was approved by Ethics Committees of the Second Clinical Hospital of Chongqing Medical University and the Suining Central Hospital, and all participants gave written informed consent.
Reagents
16HBE cells were purchased from the American Type Culture Collection (ATCC). Lipofectamine RNAiMAX reagent was acquired from Invitrogen (USA). RPMI 1640 and 10% fetal bovine serum were from Gibco, USA. 100 units/mL penicillin and 100 mg/mL streptomycin were from Boster, China. ELISA kits of TGF-β1 and MMP-9 were purchased from R&D Systems, Minneapolis, MN, USA. TRPC1 siRNAs were obtained from Ribo Biotechnology Co, Ltd. SYBR PremixEX Taq was purchased from Takara Biotechnology. The bicinchoninic acid (BCA) assay kit, Cell counting kit-8 assay and Fluo‑4 AM were from Beyotime Institute of Biotechnology and Trizol reagent was acquired from Invitrogen. Rabbit anti-human TRPC1 antibody, rabbit anti-human TGF-β1 antibody, rabbit anti-human MMP-9 antibody and anti-human GAPDH monoclonal antibody were purchased from Abcam and R&D Systems, secondary antibody goat anti-rabbit IgG conjugated horseradish peroxidase was obtained from Santa Cruz Biotechnology.
Endotracheal Aspirates (ETAs) Collection and Processing
All the ETAs were collected by trained nurses using aseptic techniques according to a standardized procedure before and after 2 hours of mechanical ventilation. A 50 cm suction catheter (Dare, 20192080031, ShanXi) was introduced into the endotracheal tube and driven forward until a resistance occurred, then it was pulled back for 1 cm, 5 mL of sterile 0.9% saline solution instilled and the sample was aspirated by gentle suction into a plastic 15 mL Falcon tube. The ETA samples were immediately labeled and stored at −80°C for further analysis.
Each ETA was filtered using a clean funnel and a sterilized nylon gauze (4 × 4 cm2, pores 52 µM), transferred to a 1.5 mL Eppendorf and centrifuged for 15 min (at 4°C, 1500 rpm/min). The resulting pellet was resuspended in phosphate buffer saline (PBS) and loaded on glass slides using a cytocentrifuge. Cytospin slides were prepared for each sample, air-dried for 30 min and stained with May-Grünwald-Giemsa. Four hundred cells per cytospin were examined under light microscopy.
Protected Specimen Brushing Sample Collection
To obtain the bronchial epithelial cells, all patients accept bronchoscopy-guided protected specimen brush (PSB). After bronchoscope introduced into the endotracheal tube, the PSB catheter (Anrei, LAMHCB-2012-2010, HangZhou) was advanced into subsegmental bronchus of right middle lobe. The brush was advanced 2–3cm beyond of the catheter, then into a wedge position with slowly rotation and retracted into the inner cannula. The whole unit was removed after sample collection. Subsequently, the PSB was immersed in PBS and the bronchial epithelial cells were washed out of the brush. Then the solution was centrifuged for 10 mins at 3000r/min and the cells mass was stored at −80°C for further Western blot and RT-qPCR to detect the TRPC1 protein and mRNA level.
Cell Culture and Treatment
The 16HBE cells were cultured in RPMI-1640 with 10% fetal bovine serum, 100 units/mL penicillin, and 100 mg/mL streptomycin and incubated at 37°C in a humidified water‑jacketed incubator containing 95% air and 5% CO2. Cells from passages three to six were used in the next experiment. Cultured cells were divided into 4 groups: a control group, a mechanical stretch group, a mechanical stretch+TRPC1 siRNA group, a stretch+NC siRNA group. Cells in the control group were cultured on similar plates in the same incubator without any additional intervention, whereas cells in the mechanical stretch group were subjected to sinusoidal stretching for 1.5 h at a frequency of 60 cycles per min at a 15% elongation using a FX‑4000 Flexcell tension system (Flexcell International Corp.) (see the mechanical stretch system section below for detailed explanation). To inhibit the activities of TRPC1, 16HBE cells were transfected with TRPC1 siRNA/NC siRNA before mechanical stretch. Furthermore, 16HBE cells were pretreated with Dexamethasone (Dex) at concentrations of 100 μM for 24 h.
