Back to Journals » Orthopedic Research and Reviews » Volume 15
Genetic Role in Recurrence of Idiopathic CTEV: A Systematic Review
Authors Muhammad H , Haryana SM, Magetsari R, Kurniawan A, Baikuni B, Saraswati PA
Received 5 December 2022
Accepted for publication 3 March 2023
Published 9 March 2023 Volume 2023:15 Pages 19—25
DOI https://doi.org/10.2147/ORR.S400243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Hilmi Muhammad,1,2 Sofia Mubarika Haryana,2 Rahadyan Magetsari,1,2 Aryadi Kurniawan,3 Bima Baikuni,1,2 Paramita Ayu Saraswati2
1Department of Surgery, Orthopaedics and Traumatology Division, Sardjito General Hospital, Yogyakarta, Indonesia; 2Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; 3Department of Orthopaedic and Traumatology, Cipto Mangunkusumo General Hospital, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Correspondence: Hilmi Muhammad, Department of Surgery, Orthopedics and Traumatology Division, Sardjito General Hospital, Jl. Kesehatan No. 1, Yogyakarta, 55281, Indonesia, Email [email protected]
Background: Congenital Talipes Equinovarus (CTEV) is a multitude of deformities involving equinus, varus, adductus, and cavus deformities. Clubfoot affects 1 in every 1000 infants born worldwide, with various incidences according to geographical areas. It has been previously hypothesized that the possible genetic role in Idiopathic CTEV (ICTEV) might have a treatment-resistant phenotype. However, the genetic involvement in recurrent ICTEV cases is yet to be determined.
Aim: To systematically review existing literature regarding the discovery of genetic involvement in recurrent ICTEV to date to further understand the etiology of relapse.
Methods: A comprehensive search was performed on medical databases, and the review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive search was performed on several medical databases: PubMed (MEDLINE), Scopus, the Cochrane Library, and European PMC on May 10, 2022. We included studies reporting patients with recurring idiopathic CTEV or CTEV of unknown cause after treatment, reporting whole-genetic sequencing, whole-exome sequencing, Polymerase Chain Reaction, or Western blot analysis as methods of genetic analysis (intervention) and providing results of idiopathic CTEV genetic involvement. Non-English studies, literature reviews, and irrelevant articles were excluded. Quality and risk of bias assessments were performed using Newcastle-Ottawa Quality Assessment Scale for non-randomized studies where appropriate. The authors discussed data extracted with the primary outcome of gene(s) frequency being reported of their involvement in recurrent ICTEV cases.
Results: Three pieces of literature were included in this review. Two studies analyzed the genetic involvement in CTEV occurrence, while one analyzed the protein types found.
Discussion: With included studies of less than five, we could not perform other forms of analysis apart from qualitatively.
Conclusion: The rarity of literature exploring the genetic etiology of recurrent ICTEV cases has been reflected in this systematic review, giving opportunities for future research.
Keywords: recurrent, idiopathic CTEV, idiopathic clubfoot, genetics, clubfoot
Introduction
Congenital Talipes Equinovarus (CTEV) is a multitude of deformities involving equinus, varus, adductus, and cavus deformities.1 CTEV, or clubfoot, is one of the most common pediatric orthopedic conditions. Clubfoot affects 1 in every 1000 infants born worldwide, with various incidences according to geographical areas.2 For instance, the New Zealand area, with primarily Polynesian ethnicity, reported a higher incidence number than the rest of the world.3 Male infants tend to be more affected by this anomaly, with bilateral cases occurring more often than not. Another intrinsic factor contributing to CTEV occurrence is proven through occurrence in monozygotic twins.1 A further extensive study successfully included five-generation family and proven the autosomal-dominant inheritance pattern, which raised the ultimate question of genetic involvement.1,22
The Ponseti method has now been used commonly as the non-surgical treatment of clubfoot.4 Numerous centers have reported the success of Ponseti treatment programs, with a recurrence rate as low as 6% within a 5-year follow-up time.5 However, recurrence of ICTEV can be seen in up to one-fifth of patients who have had successful conservative treatment.6 A systematic review by Thomas et al reported that the CTEV relapse rate occurs in up to 67.3% of patients and its positive correlation with follow-up duration, which in most studies was limited to up to 5 years. The same review identified varying definitions of “relapse” between studies. It concluded that the term should be defined as “recurrent deformity requiring any further treatment” which may occur following the initial correction of clubfoot managed using the Ponseti method.7
Studies have attempted to predict the possibility of relapses after Ponseti casting had been done in infants. Environmental factor of parents in casting adherence significantly impacts the relapse of ICTEV, with a 41% rate of early recurrence following conservative treatment.3 As recurrence can also occur post-surgery, incomplete posterior release and closure under tension, which can cause complicated superficial wound healing, may result in relapses of ICTEV.1 Several scoring systems exist to help assess the severity of CTEV. Goriainov et al showed a significant association between a high Total Pirani Score (TPS) and early relapse rates.8
Genetically, several studies have tried to determine genetic role in the incidence of clubfoot. Several studies have attempted to isolate the individual genetic cause from clubfoot patients, which some of the most commonly reported were PITX1 and MTHFR.13,21 The HOX gene family have also been frequently reported to cause clubfoot deformity due to their mutations.12 Gurnett and Dobbs (2012) believed that ICTEV is highly related to multifactorial causes which involve a number of genetic mutations occurring at the same time.21 As the genes responsible for idiopathic clubfoot are still being explored, genetic involvement on its recurrence is also being questioned. Haft et al previously hypothesized the possible genetic role in ICTEV relapses as Polynesian ethnicity showed a higher incidence of clubfoot and thus might have a treatment-resistant phenotype. Although this hypothesis was proved to be untrue for ethic-related genes, the role of specific genetic change in recurrent ICTEV cases is yet to be determined.3
This paper aims to systematically review existing works of literature regarding the discovery of genetic involvement in recurrent ICTEV to date in order to further understand the etiology of relapse.
