Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 16
Genetic Variants of CYP4F2 Associated with Ischemic Stroke Susceptibility in the Han Population from Southern China
Authors Huang K, Ma T, Li Q, Zhou Y, Qin T, Zhong Z, Tang S, Zhang W, Zhong J, Lu S
Received 23 March 2023
Accepted for publication 26 May 2023
Published 15 June 2023 Volume 2023:16 Pages 599—607
DOI https://doi.org/10.2147/PGPM.S413632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Kang Huang,1,* Tianyi Ma,1,* Qiang Li,1 Yilei Zhou,2 Ting Qin,1 Zanrui Zhong,1 Shilin Tang,1 Wei Zhang,1 Jianghua Zhong,1 Shijuan Lu1
1Department of Cardiovascular Medicine, Central South University Xiangya School of Medicine Affiliated Haikou Hospital, Haikou, People’s Republic of China; 2Medical College, Jingchu University of Technology, Jingmen, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shijuan Lu, Department of Cardiovascular Medicine, Central South University Xiangya School of Medicine Affiliated Haikou Hospital, No. 43, Renmin Avenue, Haidian Island, Haikou, Hainan, 570100, People’s Republic of China, Tel/Fax +86-13005013999, Email [email protected]
Background: The pathophysiological mechanism of ischemic stroke is complex. Traditional risk factors cannot fully or only partially explain the occurrence and development of IS. Genetic factors are getting more and more attention. Our study aimed to explore the association between CYP4F2 gene polymorphism and susceptibility to IS.
Methods: A total of 1322 volunteers were enrolled to perform an association analysis through SNPStats online software. Using FPRP (false-positive report probability) to detect whether the result is a noteworthy finding. The interaction of SNP-SNP in IS risk was assessed by multi-factor dimensionality reduction. Statistical analysis of this study was mainly completed by SPSS 22.0 software.
Results: Mutant allele “A” (OR = 1.24) and genotype “AA” (OR = 1.49) or “GA” (OR = 1.26) of CYP4F2-rs2108622 are risk genetic factors for IS. Rs2108622 is significantly associated with an increased risk of IS among subjects who are females, aging > 60 years old, with BMI ≥ 24 kg/m2, and smoking or drinking volunteers. CYP4F2-rs3093106 and -rs3093105 are associated with susceptibility to IS among smoking, drinking subjects, or IS patients complicated with hypertension.
Conclusion: CYP4F2-rs2108622, -rs3093106, and -rs3093105 are associated with an increased risk of IS.
Keywords: ischemic stroke, susceptibility, CYP4F2, genetic variants, association analysis
Introduction
Ischemic stroke (IS) is a serious disease that endangers human health. The high cost of treatment and poor prognosis of IS bring serious pain and burden to patients and society. IS is one of the leading causes of death worldwide, especially in lower-income countries.1 IS is a complex disease in pathophysiology. At present, traditional risk factors have been found to be hypertension, hyperglycemia, hyperlipidemia, smoking, atrial fibrillation, hyperlipidemia, obesity, and so on.2–4 In addition, timely diagnosis of blood system diseases is also very important for the timely and appropriate treatment of stroke patients.5 The study found that although the prevalence of IS improved with the implementation of preventive measures targeting these traditional risk factors, the risk of recurrence remained.6,7 The above may be related to a lack of awareness and control of new potential risk factors.8 Although less appreciated, genetic causes contribute significantly to the development of ischemic stroke, especially in early-onset stroke.9 Therefore, it IS of great significance to explore the genetic factors related to IS.
At present, with the development of technology, genes related to ischemic stroke have been reported a lot, but the results cannot be repeated or have not been studied to repeat. Excavation of genetic susceptibility genes for IS in specific populations will help to find more accurate IS predictors and new biomarkers, which in turn provide earlier diagnosis and prognosis for IS patients. CYP4 is one of the cytochrome P450 subfamily, mainly responsible for fatty acids and related metabolism. CYP4F2 is a member of the CYP4 subfamily and is the main synthase metabolized by arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE).10–12 For patients with cerebral hemorrhage, 20-HETE can lead to a sharp decrease in blood flow, which has been confirmed in related animal experiments.13 The production of a large amount of 20-HETE can lead to oxidative stress and endothelial cell damage, thereby increasing the incidence of ischemic stroke.14 The above suggests that CYP4F2 is expected to become a therapeutic target for ischemic stroke.
