Back to Journals » Infection and Drug Resistance » Volume 16
Genome Analysis of ST1 Bartonella henselae, a Zoonotic Pathogen Causing Endocarditis in an Elderly Patient in China
Authors Mu X, Liang J, Qian L, Zhou B, Zou X , Fu Y, Zhu Y, Li X , Shi J
Received 15 June 2023
Accepted for publication 29 August 2023
Published 11 September 2023 Volume 2023:16 Pages 6079—6084
DOI https://doi.org/10.2147/IDR.S422345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Sandip Patil
Xinli Mu,1,* Jianghong Liang,2,* Linyan Qian,3 Bing Zhou,4 Xuehan Zou,5 Ying Fu,6 Yongze Zhu,2 Xi Li,2 Jiana Shi7
1Department of Infectious Diseases, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Laboratory Medicine Center, Department of Clinical Laboratory, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, People’s Republic of China; 3Heart Center, Department of Cardiovascular Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, People’s Republic of China; 4Heart Center, Department of Cardiovascular Surgery, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, People’s Republic of China; 5Center for General Practice Medicine, Department of Infectious Diseases, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 6Department of Clinical Laboratory, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China; 7Center for Clinical Pharmacy, Cancer Center, Department of Pharmacy, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xi Li, Laboratory Medicine Center, Department of Clinical Laboratory, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, 310014, People’s Republic of China, Tel/Fax +86-571-8589-3267, Email [email protected] Jiana Shi, Center for Clinical Pharmacy, Cancer Center, Department of Pharmacy, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, Zhejiang, 310014, People’s Republic of China, Tel +86-571-8589-3899, Email [email protected]
Abstract: Infective endocarditis (IE) is a rare disease but with high associated mortality. Currently, the mainstays of diagnosis are still echocardiography and blood cultures. Here, we reported a case of infective endocarditis with negative blood cultures, and blood and aortic valve tissue metagenomic next-generation sequencing (mNGS) results suggested Bartonella henselae. In addition, we obtained the whole genomic sequence of B. henselae ZJBH strain. To our knowledge, this is the first report of B. henselae genomic analysis isolated from clinic in China. Furthermore, we described the whole genome sequencing (WGS) data incorporating all B. henselae from diverse sources worldwide and shed light on underlying risk of B. henselae transmitted between cats and humans.
Keywords: infective endocarditis, Bartonella henselae, metagenomic next-generation sequencing
Introduction
Infective endocarditis (IE), as a disease first recognized in 1885, is defined by infection of a native or prosthetic heart valve, the endocardial surface, or an indwelling cardiac device.1–3 Despite advances in diagnostic capabilities and treatment options, IE remains a rare condition but with high associated mortality. And epidemiological studies have shown that 1-year mortality in IE has not improved over the past 20 years.4–6 One of the key reasons is that the diagnosis of pathogens is difficult. Current diagnosis is still based on echocardiography and blood culture.7 Imaging examinations have irreplaceable value for the diagnosis of IE. Correspondingly, etiological examination also plays an important role in the treatment of IE, especially the formulation of antibiotic regimens.8 The commonly described IE-associated pathogens are Staphylococci, Streptococci, and Enterococci, with Staphylococcus aureus being the most frequently diagnosed species.9 These microorganisms were mostly identified in blood cultures.9,10 Notably, blood-culture negative endocarditis (BCNE) is reported to be up to 31% of all cases of IE in the current guidelines.11 For patients with suspected infective endocarditis with negative blood cultures, traditional investigations include serological studies, polymerase chain reaction (PCR) assays of heart valves, and histopathology. In addition, metagenomic next-generation sequencing (mNGS) is a promising technology that has been widely used in the detection of pathogens in IE in recent years.12–16 mNGS is a technology capable of sequencing extremely large numbers of DNA fragments (thousands to millions) simultaneously. A chief advantage of mNGS is unbiased sampling, which enables broad identification of known as well as unexpected pathogens or even the discovery of new organisms.