Materials and Methods
Research Strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.19 The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO; CRD42022324761) database prior to the start of this study. A comprehensive search was performed on several medical databases: PubMed (MEDLINE), Scopus, the Cochrane Library, and European PMC on May 10, 2022. The search strategy terms are listed in the Supplementary Material.
Study Selection Criteria
Included were studies that fulfilled the following: (1) the Study group is patients with recurring idiopathic CTEV or CTEV of unknown cause after treatment, (2) the Study design are a randomized clinical trial, controlled clinical trial, case review, case report, systematic review, case series, research studies, research article, follow-up study or cohort studies, (3) The interventions are whole-genetic sequencing, whole-exome sequencing, PCR or Western blot analysis, (4) Outcomes of the study is Idiopathic CTEV genetic involvement, (5) Non-English studies, literature review and irrelevant articles were excluded.
Screening
After removing duplicates, titles and abstracts were scanned for eligibility by two authors independently. Senior authors were consulted if there was disagreement over the suitability of a text for inclusion. Disagreements were resolved through discussion. Full texts of screened studies were then read, and reviewers summarized the study selection.
Data Extraction
Extracted data included basic information regarding the study (author, year, title, type of study, main objective), participant demographics, intervention data of genetic testing method, and analysis. The frequency of gene(s) being reported of its involvement in recurrent ICTEV cases were the measure for our primary outcome. Furthermore, subgroup analysis depending on the type of genetic analysis method performed was attempted. We independently compiled extracted data from authors using Microsoft Excel (Microsoft, Redmond, WA). Synthesized results were presented individually according to article references to anticipate missing data encountered by authors throughout the extraction. Heterogeneity assessment was planned to be conducted visually according to the authors’ judgment and, if possible, through statistical heterogeneity assessment. We anticipated the data to be descriptive; if numerical data were to be obtained, meta-analysis would be attempted with sensitivity analysis using random-effect analysis if the heterogeneity of data is high and fixed-effect analysis if not.
Methodological Quality and Risk of Bias Assessment
Quality and risk of bias assessment of individual studies were performed using the Cochrane Risk of Bias tool for randomized studies and the Newcastle-Ottawa Quality Assessment Scale for non-randomized studies where appropriate.
Results
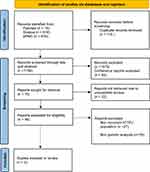
The initial search resulted in a total of 1312 articles. The PRISMA flowchart for study selection is presented in Figure 1. A total of 48 potentially relevant articles were assessed for eligibility criteria. After reading the full texts and applying the eligibility criteria, three studies were left to be included in the review. Reasons for full-text exclusion were due to the criteria of the study population (patients with recurrent ICTEV) and intervention of genetic analysis not being met.
The study demographic is summarized in Table 1. Since the studies were not randomized, Newcastle-Ottawa Quality Assessment Scale was used, with total scores ranging from good to fair quality. Due to the limited studies included and high heterogeneity of data (ie, Type of intervention, outcome measure), subgroup analysis, correlation analysis, and meta-analysis were unable to be conducted. Data from included studies were initially extracted into a shared Microsoft excel sheet which we presented in Table 2. All materials mentioned in this study have been presented in the full text and Supplementary Material.
 |
Table 1 Included Study Characteristics |
 |
Table 2 Data Extracted from Included Studies |
Three pieces of literature were included in this review. Two studies analyzed the genetic involvement in CTEV occurrence, while one analyzed the protein types found. We found no missing data of interest from these three articles. Due to the study limitation, the three studies reported different genes or pathways that individually and equally contribute to their single frequency as reported potential pathophysiology of ICTEV recurrency. A meta-analysis, sensitivity analysis, risk of bias due to missing results, and certainty were not performed as our extracted data were descriptive.