Several studies have shown that single nucleotide polymorphism (SNP) CYP4F2-rs2108622 can inhibit the conversion of arachidonic acid to 20-HETE.10,15 Although there are more and more studies on these SNPs and ischemic stroke susceptibility, the results are not consistent,16,17 which may be related to the small sample size and the limitations of the genetic background of the subjects. No study has been reported on the association between CYP4F2 genetic polymorphism and IS risk in population from southern China.
One of the purposes of this study is to conduct replicate test in different populations based on previous studies, which will further clarify the association between CYP4F2-rs2108622 and IS in population from southern China. We will also explore new genetic polymorphisms associated with IS susceptibility. Our study will provide a reference for the genetic etiology of ischemic stroke, and then provide effective help for the diagnosis and treatment of IS in specific populations.
Methods
Sample Source
A total of 1322 volunteers were enrolled in this case-control study, consisting of 661 IS patients and 661 healthy individuals. All volunteers were recruited in Central South University Xiangya School of Medicine Affiliated Haikou Hospital during the same period. The case group consisted of patients with newly diagnosed ischemic stroke and was confirmed by rigorous computed tomography (CT) or magnetic resonance (MR) brain imaging. Patients with IS confirmed to have cerebral hemorrhage or other accompanying serious medical diseases, such as malignant tumors, severe liver and kidney dysfunction, malignant anemia and autoimmune diseases, were excluded. The control group was healthy individuals with no history of stroke.
After fully informing all subjects of the purpose and significance of this study and obtaining their informed consent, 5mL of peripheral venous blood was collected.
Selection of SNPs and Genotyping
CYP4F2-rs2108622 obtained from previous studies18 was selected as a candidate SNP to explore whether it is associated with susceptibility to IS among the Han population from southern China.
We searched the chromosome location of the CYP4F2 gene in the Ensembl genome browser of the GRCH38.p13 version to avoid missing genetic variants (https://asia.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000186115;r=19:15878023-15898077). Then, inputting the information related to “CYP4F2”, chromosome position and the population (CHS or CHB) in the “VCF to PED” module, files with the filename suffix “.info” and “.ped” will be downloaded. After importing the downloaded two files into Haploview software, set specific parameters in the “Tagger” module to obtain tagSNPs. Specific parameters included r2 > 0.8, Min Genotype > 75%, MAF > 0.05 and HWE > 0.01. Finally, candidate SNPs for this study were randomly selected from tagSNPs (rs3093106, rs3093105). Designing primers was performed through MassARRAY Assay Design software (Supplemental Table 1). Genotyping was completed by MassARRAY ® -IPLEX SNP genotyping technology. Genotyping success rate reached 100%.
Data Analysis
Measurement data was expressed as mean ± standard deviation, count data was expressed as frequency (percentage). The χ² goodness-of-fit was used to test whether the candidate genetic polymorphisms were consistent with Hardy-Weinberg equilibrium (HWE P value >0.05 was considered to be consistent with HWE). SNPStats online tool was used for association analysis between susceptibility to IS and candidate SNPs (https://www.snpstats.net/start.htm?q=snpstats/start.htm). Using odds ratios (OR) and 95% confidence intervals (CI) values to evaluate the association between candidate SNPs and susceptibility to IS. Results were adjusted by age, gender, BMI, smoking or drinking status, helping to avoid influence of confounding factors. Using online database to visualize the significant results of the stratified analysis (SangerBox: http://sangerbox.com/home.html#), and the forest map will be drawn.19 In addition, since multiple hypothesis tests may increase the false-positive probability, we used FPRP (false-positive report probability) to detect whether positive result is noteworthy finding (FPRP threshold is 0.2 and prior probability level is 0.25).20 Multi-factor dimensionality reduction (MDR) was chosen to evaluate interaction of SNP-SNP. “p < 0.05” indicates statistical significance.