Cell Viability Assay
The viabilities of Dex treatment on 16HBE cells were detected by the Cell Counting Kit-8 assay. 16HBE cells were seeded into 96-well plate with 1×105 cells per well and added with Dex for 24 h. The CCK-8 reagent (10μL) was added into each well diluted by 100μL RPMI 1640 medium and incubated for 1 hour at 37°C. After that, the optical density of each well at 450 nm was measured using Microplate Reader (Bio-Rad iMark Microplate Reader, Hercules, CA, USA).
Transient Transfection with TRPC1 siRNA
16HBE cells were seeded in a 6-well plate at a density of 2 × 105 wells in medium containing 10% fetal bovine serum and incubated overnight. These cells were then cultured in serum‑free medium before being divided into the control group, the TRPC1 siRNA group and the NC siRNA group.16HBE cells were transfected with 100 pmol TRPC1 siRNA or NC siRNA group using Lipofectamine RNAiMAX reagent according to the manufacturer’s recommendations. At 6 hours after transfection, the cells were fed with medium containing 10% fetal bovine serum and incubated at 37°C for another 48 h. The Western blot analysis was then performed to determine the efficiency of TRPC1 knockdown.
Mechanical Stretch System
In the experiments, the 16HBE cells were stimulated using a FX-4000 Flexcell tension system (Flexcell International Corp., USA) as previously described to imitate the pathological condition which was associated with airway mechanical stress.22 The cells were first seeded in Flexcell biaxial 6-well plates coated with type I collagen (Flexcell International Corp., USA) at a density of 4×105 cells per well in medium containing 10% fetal bovine serum for 24 hours. Secondly, the cells were cultured in a serum-free medium and divided into control group, a mechanical stretch group. Cells in the control group were cultured in the same incubator without any additional intervention, whereas cells in the mechanical stretch group were subjected to a sinusoidal cyclic stretch of 15% elongation at a frequency of 60 cycles per min using a Flexcell FX-4000 Tension System for 1.5h. Levels of TGF-β1, MMP-9 in the culture supernatant and cell lysates were verified by ELISA and RT‑PCR.
Intracellular Calcium Measurement
16HBE cells were seeded at a density of 2 × 105 cells/well in complete growth medium in a 6-well plate for 24h. The following day, cells were treated with 100μM Dex for 24 h, and then cells in the stretch group were under mechanical stretch system for 1.5h. After cells were washed three times with PBS without Ca2+. Subsequently, all cells were incubated with 2.5μmol/L Fluo-4AM for 30 min at 37°C in the dark, then washed 3 times with PBS without Ca2+ to remove extracellular Fluo-4AM. Images were obtained using an EVOS M5000 microscope (Invitrogen by Thermo Fisher Scientific). The intensity of calcium fluorescence was quantified by Image-J software.
ELISA Assay for TGF-β1, MMP-9
After ETAs and the supernatant of cultured cells centrifuged for 15 mins at 1500r/min in 4°C, the supernatants were for analysis by ELISA. The concentrations of TGF-β1, MMP-9 in ETAs were determined using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA). ELISAs were performed according to the manufacturer’s instructions. The minimal detectable concentrations were 31.2ng/L for TGF-β1 and 0.30 μg/L for MMP-9.
Western Blotting
The bronchial epithelial cells were lysed in RIPA lysis buffer (Roche Diagnostics, Basel, Switzerland). The supernatant was collected after homogenates were centrifuged at 12,000 × g at 4°C. Protein concentrations were measured using BCA Protein Assay Kit. Afterwards, proteins were separated by 10% SDS-PAGE and then transferred to the polyvinylidene difluoride (PVDF) membranes. The membranes were blocked by tris-buffered saline (TBS) with 5% nonfat milk at room temperature for 1 h. Then the membranes were cut according to molecular size and incubated with antibodies specific for rabbit anti-human TRPC1 (1:1000 dilution), rabbit anti-human MMP-9 antibody (1:1000 dilution), rabbit anti-human TGF-β1 antibody (1:1000 dilution) and anti-human GAPDH monoclonal antibody (1:1000 dilution) in the blocking buffer overnight at 4°C. With continuous shaking, blots were washed with 0.1% Tris-buffered saline with Tween 3 times and subsequently incubated with goat anti-rabbit IgG conjugated horseradish peroxidase (1:1000 dilution) for 1 h. The blots were visualized using electrochemical luminescence assay kit (Millipore, Bedford, MA, USA). The densitometric evaluation was carried out using Quantity One software and the relative quantity of proteins was expressed as the ratio of the gray scale respective to that of GAPDH.