Bartonella emerged in the form of trench fever in World War I, but the disease it causes has been less well understood. Until the early 1990s, in the form of opportunistic infections in AIDS patients and homeless patients.17 Bartonella species are gram-negative and intracellular pathogens with a unique erythrocytic intracellular lifestyle.18 They are often found in the gut of obligate blood-feeding arthropod carriers and in the blood of mammalian hosts.19 It has been reported that its animal host range has steadily expanded in recent years, such as cats, sheep, rodents, humans, and Marine mammals.20–23 Bartonella endocarditis is a serious disease that can cause clinical complications and has a high mortality rate.18,24 Bartonella henselae accounts for about one-fourth of Bartonella endocarditis cases.25
Here, we report a case of infective endocarditis with negative blood cultures, and blood and aortic valve tissue mNGS results suggested B. henselae. In addition, the whole-genome sequence of B. henselae were obtained. To our knowledge, this is the first report of B. henselae genomic analysis isolated from clinic in China.
Case Presentation
A 64-year-old man was admitted to Zhejiang Provincial People’s Hospital from a community (28°70’ 66’ N; 118°31’ 98’ E), Yushan county of Jiangxi Province. Fifteen days before presentation, the patient developed chest tightness, shortness of breath, and fatigue during activities, which improved with rest. During the period accompanied by fever, cough, headache, and expectoration. He had a history of smoking and drinking for more than 40 years.
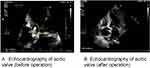
On admission, his vital signs were as follows: blood pressure, 106/51 mmHg; heart rate 81 beats/min; body temperature, 37.7 °C; respiratory rate, 20/min. Physical examination revealed a diastolic murmur in the patient’s aortic valve auscultation area. Laboratory test results revealed normal white blood cell count, neutrophil count, C-reactive protein and procalcitonin levels, decreased hemoglobin (103 g/L, normal 130–175 g/L) and albumin (30.8 g/L, normal 40.0–55.0 g/L), increased cardiac troponin I (TnI 0.580 ug/L, normal ≤0.050 ug/L) and brain natriuretic peptide (BNP 1405.4 pg/L, normal ≤119.0 pg/L). Blood cultures were drawn on admission. The next day, echocardiography revealed severe regurgitation of aortic valve with vegetation formation (Figure 1). The patient was diagnosed with infective endocarditis and acute heart failure. The most common pathogen of infective endocarditis outside the hospital is gram-positive bacteria. Ceftriaxone combined with daptomycin was used for anti-infection treatment, and blood cultures were collected at the same time.
After 1 week of treatment with the above-mentioned antibiotic regimen, the patient’s symptoms of infection, such as low-grade fever, remained unrelieved. In addition, multiple blood/sputum cultures and smears were negative on the first, third, and seventh days of admission. Due to the need for an effective antibiotic regimen, the peripheral blood mNGS examination was performed on the sixth day of admission to clarify the pathogen, which showed: B. henselae. Afterward, the patient’s contact history was carefully inquired again, and it was found that the patient lived with a juvenile for a long time, which is the most common source of Bartonella infection.26 He recalled pet scratches and bites.
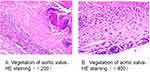
Subsequently, the antibiotic regimen was changed to amikacin combined with doxycycline, and aortic valve prosthetic valve replacement was performed. The pathological examination of the aortic valve tissue after surgery showed that the valve tissue had fibrinous necrosis, calcification, with hyaline degeneration, and local infiltration of a large number of inflammatory cells, which was in line with the pathology of infective endocarditis (Figure 2). Concurrently, mNGS of aortic valve tissue was still suggestive of B. henselae. After surgery, the patient continued to receive targeted anti-infective treatment with the antibiotic regimen in addition to ceftriaxone treatment, intravenous amikacin (0.2 g/12 hrs) and doxycycline (0.1 g/12 hrs). His symptoms improved quickly, and he finally recovered and was discharged from hospital. At the 4-week and 2-month follow-up, the patient had no sign of heart failure or ongoing infection.
Genomic Analysis of ST1 Bartonella henselae
PCR-free library preparation and metagenomic sequencing were performed according to the previous study.27 Shotgun sequencing was carried out on illumina Nextseq platform (Illumina Inc., San Diego, CA). The raw reads of B. henselae ZJBH were assembled into draft genomes using CLC Genomics Workbench v.10.0 software (QIAGEN, Hilden, Germany; https://www.qiagenbioinformatics.com/products/clc-genomics-work-bench). The assembled B. henselae ZJBH strain genome, with a size of 1.73 Mb, is similar to the generally observed genome sizes of other Bartonellae.