Results
Basic Information of Subject and Candidate SNPs
As shown in Table 1, there were no significant difference in age, gender, BMI, and drinking/smoking status between case and control group, indicating that the two groups were comparable.
 |
Table 1 Characteristics of Patients with IS and Healthy Individuals |
We found that of CYP4F2 is on the Chromosome 19: 15,878,023–15,898,077 from Ensembl Genome browser for GRCH38 version, including 5931 genetic variants. According to the flow chart shown in Figure 1, CYP4F2-rs3093105 (chromosome 19: 15,897,578) and CYP4F2-rs3093106 (chromosome 19: 15,897,447) were randomly selected as candidate SNPs for this study. The genotypic frequencies of the three candidate SNPs in the control group were consistent with Hardy-Weinberg equilibrium. Minor allele frequencies (MAF) of candidate SNPs in the African, European, and Han Chinese in Beijing/Shanghai population (1000 genomes) obtained through e!Ensembl genome browser can be seen in Table 2. The results showed that the MAF of candidate SNPs is different in populations with different genetic backgrounds (Table 2).
 |
Table 2 The Basic Information and HWE About the Candidate SNPs of CYP4F2 |
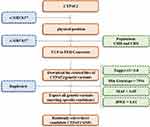 |
Figure 1 Flow chart of candidate SNP screening (rs3093106 and rs3093105). |
Association Between CYP4F2 SNPs and Susceptibility to IS
We found only CYP4F2-rs2108622 has an association with susceptibility to IS among whole subjects (Table 3). Compared with wild-type allele “G” and wild homozygous genotype “GG”, mutant allele “A” (OR = 1.24) and genotype “AA” (OR = 1.49) or “GA” (OR = 1.26) of rs2108622 are risk genetic factors for IS. Rs2108622 also has a significant association with increased risk of IS under dominant (OR = 1.30) and log-additive genetic models (OR = 1.24). As shown in Figure 2, CYP4F2-rs2108622 is significantly associated with an increased risk of IS among subjects who are females, aging >60 years old, BMI ≥ 24 kg/m2, and smoking or drinking volunteers. Interestingly, the mutant allele (A) is a genetic risk factor for IS in all of the above volunteers.
 |
Table 3 Association Between Candidate SNPs in CYP4F2 and Susceptibility to IS |
 |
Figure 2 Forest map based on the positive results observed in stratified analysis. |
Stratified analysis showed (Figure 2) that CYP4F2-rs3093106 had a significant association with susceptibility to IS among smoking (CT VS TT: OR=1.52), drinking volunteers (CT VS TT: OR=1.50) or IS patients complicated with hypertension (CT VS TT: OR=1.70). Similarly, mutant genotype “CA” of CYP4F2-rs3093105 is associated with susceptibility to IS among smoking (OR = 1.60), drinking volunteers (OR = 1.55), or IS patients complicated with hypertension (OR = 1.69).
We have also divided IS patients according to whether they were complicated CHD or diabetes for stratified analysis, but there was no positive finding (Supplemental Table 2).
Analysis of False-Positive Report Probability
Statistical power for positive results of the overall analysis ranges from 92.61% to 100.0%, indicating that the sample size of this study is large enough to effectively prevent the occurrence of false-positive results. Results showed that the prior probability of all positive results found in our study is less than 0.2 (Supplemental Table 3), indicating that the significant association between candidate SNPs and susceptibility to IS found in our study are noteworthy findings.