RT-qPCR Analysis
Total RNA was extracted from bronchial epithelial cells by using Trizol reagent (Invitrogen, CA, USA). Extracted RNA was reverse transcribed to cDNA using a reverse transcription kit (Takara, China). TRPC1 forward, 5’-GAAGATTTTGGGAAATTTCTGG −3’, TRPC1 reverse, 5’-CTTATCCTCATGTTTGCTAT-3’, TGF-β1 forward,5-CGACTCGCCAGAGTGGTTAT-3′, TGF-β1 reverse, 5’-CAGTAGTGAACCCGTTGACCT-3’, MMP-9 forward, 5’-CAACTCTGGAGGTTCGAC-3’, MMP-9 reverse, 5’-CATTCACGCGCCAGTAGCCG-3’, GAPDH forward 5’-AATCCCATCATCACCATCTTCCA-3’, GAPDH reverse 5’-CCTGCTTCACCACCTTCTTGAGG-3’. RT-qPCR was performed using the SYBR Premix Ex TaqTM II PCR kit (Takara, China). The reaction condition was incubated at 95°C for 3 min, followed by 40 cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 30 sec, and then elongation at 72°C for 30 sec. GAPDH was selected for normalization. The relative expression of TRPC1 was calculated by using the 2−ΔΔCt.
Statistical Analysis
Data are expressed either as mean±SD or absolute numbers. The Student’s t-test or chi-square test was used to assess differences between the groups. One-way ANOVA with Tukey-Kramer post-test was used for statistical comparison in more than two groups of variables. The correlation between variables was assessed by Spearman’s rank correlation coefficient. P values were judged significant if they were less than 0.05. All data analyses were performed using SPSS version 17.0.
Results
Clinical Parameters
The lung function and clinical characteristics of all patients included in this study are listed in Table 1. There were significant differences in FEV1/FVC%, FEV1%pred, FEV1, FVC, PEF25%, PEF75% parameters between the control group and COPD group (Table 1).
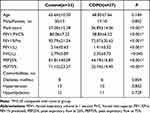 |
Table 1 Characteristics of Patients |
Cellular Composition in the ETAs
The counts of total cells, macrophages, eosinophils, lymphocytes as well as neutrophils are shown in Table 2. The total cells, macrophages, lymphocytes and neutrophils in ETAs of COPD group were greater than those in control group. However, the count of eosinophils in ETAs showed no significant difference between the control group and COPD group (Table 2).
 |
Table 2 Endobronchial Aspiration Total Cells Count and Cells Differential Count (×106 Cells/ML, Mean±SD) |
TRPC1 Levels in Bronchial Epithelial Cells
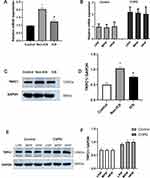
RT-qPCR and Western blotting analysis were used to quantify the expression of TRPC1 mRNA and protein in bronchial epithelial cells. We observed that the COPD group showed significantly higher levels of both mRNA and protein of TRPC1 than control group. Additionally, we also found that the expression levels of TRPC1 were higher in Non-ICS subgroup than ICS subgroup (P<0.01, respectively, Figure 1A, C and D). However, there was no difference in subgroups respectively in control group and COPD group before mechanical ventilation (P> 0.05, Figure 1B, E and F).
The Expression of TGF-β1 and MMP-9 in ETAs
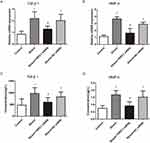
To investigate whether mechanical ventilation contribute to influence the factors of airway remodeling, we performed ELISA to measure the protein levels of TGF-β1, MMP-9 in ETAs. As shown in Figure 2, before mechanical ventilation, the levels of TGF-β1 (control vs COPD, 228.12±73.56 vs 407.74±95.58, P < 0.001) and MMP-9 (control vs COPD, 1.86±0.88 vs 6.27±1.73, P < 0.001) in COPD group are significantly higher than that in control group, and there are no differences for TGF-β1 or MMP-9 expression in subgroups of control or COPD group (all P>0.05). After 2 hours of mechanical ventilation, the expressions of TGF-β1 and MMP-9 were significantly upregulated (P<0.05) in HPIP control subgroup but not in LPIP or MPIP control subgroups. In addition, TGF-β1 and MMP-9 protein levels in all the LPIP, MPIP and HPIP COPD subgroups were marked increased after 2 hours of mechanical ventilation, moreover, both protein levels of TGF-β1 and MMP-9 in HPIP COPD subgroup were significantly higher than those in MPIP and LPIP COPD subgroups (P<0.05, Figure 2). These data demonstrate that mechanical pressure can upregulate the levels of airway remodeling-related cytokines.