In order to shed some light on the molecular epidemiology and evolutionary relatedness of B. henselae around the world, we used whole-genome phylogenetic analyses to examine the phylogenetic relatedness between B. henselae and other 38 B. henselae strains from the National Center of Biotechnology Information (NCBI) GenBank database (as of 09 October 2022). The basic information of B. henselae strains was downloaded from NCBI GenBank in Supplementary Table 1. All B. henselae strains obtained from at least six countries (China, France, Denmark, Switzerland, Germany and the USA) could be assigned into five distinct clades. It was noted that B. henselae ZJBH strain was the first genome sequence of B. henselae isolated from human in China (Figure 3). B. henselae ZJBH strain showed close relationship with human and felis catus-derived strains detected in three countries (Denmark, Germany and USA) in the same clade. Furthermore, these B. henselae strains belonged to several diverse ST types. A previous report described that different STs may be associated with different clinical manifestations, such as bacillary angiomatosis and endocarditis.16 Multilocus sequence typing (MLST) analysis indicated that the B. henselae ZJBH belonged to ST1 using the bioinformatics tools MLST v.2.0 from the Center for Genomic Epidemiology (http://www.genomicepidemiology.org/), which is the most frequently found sequence type in China by molecular detection. The other three strains (A20, A71 and F1) collected from cats also belonged to ST1, but they were rarely assigned to the same clade. These findings implicated that Bartonella infection among cats could pose a potential risk to human.
 |
Figure 3 The evolutionary tree of B. henselae. Abbreviations: CSD, cat-scratch disease; NA, not available. |
Discussion
Bartonella species are facultative intracellular, small fastidious Gram-negative bacilli responsible for one of the rare causes of IE. The genus Bartonella contains aerobic or microaerophilic, fastidious, Gram-negative bacilli, and belongs to the alpha-2 subgroup of the class Proteobacteria. Of the 13 species known to be associated with human diseases, at least 8 of them can cause infective endocarditis, the most important of which are B. henselae and B. quintana. The majority, but not all, of B. henselae endocarditis cases have a history of contact or interaction with a cat.18 Felis catus plays a vital role since it is the major reservoir of B. henselae, and the cat fleas are considered the principal vector for transmission between cats and humans.28 Like our case, IE caused by B. henselae may be associated with cats or cat fleas because the patient lived with a cat for a long time and recalled pet scratches and bites. Furthermore, Bartonella species have been found in ticks from China,29 and tick-borne Bartonellosis in our case is a possibility.
Notably, a recent study described at least two diverse STs of B. henselae isolates from cats samples in Brazil by MLST and microbiological analyses.30 Even though B. henselae has been extensively studied by serological and molecular detection in humans and cats around the world, the genomic analyses of B. henselae and evolutionary trajectory of this zoonotic pathogen among humans and mammals have yet to be elucidated.31,32 To our knowledge, this is the first genomic report of B. henselae in patient from China. MLST results showed the B. henselae ZJBH strain belonged to ST1, which is commonly identified in cats from other countries. Furthermore, we described whole genome sequencing (WGS) data incorporating all B. henselae from diverse sources worldwide and shed light on underlying risk of B. henselae transmitted between cats and humans. In addition, many virulence factors could contribute to host-specific pathogenesis of B. henselae ZJBH strain, mainly including bacterial type IV secretion system (T4SS) proteins VirB and Trw. The VirB/T4SS system in B. henselae strain mediates invasion, proinflammatory activation, and anti-apoptotic protection of endothelial cells. Trw type T4SS mediates adhesion to erythrocytes and diversifies the host specificity in B. henselae.33 Overall, our results suggest that the presence of these virulence factors may be an important determinant to the pathogenicity of B. henselae strains.
Historically, patients with Bartonella IE had a high mortality rate despite surgery,34 so early diagnosis and timely treatment were very necessary. Nowadays, the rapidly developing mNGS technology can rapidly detect unknown pathogenic microorganisms, breaking through the limitations of traditional microbial detection, showing broad prospects in the field of clinical microbiology. Taking the case reported above as an example, the rapid and accurate identification of pathogenic microorganisms through mNGS detection helped us formulate a targeted antibiotic regimen. We also highlight the major role of zoonotic agents and the underestimated role of infective diseases in BCNE. Clinicians should consider Bartonella serology, echocardiography and infectious disease consultation when patients present unwell with a history of body lice infestation.