SNP-SNP Interaction and IS Risk
MDR analysis showed that the single-locus model composed by CYP4F2-rs2108622 has the highest test-balanced accuracy. Fruchterman-Reingold showed that the Information Gain (IG) value of rs2108622 is the highest (Supplemental Figure 1). The above means that the single-locus model composed by CYP4F2-rs2108622 can be chosen as the best model for predicting susceptibility to IS, with perfect CVC (10/10) and the highest test accuracy of 0.533 (Table 4).
 |
Table 4 CYP4F2 SNP–SNP Interaction Models Analyzed by the MDR Method |
Discussion
CYP4F2 is the main metabolic enzyme of 20-HETE. There are relatively many studies on associations between CYP4F2-rs2108622 and susceptibility to various cardiovascular diseases including ischemic stroke.18,21,22 Considering that the minimum allele frequency of rs2108622 is significantly different in populations with different genetic backgrounds, it is necessary to study the relationship between rs2108622 and IS risk in different populations. There are no related studies have been reported among the Han population from southern China. This is the first study to investigate the association of CYP4F2-rs2108622, -rs3093106, and-rs3093105 with ischemic stroke risk in the population from southern China, and some noteworthy results have been found.
Among subjects, allele “A” of CYP4F2-rs2108622 is risk genetic factor for IS. We have observed that this SNP was associated with IS risk in previous studies.23,24 It is worth noting that rs2108622 was only significantly associated with IS in men in a previous association study conducted in a northern Chinese Han population, and no positive results were found in all subjects and female participants.25 However, we found CYP4F2-rs2108622 was significantly associated with IS risk in whole or female subjects, and no positive results were found in males. We speculate that the reason for this may be that, although both Chinese Han populations, but the genetic background of the northern and southern populations are different. This further illustrates the need to identify IS susceptibility genes in a specific population, which will contribute to the accurate prevention, diagnosis, and treatment of IS.
In addition, we have investigated the association between CYP4F2-rs3093106, -rs3093105, and IS risk to identify new susceptibility genetic loci of IS. We only found that -rs3093106 and -rs3093105 were associated with an increasing risk of IS in stratified analyses. Nevertheless, FPRP results suggested that positive results found in stratified analyses are noteworthy new findings. The study has reported that CYP4F2-rs3093105 is potentially associated with the course of coronary heart disease.26 CYP4F2-rs3093105 and rs3093106 have been reported to be significantly associated with reduced lung cancer risk.27 No studies have been reported on the relationship between the above two genetic polymorphisms and IS risk. We are the first to find evidence that CYP4F2-rs3093106 and-rs3093105 can be used as novel susceptibility loci for IS. It will provide new ideas for further targeting CYP4F2 gene polymorphism to treat IS.
The level of 20-HETE is higher in IS patients.28 Inhibition of 20-HETE may reduce infarct size by reducing oxidative stress and apoptosis in neurons.29–32 However, synthetase of 20-HETE increased after 7 days of ipsilateral ischemic injury in rats,33 which suggested that up-regulation of the CYP4F family of 20-HETE synthetase may be involved in angiogenesis and neuroinflammation.34 Combined with our study, we speculate that CYP4F2-rs2108622, -rs3093106, and -rs3093105 were significantly associated with increased risk of IS, which may be the contribution of these three genetic polymorphisms have up-regulated CYP4F2 gene expression, leading to the increased level of 20-HETE, which in turn affected the susceptibility to ischemic stroke. However, the above is just a speculation. It will be very interesting and meaningful to further design functional verification tests to explore specific mechanisms of three candidate genetic polymorphisms in the occurrence and development of IS.
There are still some limitations in this study. Firstly, large sample size is necessary to ensure the reliability and repeatability of the results. Secondly, it would be interesting to further design functional validation experiments to clarify the specific mechanism of three candidate CYP4F2 SNPs in the occurrence and development of IS. Thirdly, different stroke subtypes such as large vessel disease, small vessel ischemic strokes, or lacunar strokes are also worthy of study.35 In subsequent studies, it will be very meaningful to further explore the association between CYP4F2 SNPs and the susceptibility of different stroke subtypes, which will lay a reliable theoretical foundation for diagnosing and managing different stroke subtypes in the clinic. Nevertheless, we are the first to explore the association between candidate CYP4F2 SNPs (rs2108622, rs3093106, and rs3093105) and susceptibility to IS among the population from southern China and noteworthy positive results have been found.