Correlation Analysis
Results of Spearman rank correlation analysis indicated that the expression of TRPC1 protein and mRNA in bronchial epithelial cells was negatively correlated with FEV1% pred in COPD group (r = −0.582, r = −0.476, all P<0.05, Figure 3). However, TRPC1 protein and mRNA levels were positively correlated with the counts of macrophages (r = 0.538 and r = 0.647), lymphocytes (r = 0.486 and r = 0.556) and neutrophils (r = 0.599 and r = 0.604) in ETAs in COPD group (all P<0.05, Figure 4). Furthermore, a positive correlation between TRPC1 protein levels and the levels of TGF-β1 and MMP-9 (r = 0.464, r = 0.613, all P<0.05, in LPIP group; r = 0.478, r = 0.677, all P<0.05, in MPIP group; r = 0.597, r = 0.546, all P<0.05, in HPIP group) was observed after mechanical ventilation (Figure 5).
TRPC1 siRNA Decreased the TRPC1 Protein Expression in 16HBE Cells
The knockdown efficiency was determined by Western blot analysis. We observed that TRPC1 protein levels dramatically suppressed in the16HBE cells stably transfected with TRPC1 siRNA than untransfected 16HBE cells (P<0.01, Figure 6). However, there was no significant difference between the control group and the NC siRNA transfection group (P>0.05, Figure 6).
Genetic Knockdown of TRPC1 in 16HBE Cell Attenuates the Mechanical Stretch-Induced TGF-β1 and MMP-9 Upregulation
RT-qPCR and ELISA analysis were used to quantify changes in the mRNA and protein levels of TGF-β1 and MMP-9 in 16HBE cells. As shown in Figure 7, compared with control group, levels of both mRNA and protein of TGF-β1 and MMP-9 (P<0.001, respectively) in the mechanical stretch group are increased. Further analysis revealed that these increases in mRNA and protein levels of TGF-β1 and MMP-9 after mechanical stretch were significantly attenuated by transfection of TRPC1 siRNA, whereas the transfection of NC siRNA had no effect on the mRNA or protein level of these cytokines (Figure 7).
Toxicity of Dexamethasone
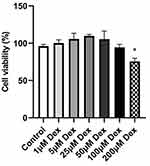
To investigate the effect of Dex on cell proliferation, 16HBE cells were treated with different concentrations of Dex (1, 5, 25, 50, 100, 200μM) and incubated for 24h, respectively. It was found that the cellular viability of 16HBE cells were efficiently reduced at 200μM (Figure 8). Thus, Dex concentrations of 100 µM were utilized in the further experiment.
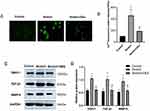
Dex Reduced Calcium Levels and the Levels of Airway Remodeling-Associated Cytokines Induced by Mechanical Stress in 16HBE Cells
We examined the Dex effect on calcium levels in 16HBE under mechanical stress. Results shown that mechanical stress increased intracellular Ca2+ levels compared with the control group, while the fluorescence intensity in the 16HBE cells was significantly reduced after treatment with 100μM Dex (Figure 9A and B). To explore the effect of Dex on the expression levels of airway remodeling-associated cytokines, the expressions of TGF-β1 and MMP-9 were determined by Western blotting. As shown in Figure 9, compared to the control group, the expression levels of TRPC1, TGF-β1 and MMP-9 are increased underwent mechanical stress. However, the expression levels of these proteins were decreased treatment with Dex, compared with the stress group (Figure 9C and D). These results suggested that Dex might protect against airway remodeling via TRPC1 channels.