Conclusions
This is the first genomic report of B. henselae in patient from China, to the best of our knowledge. Early diagnosis of Bartonella spp. infectious endocarditis, is challenging, especially for patients with preexisting valvular heart disease. The epidemiology and management of infective endocarditis are continually changing and many uncertainties remain. Traditional etiological testing has not worked and blood-culture is negative, mNGS examination may be a good option for BCNE. Prompt diagnosis and effective treatment can improve the prognosis.
Abbreviations
IE, Infective endocarditis; mNGS, metagenomic next-generation sequencing; BCNE, blood-culture negative endocarditis; PCR, polymerase chain reaction; NCBI, National Center of Biotechnology Information; MLST, Multilocus sequence typing; WGS, whole genome sequencing.
Data Sharing Statement
The complete genome sequences of the B. henselae ZJBH reported here have been deposited at DDBJ/ENA/GenBank under accession no JARVXB000000000. The version described in this paper is the first version JARVXB000000000.
Ethics Approval and Consent for Publication
This case report was approved by Zhejiang Provincial People’s Hospital Research Ethics Board (QT2022429). This patient provided consent for publication of the clinical details, and written informed consent was obtained.
Funding
The work was supported by the Research Fund for Applications of High-throughput Sequencing Technology in Infectious Diseases (No. 2024645138), Zhejiang Provincial CONBA Hospital Management Soft Science Research Project (No. 2019ZHA-KEB313) and Zhejiang Provincial Natural Science Foundation of China (LQ21H310004).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882–893. doi:10.1016/S0140-6736(15)00067-7
2. Osler W. The gulstonian lectures, on malignant endocarditis. Br Med J. 1885;1(1262):467–470. doi:10.1136/bmj.1.1262.467
3. Shmueli H, Thomas F, Flint N, Setia G, Janjic A, Siegel RJ. Right-sided infective endocarditis 2020: challenges and updates in diagnosis and treatment. J Am Heart Assoc. 2020;9(15):e017293. doi:10.1161/JAHA.120.017293
4. Baddour LM, Wilson WR, Bayer AS, et al. Infective Endocarditis In Adults: Diagnosis, Antimicrobial Therapy, And Management Of Complications: A Scientific Statement For Healthcare Professionals From the American Heart Association. Circulation. 2015;132(15):1435–1486. doi:10.1161/CIR.0000000000000296
5. Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: challenges and perspectives. Lancet. 2012;379(9819):965–975. doi:10.1016/S0140-6736(11)60755-1
6. Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med. 2020;20(1):31–35. doi:10.7861/clinmed.cme.20.1.1
7. Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol. 2017;69(3):325–344. doi:10.1016/j.jacc.2016.10.066
8. Liesman RM, Pritt BS, Maleszewski JJ, Patel R, Kraft CS. Laboratory diagnosis of infective endocarditis. J Clin Microbiol. 2017;55(9):2599–2608. doi:10.1128/JCM.00635-17
9. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128. doi:10.1093/eurheartj/ehv319
10. Rodríguez-Pastor R, Shafran Y, Knossow N, et al. A road map for in vivo evolution experiments with blood-borne parasitic microbes. Mol Ecol Resour. 2022;22(8):2843–2859. doi:10.1111/1755-0998.13649
11. Shi S, Huang Q, Xiao K. Letter to the Editor: rare pathogen of infective endocarditis: Bartonella henselae. Surg Infect. 2023;24(2):203. doi:10.1089/sur.2022.305
12. Guo Y, Li H, Chen H, et al. Metagenomic next-generation sequencing to identify pathogens and cancer in lung biopsy tissue. EBioMedicine. 2021;73. doi:10.1016/j.ebiom.2021.103639
13. Li M, Yan K, Jia P, Wei E, Wang H. Metagenomic next-generation sequencing may assist diagnosis of cat-scratch disease. Front Cell Infect Microbiol. 2022;12:946849. doi:10.3389/fcimb.2022.946849
14. Drummond MR, Lania BG, Diniz PPVDP, et al. Improvement of Bartonella henselae DNA detection in cat blood samples by combining molecular and culture methods. J Clin Microbiol. 2018;56(5):e01732–17. doi:10.1128/JCM.01732-17
15. Next-generation sequencing combined with routine methods to detect the pathogens of encephalitis/meningitis from a Chinese tertiary pediatric neurology center - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/30797792/.