Conclusions
To sum up, we confirmed that CYP4F2 SNPs (rs2108622, rs3093106, and rs3093105) have a significant association with the occurrence of IS in the population from southern China. This may provide new ideas for the prevention and diagnosis of IS.
Data Sharing Statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The use and protocol of human tissue in the study strictly followed the principles expressed in the Declaration of Helsinki and was approved by the Ethics Committee of Haikou People’s Hospital (2020-(Ethics Review)-048). All participants signed informed consent forms before participating.
Acknowledgments
We thank all authors for their contributions and support.
Author Contributions
Kang Huang and Tianyi Ma are co-first authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the specific research fund of the Innovation Platform for Academicians of Hainan Province: Dynamic monitoring and genetic correlation of blood biochemical indexes related to cardiovascular and cerebrovascular diseases in “migratory bird population” in Hainan (No. YSPTZX202032).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6–S16. doi:10.1212/WNL.0000000000012781
2. Su EJ, Lawrence DA. Diabetes and the treatment of ischemic stroke. J Diabetes Complications. 2022;36(11):108318. doi:10.1016/j.jdiacomp.2022.108318
3. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
4. Ferrari F, Villa RF. Brain bioenergetics in chronic hypertension: risk factor for acute ischemic stroke. Biochem Pharmacol. 2022;205:115260. doi:10.1016/j.bcp.2022.115260
5. Arboix A, Jiménez C, Massons J, Parra O, Besses C. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9(9):891–901. doi:10.1080/17474086.2016.1208555
6. Wang YJ, Li ZX, Gu HQ, et al. China stroke statistics 2019: a report from the National Center for hEalthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2020;5(3):211–239. doi:10.1136/svn-2020-000457
7. Collaborators GN. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–480. doi:10.1016/S1474-4422(18)30499-X
8. Xu J, Zhang X, Jin A, et al. Trends and risk factors associated with stroke recurrence in China, 2007–2018. JAMA Netw Open. 2022;5(6):e2216341. doi:10.1001/jamanetworkopen.2022.16341
9. Ekkert A, Šliachtenko A, Grigaitė J, Burnytė B, Utkus A, Jatužis D. Ischemic stroke genetics: what is new and how to apply it in clinical practice? Genes. 2021;13(1):48. doi:10.3390/genes13010048
10. Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem. 2000;275(6):4118–4126. doi:10.1074/jbc.275.6.4118
11. Kikuta Y, Kusunose E, Kusunose M. Characterization of human liver leukotriene B(4) omega-hydroxylase P450 (CYP4F2). J Biochem. 2000;127(6):1047–1052. doi:10.1093/oxfordjournals.jbchem.a022696
12. Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277(28):25290–25296. doi:10.1074/jbc.M201466200
13. Kehl F, Cambj-Sapunar L, Maier KG, et al. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol. 2002;282(4):H1556–H1565. doi:10.1152/ajpheart.00924.2001
14. Williams JM, Fan F, Murphy S, et al. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from brown Norway to dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):H1509–H1518. doi:10.1152/ajpregu.00604.2011
15. Stec DE, Roman RJ, Flasch A, Rieder MJ. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics. 2007;30(1):74–81. doi:10.1152/physiolgenomics.00003.2007
16. Di Fusco D, Ciccacci C, Rufini S, Forte V, Novelli G, Borgiani P. Resequencing of VKORC1, CYP2C9 and CYP4F2 genes in Italian patients requiring extreme low and high warfarin doses. Thromb Res. 2013;132(1):123–126. doi:10.1016/j.thromres.2013.05.002
17. Zhuang W, Wen W, Xuan B, et al. Effect of CYP2C9, CYP4F2 and VKORC1 genetic polymorphisms on pharmacokinetics and pharmacodynamics of mean daily maintenance dose of warfarin in Chinese patients. Blood Coagul Fibrinolysis. 2015;26(2):167–174. doi:10.1097/MBC.0000000000000213