Discussion
Excessive mechanical stress to the bronchial epithelial cells is one of the most common characters of the chronic inflammatory airway diseases, such as COPD, with the well-established features of airway remodeling and chronic airway inflammation.23 It has been shown that the upregulated mechanical stress further aggravated airway remodeling24 and airway inflammation.12,25 Thus, an in-depth insight into the regulatory mechanisms of mechanical stress in airway remodeling and airway inflammation is crucial for improving the prognosis of patients with COPD. In our study, by means of mechanical ventilation and FX-4000 Flexcell tension system in patients and in vitro, we investigated the effect of the pathological high mechanical pressure in COPD on the airway remodeling and airway inflammation, we found that PIP could upregulate the expression of airway remodeling-related cytokines TGF-β1 and MMP-9 in COPD, and the level of TRPC1 was significantly increased and positively correlated with the level of TGF-β1, MMP-9 as well as the count of macrophages, lymphocytes and neutrophils. Our further result in vitro demonstrated that the increased TGF-β1, MMP-9 expression induced by high mechanical stretch was mediated by TRPC1. Our results suggested that the increased TRPC1 plays a crucial role in the aggravated airway inflammation and airway remodeling in COPD due to increased airway mechanical stress, thus, this molecule may serve as a novel therapeutic target for the progression of COPD.
The regular respiratory cycle causes the airway epithelium to stretch and relax; high stress amplifies this impact and changing the physiology of the lungs. In actuality, airway stretching affects lung function and the release of airway mediators, which may then result in a potentially pathological response. Mechanosensitive cation channels (MscCa) transduce membrane stretch into biochemical and biomolecular signaling and play a key role in various physiological and pathological processes associated with mechanical stress including cell-volume regulation, cell locomotion, muscle dystrophy and cardiac arrhythmias. TRPC1 functions as a mechanosensitive channel that mediates Ca2+entry in response to increased membrane stretch. Our previous study has demonstrated that TRPC1 was localized at the basal surface of airway epithelial cells and was significantly upregulated in patients with COPD,19 and furthermore, the increased mechanical stretch induced obvious upregulation of TRPC1 in 16HBE cells and remarkable increased intracellular Ca2+ levels.26 Consistent with Xu’s study,20 our present study also found that the level of TRPC1 in bronchial epithelia was significantly higher in patients with COPD and was negatively correlated with FEV1%pred. Taken together, these results indicated the role of increased expression of TRPC1 in the progression of COPD under increased mechanical stress.
Airway remodeling is one of the most important pathological features of COPD, which leads to poor clinical outcomes.27 Thus, a better mechanistic understanding of airway remodeling in COPD can significantly improve prognosis. MMP-9 and TGF-β1 are essential factors in airway remodeling. TGF-β1 promotes the differentiation and proliferation of fibroblasts to myofibroblast cells and also increases the deposition of epithelial–mesenchymal transition.28,29 In addition, it was shown that TGF-β1 induced the proliferation of airway smooth muscle (ASM) cells, which contributed to airway remodeling.30 Furthermore, MMP-9 play an important role in extracellular matrix metabolism which is an essential factor for the thickening and fibrosis of small airway in COPD.31 The activated MMPs degrade extracellular fibrous proteins, including type IV collagen, laminin and alveolar basement membrane,32 which could lead to airway remodeling. Our study found that the levels of TGF-β1 and MMP-9 in ETAs of COPD patients were higher than that in control group. Interestingly, Xu F et al found similar variability in their study, compared with the control group, the bronchoalveolar lavage fluid (BALF) levels of TGF-β1, MMP-9 in the COPD group significantly increased.33 This higher expression of airway remodeling-related factors in COPD would lead to further development of airway remodeling, which eventually aggravate irreversible airflow limitation and the decline of lung function.
In the meanwhile, previous research has showed that intracellular Ca2+ was essential for TGF-β1 expression.16 It has also been reported that the Ca2+ entry through TRPV4 activation promoted MMP-2, MMP-9 release and activation.17 As a mechanosensitive Ca2+ permeable channel, TRPC1 has been reported to be activated by mechanical stretch and subsequently induced a remarkable increase in intracellular Ca2+ level. Thus, TRPC1 may play a key role in the occurrence of airway remodeling in response to mechanical stretch. Transfection of siRNA targeting TRPC1 was used in our present study to confirm essential role of TRPC1 in mechanical stretch which further induced the expression of TGF-β1 and MMP-9. We concluded that the mechanisms underlying these changes were mediated, at least in part, by a TRPC1-mediated intracellular Ca2+ increase, since transfection with TRPC1 siRNA abolished these alterations in TGF-β1 and MMP-9 expression induced by mechanical stress.