16. Clinical metagenomic next-generation sequencing for pathogen detection - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/30355154/.
17. The centenary of the discovery of trench fever, an emerging infectious disease of World War 1 - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/27375211/.
18. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. 2017;30(3):709–746. doi:10.1128/CMR.00013-17
19. Roden JA, Wells DH, Chomel BB, Kasten RW, Koehler JE, McCormick BA. Hemin binding protein C is found in outer membrane vesicles and protects Bartonella henselae against toxic concentrations of hemin. Infect Immun. 2012;80(3):929–942. doi:10.1128/IAI.05769-11
20. Ereqat S, Nasereddin A, Vayssier-Taussat M, et al. Molecular evidence of Bartonella species in ixodid ticks and domestic animals in Palestine. Front Microbiol. 2016;7:1217. doi:10.3389/fmicb.2016.01217
21. Maggi RG, Raverty SA, Lester SJ, et al. Bartonella henselae in captive and hunter-harvested beluga (Delphinapterus leucas). J Wildl Dis. 2008;44(4):871–877. doi:10.7589/0090-3558-44.4.871
22. Molecular evidence of Bartonella species in ixodid ticks and domestic animals in Palestine - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/27540374/.
23. Hertig MP. Phlebotomus and Carrion’s disease. Am J Trop Med. 1942;22(Suppl):1–80.
24. Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents. 2014;44(1):16–25. doi:10.1016/j.ijantimicag.2014.04.006
25. Shapira L, Rasis M, Binsky Ehrenreich I, et al. Laboratory diagnosis of 37 cases of Bartonella endocarditis based on enzyme immunoassay and real-time PCR. J Clin Microbiol. 2021;59(6):e02217–20. doi:10.1128/JCM.02217-20
26. Brown LD, Maness R, Greer K. Detection of Bartonella spp. and Rickettsia spp. in cat fleas (Ctenocephalides felis) collected from free-roaming domestic cats in southeastern Georgia, USA. Vet Parasitol Reg Stud Rep. 2022;32:100743. doi:10.1016/j.vprsr.2022.100743
27. Luan Y, Hu H, Liu C, et al. A proof-of-concept study of an automated solution for clinical metagenomic next-generation sequencing. J Appl Microbiol. 2021;131(2):1007–1016. doi:10.1111/jam.15003
28. Furquim MEC, Do Amaral R, Dias CM, et al. Genetic diversity and multilocus sequence typing analysis of Bartonella henselae in domestic cats from Southeastern Brazil. Acta Trop. 2021;222:106037. doi:10.1016/j.actatropica.2021.106037
29. Cao XQ, Gu XL, Zhang L, Xu J, Han HJ, Yu XJ. Molecular detection of Rickettsia, Anaplasma, and Bartonella in ticks from free-ranging sheep in Gansu Province, China. Ticks Tick Borne Dis. 2023;14(3):102137. doi:10.1016/j.ttbdis.2023.102137
30. Dias CM, Do Amaral RB, Perles L, et al. Multi-locus Sequencing Typing of Bartonella henselae isolates reveals coinfection with different variants in domestic cats from Midwestern Brazil. Acta Trop. 2023;237:106742. doi:10.1016/j.actatropica.2022.106742
31. Segers FH, Kešnerová L, Kosoy M, Engel P. Genomic changes associated with the evolutionary transition of an insect gut symbiont into a blood-borne pathogen. ISME J. 2017;11(5):1232–1244. doi:10.1038/ismej.2016.201
32. Nystedt B, Frank AC, Thollesson M, Andersson SGE. Diversifying selection and concerted evolution of a type IV secretion system in Bartonella. Mol Biol Evol. 2008;25(2):287–300. doi:10.1093/molbev/msm252
33. Mullins KE, Hang J, Clifford RJ, et al. Whole-genome analysis of bartonella ancashensis, a novel pathogen causing Verruga Peruana, Rural Ancash Region, Peru. Emerg Infect Dis. 2017;23(3):430–438. doi:10.3201/eid2303.161476
34. Raoult D, Fournier PE, Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125(8):646–652. doi:10.7326/0003-4819-125-8-199610150-00004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.