18. Hirata TDC, Dagli-Hernandez C, Genvigir FDV, et al. Cardiovascular pharmacogenomics: an update on clinical studies of antithrombotic drugs in Brazilian patients. Mol Diagn Ther. 2021;25(6):735–755. doi:10.1007/s40291-021-00549-z
19. Peng D, Wei C, Zhang X, et al. Pan-cancer analysis combined with experiments predicts CTHRC1 as a therapeutic target for human cancers. Cancer Cell Int. 2021;21(1):566. doi:10.1186/s12935-021-02266-3
20. Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96(6):434–442. doi:10.1093/jnci/djh075
21. Zhang T, Yu K, Li X. Cytochrome P450 family 4 subfamily F member 2 (CYP4F2) rs1558139, rs2108622 polymorphisms and susceptibility to several cardiovascular and cerebrovascular diseases. BMC Cardiovasc Disord. 2018;18(1):29. doi:10.1186/s12872-018-0763-y
22. Colàs-Campàs L, Royo JL, Montserrat MV, et al. The rs2108622 polymorphism is related to the early risk of ischemic stroke in non-valvular atrial fibrillation subjects under oral anticoagulation. Pharmacogenomics J. 2018;18(5):652–656. doi:10.1038/s41397-017-0007-z
23. Munshi A, Sharma V, Kaul S, et al. Association of 1347 G/A cytochrome P450 4F2 (CYP4F2) gene variant with hypertension and stroke. Mol Biol Rep. 2012;39(2):1677–1682. doi:10.1007/s11033-011-0907-y
24. Liao D, Yi X, Zhang B, Zhou Q, Lin J. Interaction between CYP4F2 rs2108622 and CPY4A11 rs9333025 variants is significantly correlated with susceptibility to ischemic stroke and 20-hydroxyeicosatetraenoic acid level. Genet Test Mol Biomarkers. 2016;20(5):223–228. doi:10.1089/gtmb.2015.0205
25. Deng S, Zhu G, Liu F, et al. CYP4F2 gene V433M polymorphism is associated with ischemic stroke in the male Northern Chinese Han population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):664–668. doi:10.1016/j.pnpbp.2010.03.009
26. Liang T, Liang A, Zhang X, et al. The association study between CYP20A1, CYP4F2, CYP2D6 gene polymorphisms and coronary heart disease risk in the Han population in southern China. Genes Genomics. 2022;44(9):1125–1135. doi:10.1007/s13258-021-01125-9
27. He R, Li M, Li A, et al. CYP4F2 and CYP3A5 gene polymorphisms and lung cancer in Chinese Han population. Clin Exp Med. 2020;20(3):461–468. doi:10.1007/s10238-020-00631-6
28. Ward NC, Croft KD, Blacker D, et al. Cytochrome P450 metabolites of arachidonic acid are elevated in stroke patients compared with healthy controls. Clin Sci. 2011;121(11):501–507. doi:10.1042/CS20110215
29. Fan F, Ge Y, Lv W, et al. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci. 2016;21(7):1427–1463. doi:10.2741/4465
30. Roman RJ, Fan F. 20-HETE: hypertension and Beyond. Hypertension. 2018;72(1):12–18. doi:10.1161/HYPERTENSIONAHA.118.10269
31. Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295(6):H2455–H2465. doi:10.1152/ajpheart.00512.2008
32. Omura T, Tanaka Y, Miyata N, et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37(5):1307–1313. doi:10.1161/01.STR.0000217398.37075.07
33. Kawasaki T, Marumo T, Shirakami K, et al. Increase of 20-HETE synthase after brain ischemia in rats revealed by PET study with 11C-labeled 20-HETE synthase-specific inhibitor. J Cereb Blood Flow Metab. 2012;32(9):1737–1746. doi:10.1038/jcbfm.2012.68
34. Shekhar S, Varghese K, Li M, et al. Conflicting roles of 20-HETE in hypertension and stroke. Int J Mol Sci. 2019;20(18):4500. doi:10.3390/ijms20184500
35. Rudilosso S, Rodríguez-Vázquez A, Urra X, Arboix A. The potential impact of neuroimaging and translational research on the clinical management of lacunar stroke. Int J Mol Sci. 2022;23(3):1497. doi:10.3390/ijms23031497
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