In addition, in patients experiment, we found that the expression of TGF-β1 and MMP-9 were upregulated in all the COPD subgroups but only in HPIP control subgroup, and the higher the peak airway pressure, the higher levels of TGF-β1 and MMP-9. Considering the increased TRPC1 level in COPD group and the positive correlation between the TRPC1 level and the expression of TGF-β1 and MMP-9, it indicated that TRPC1 plays a key role in the high airway pressure-induced upregulation of airway remodeling related factors TGF-β1 and MMP-9, and the higher the TRPC1 level, the more sensitive to the high airway pressure.
Several inflammation cells, such as macrophages, neutrophils and lymphocytes, play an important role in chronic airway inflammation as well as in airway remodeling.34–38 Neutrophil-derived chemokines such as IL-1 and IL-8 are proven to be involved in airway remodeling,3 and neutrophil elastase-induced FGF-2 prompt smooth muscle hyperplasia that contribute to airway remodeling.35 Lymphocytes and macrophages release a gradient of inflammatory cytokines which involves in goblet cell hyperplasia, increase in vascular permeability, mucus secretion and collagen deposition which play a significant role in airway remodeling as well.28–30 Our experiment demonstrated that the total cells, macrophages, lymphocytes as well as neutrophils in ETAs were significantly higher in COPD group than those in the control group and they were positively correlated with the level of TRPC1 in ETAs. Interestingly, previous experiments revealed a reduced number of leukocytes, eosinophils, and macrophages in the BALF of TRPC1 deficient mice,39 suggested a crucial role of TRPC1 in the lung inflammation. This was further supported by our result that the expression levels of TRPC1 were positively correlated with macrophages, lymphocytes and neutrophils concentrations.
The percent predicted of the FEV1 (FEV1% pred) is the most commonly used measurement to evaluate the severity and prognosis of COPD.40 It has been known that lower FEV1% pred was associated with higher rate of exacerbations and mortality.41,42 This present study demonstrated a negative correlation between TRPC1 level and the values of FEV1% pred, which indirectly indicated the adverse effect of high level of TRPC1 in COPD.
Our previous study suggested the application of budesonide, one of the most widely used inhaled corticosteroid (ICS), resulted in decreased TRPC1 expression at both the mRNA and protein levels.18 This was consistent with our present study that COPD patients in ICS subgroup showed both lower mRNA and protein levels of TRPC1 than that in the non-ICS subgroup. In addition, treatment with Dex decreased the production of TRPC1, Ca2+ and airway remodeling-associated cytokines induced by mechanical stress in vitro study. Above all, we inferred that the increase of airway remodeling-associated cytokine in COPD patients was related to the upregulation of TRPC1 and glucocorticoid may be useful in the inhibition of this process by regulation of TRPC1 levels.
The limitations of this study include the lack of results from lung tissues in control patients and COPD patients, thus, we were unable to explore the expression of TGF-β1 and MMP-9 in lung samples. Moreover, our study demonstrated that mechanical pressures increased Ca2+ flux and airway remodeling cytokines. More studies are needed to explore the detailed mechanisms of Ca2+ regulating airway remodeling factors under stretch stimulated. Finally, the sample size of this study was relatively small. To verify the impact of mechanical stress on COPD, a multicenter investigation with larger samples is required.
Conclusion
In summary, our study has revealed that increased mechanical stress in COPD airway could increase the expression of airway remodeling-related cytokines MMP-9 and TGF-β1 as well as the counts of airway inflammatory cells through upregulation of TRPC1. Thus, TRPC1 may become a novel therapeutic target for the treatment in COPD and glucocorticoid could alleviate airway remodeling via inhibition of TRPC1.
Ethics Approval and Consent to Participate
The study was conducted following the Declaration of Helsinki and has been approved by the Ethics Committee of Suining Central Hospital (No. LSLH20180001) and the Second Affiliated Hospital of Chongqing Medical University (No. 2014(65)), and all participants signed informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgments
We would like to acknowledge the members in the Division of Hepatobiliary and Pancreas Surgery of Suining Central Hospital, the Second Affiliated Hospital of Chongqing Medical University and the support from the hospitals in supporting this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81270102) and Chongqing medical scientific research project (Joint project of Chongqing Health Commission and Science and Technology Bureau, grant no.2022MSXM013).
Disclosure
The authors declare that they have no competing interests.
References
1. Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. 2019;54(2):1900651. doi:10.1183/13993003.00651-2019
2. Rao W, Wang S, Duleba M, et al. Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis. Cell. 2020;181(4):848–864. doi:10.1016/j.cell.2020.03.047
3. Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348. doi:10.2147/COPD.S176122
4. Li Y, Zhang L, Polverino F, et al. Hedgehog interacting protein (HHIP) represses airway remodeling and metabolic reprogramming in COPD-derived airway smooth muscle cells. Sci Rep. 2021;11(1):9074. doi:10.1038/s41598-021-88434-x
5. Cabrera-Benitez NE, Parotto M, Post M, et al. Mechanical stress induces lung fibrosis by epithelial-mesenchymal transition. Crit Care Med. 2012;40(2):510–517. doi:10.1097/CCM.0b013e31822f09d7
6. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112(12):1776–1784. doi:10.1172/JCI20530
7. Hou W, Hu S, Li C, et al. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: a Possible Link between COPD and Lung Cancer. Biomed Res Int. 2019:2025636. doi:10.1155/2019/2025636
8. Sohal SS, Mahmood MQ, Walters EH. Clinical significance of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease(COPD): potential target for prevention of airway fibrosis and lung cancer. Clin Transl Med. 2014;3(1):33. doi:10.1186/s40169-014-0033-2
9. Liu YN, Guan Y, Shen J, et al. Shp2 positively regulates cigarette smoke-induced epithelial mesenchymal transition by mediating MMP-9 production. Respir Res. 2020;21(1):161. doi:10.1186/s12931-020-01426-9
10. Mahmood MQ, Reid D, Ward C, et al. Transforming growth factor (TGF) β1 and Smad signalling pathways: a likely key to EMT-associated COPD pathogenesis. Respirology. 2017;22(1):133–140. doi:10.1111/resp.12882
11. Suki B, Sato S, Parameswaran H, Szabari MV, Takahashi A, Bartolak-Suki E. Emphysema and mechanical stress-induced lung remodeling. Physiology. 2013;28(6):404–413. doi:10.1152/physiol.00041.2013
12. Santus P, Pecchiari M, Tursi F, Valenti V, Saad M, Radovanovic D. The Airways’ Mechanical Stress in Lung Disease: implications for COPD Pathophysiology and Treatment Evaluation. Can Respir J. 2019;3546056. doi:10.1155/2019/3546056
13. Gosens R, Grainge C. Bronchoconstriction and airway biology: potential impact and therapeutic opportunities. Chest. 2015;147(3):798–803. doi:10.1378/chest.14-1142
14. Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honore E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010;460(3):571–581. doi:10.1007/s00424-010-0847-8
15. Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7(2):179–185. doi:10.1038/ncb1218
16. Jeong HJ, Hong SH, Park RK, An NH, Kim HM. Ethanol induces the production of cytokines via the Ca2+, MAP kinase, HIF-1alpha, and NF-kappaB pathway. Life Sci. 2005;77(17):2179–2192. doi:10.1016/j.lfs.2005.04.014
17. Villalta PC, Rocic P, Townsley MI. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307(8):L652–659. doi:10.1152/ajplung.00113.2014
18. Zhang X, Zhou Z, Wang D, et al. Activation of phosphatidylinositol-linked D1-like receptor modulates FGF-2 expression in astrocytes via IP3-dependent Ca2+ signaling. J Neurosci. 2009;29(24):7766–7775. doi:10.1523/JNEUROSCI.0389-09.2009
19. Li N, He Y, Yang G, Yu Q, Li M. Role of TRPC1 channels in pressure-mediated activation of airway remodeling. Respir Res. 2019;20(1):91. doi:10.1186/s12931-019-1050-x
20. Xu F, Liu XC, Li L, Ma CN, Zhang YJ. Effects of TRPC1 on epithelial mesenchymal transition in human airway in chronic obstructive pulmonary disease. Medicine. 2017;96(43):e8166. doi:10.1097/MD.0000000000008166
21. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2015 updated. Available from: http://goldcopd.org/GoogleScholar.
22. Yu Q, Li M. Effects of transient receptor potential canonical 1 (TRPC1) on the mechanical stretch-induced expression of airway remodeling-associated factors in human bronchial epithelioid cells. J Biomech. 2017;51:89–96. doi:10.1016/j.jbiomech.2016.12.002
23. Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol. 2006;68:563–583. doi:10.1146/annurev.physiol.68.072304.113102
24. Tschumperlin DJ, Drazen JM. Mechanical stimuli to airway remodeling. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S90–94. doi:10.1164/ajrccm.164.supplement_2.2106060
25. Hara J, Fujimura M, Ueda A, et al. Effect of pressure stress applied to the airway on cough-reflex sensitivity in Guinea pigs. Am J Respir Crit Care Med. 2008;177(6):585–592. doi:10.1164/rccm.200703-457OC
26. Wang J, He Y, Yang G, Li N, Li M, Zhang M. Transient receptor potential canonical 1 channel mediates the mechanical stress induced epithelial mesenchymal transition of human bronchial epithelial (16HBE) cells. Int J Mol Med. 2020;46(1):320–330. doi:10.3892/ijmm.2020.4568
27. Page C, O’Shaughnessy B, Barnes P. Pathogenesis of COPD and Asthma. Handb Exp Pharmacol. 2017;237:1–21. doi:10.1007/164_2016_61
28. Soltani A, Sohal SS, Reid D, Weston S, Wood-Baker R, Walters EH. Vessel-associated transforming growth factor-beta1 (TGF-beta1) is increased in the bronchial reticular basement membrane in COPD and normal smokers. PLoS One. 2012;7(6):e39736. doi:10.1371/journal.pone.0039736
29. Zhang C, Zhu X, Hua Y, et al. YY1 mediates TGF-β1-induced EMT and pro-fibrogenesis in alveolar epithelial cells. Respir Res. 2019;20(1):249. doi:10.1186/s12931-019-1223-7
30. Chen M, Zhang W, Shi J, Jiang S. TGF-β1-Induced Airway Smooth Muscle Cell Proliferation Involves TRPM7-Dependent Calcium Influx via TGFβR/SMAD3. Mol Immunol. 2018;103:173–181. doi:10.1016/j.molimm.2018.09.015
31. Milara J, Peiro T, Serrano A, Cortijo J. Epithelial to mesenchymal transition is increased in patients with COPD and induced by cigarette smoke. Thorax. 2013;68(5):410–420. doi:10.1136/thoraxjnl-2012-201761
32. Oikonomidi S, Kostikas K, Tsilioni I, Tanou K, Gourgoulianis KI, Kiropoulos TS. Matrix metalloproteinases in respiratory diseases: from pathogenesis to potential clinical implications. Curr Med Chem. 2009;16(10):1214–1228. doi:10.2174/092986709787846587
33. Xu F, Lin J, Cui W, et al. Scutellaria baicalensis Attenuates Airway Remodeling via PI3K/Akt/NF-kappaB Pathway in Cigarette Smoke Mediated-COPD Rats Model. Evid Based Complement Alternat Med. 2018;2018:1281420. doi:10.1155/2018/1281420
34. Huang S, Wang J, Liu F, Dong L. Alternatively activated macrophages promote airway inflammation through JAK3-STAT5-Fra2 in asthma. Inflamm Res. 2022;71(7–8):873–885. doi:10.1007/s00011-022-01585-z
35. Ogawa H, Azuma M, Tsunematsu T, et al. Neutrophils induce smooth muscle hyperplasia via neutrophil elastase-induced FGF-2 in a mouse model of asthma with mixed inflammation. Clin Exp Allergy. 2018;48(12):1715–1725. doi:10.1111/cea.13263
36. Lee YG, Jeong JJ, Nyenhuis S, et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 2015;52(6):772–784. doi:10.1165/rcmb.2014-0255OC
37. Zhang X, Kohl J. A complex role for complement in allergic asthma. Expert Rev Clin Immunol. 2010;6(2):269–277. doi:10.1586/eci.09.84
38. Khan MA, Assiri AM, Broering DC. Complement mediators: key regulators of airway tissue remodeling in asthma. J Transl Med. 2015;13:272. doi:10.1186/s12967-015-0565-2
39. Yildirim E, Carey MA, Card JW, et al. Severely blunted allergen-induced pulmonary Th2 cell response and lung hyperresponsiveness in type 1 transient receptor potential channel-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;303(6):L539–549. doi:10.1152/ajplung.00389.2011
40. Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi:10.1164/ajrccm.163.5.2101039
41. Seemungal TA, Wedzicha JA. Exacerbation frequency and FEV1 decline of COPD: is it geographic? Eur Respir J. 2014;43(5):1220–1222. doi:10.1183/09031936.00046014
42. Menezes AM, Perez-Padilla R, Wehrmeister FC, et al. FEV1 is a better predictor of mortality than FVC: the PLATINO cohort study. PLoS One. 2014;9(10):e109732. doi:10.1371/journal.pone.0109732
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.