Discussion
Socioeconomic factors and familial and treatment compliance have illustrated their effects in causing relapses in some patients.2,9–11 Unfortunately, the role of genes, which had been in question for a while, has shown to be understudied, as seen by the insufficient number of studies. Genetic causes have been linked to the occurrence of idiopathic clubfoot after studies showed linkage through positive family history, twin studies, higher incidence in certain areas and gender.21 Haft et al reported compliance as the most influential factor in the recurrence of ICTEV. However, there were still patients reporting recurrences despite compliance with bracing. This brought up the speculation that certain genes might play a role in this incidence.12
Systematic reviews by Yong et al and Pavone et al have tried to pinpoint the etiology of idiopathic CTEV. They have concluded that genetics have a strong association with the incidence. Changes in several genes, such as PITX and the HOX family genes, were linked to the incidence of ICTEV. However, due to the significant heterogeneity of study results, there has yet to be a consensus on which genetic change directly causes ICTEV. When specifying the search strategy for ICTEV cases that relapsed, recurred, or were resistant to treatment, studies reporting genetic roles were even more limited.12,13
From our search result, one study reported 5’ HOXC13 and HOXC13-AS lncRNA deletion in subjects with treatment-resistant ICTEV, which led to subsequent corrective surgeries.14 HOX genes are essential in forming limb buds within embryological development. The distal limb component (ie, foot) would be controlled by the region closer to the 5’ end.20 Other than limb formation, HOX genes also control limb muscle development. It was initially believed that HOXA and HOXD genes were responsible for limb and muscle patterning during development.15 Although HOXA9 was proven to be involved in early muscle patterning,15 there seemed to be no evidence linking the HOXD gene cluster to play a role in ICTEV.16 From the same individuals with HOXC13 gene deletion reported by Alvarado et al14 patients showed smaller calf volume within the peroneus muscle through MRI scans. This gave the idea that HOXC deletion not only resulted in treatment-resistant clubfoot phenotype but also had a role in the hypoplasia of limb muscles. As many regulators control the HOX family genes and high specificity for regional deletion would result in different colinear expression of the gene, Alvarado et al were unable to conclude the effect of a single gene deletion within the HOXC locus as the cause of specific phenotype disorder such as recurrent ICTEV.14
The rest of the study results included in this review discussed the protein changes found in patients with recurrent ICTEV, where one of them also discussed the genes responsible for these protein expressions.2 Ošťádal et al reported 19 different proteins found within relapsed clubfeet samples taken during surgery which were dominated by collagens type I and III as the primary component of the extracellular matrix. New findings of some significant collagen reported were collagens V, VI, and XII, and as the samples were all taken from recurrent ICTEV patients, meaning the quantity of these may correlate with the severity of ICTEV led to multiple series of casting and eventually surgery.17 A similar result was reported by Eckhardt et al with increased expression of these collagen types on samples taken from the medial side of relapsed ICTEV patients’ feet.18 The collagen family genes (COL) have been reported previously to play a role in ICTEV incidence, resulting in increased COL9A and COL1A1 expressions in ICTEV patients than in healthy subjects.12,13 Although information regarding the functioning of these newly-observed collagens is limited, collagen types V, VI, and XII contributed to the formation between connective tissue components, muscle functioning, and collagen fibril organization, respectively.18
The study by Novotny et al quantified protein expression and the respective genes found on samples taken from relapsed ICTEV patients. Through PCR quantification, expression of ACTA2, TGFB1, HIF1A, and FN1 genes were significantly higher on the medial side compared to the lateral side. Genes in this study were all involved in fibrosis formation as a response to tissue hypoxia in recurring ICTEV, where the process was centralized around HIF1A gene expression. The increased HIF1A gene in response to ongoing hypoxia would stimulate the production of TGFb1 as a cytokine which led to myofibroblast trans-differentiation, reflected in the raised expression of ACTA2 gene expression and its protein, SmActin.2 Similar to the previous study result discussed by Ošťádal et al, these samples’ collagen type III and VI levels on the medial clubfoot side were also increased compared to its lateral side.17 These changes in extracellular matrix protein expression, along with their corresponding genes from these feet samples, shed light on the role of hypoxia-induced fibrotic changes within recurrent ICTEV cases.18
Our systematic review consists of its flaws. With included studies of less than five, we could not perform other forms of analysis apart from qualitatively. Although our search result found quite a large number of studies, this review has shown how the recurrency of idiopathic CTEV has yet to be studied extensively in terms of possible genetic influence. Due to the different genetic analysis methods from these studies, subgroup analysis was not possible, limiting its correlation with the incidence of recurrent ICTEV. Although one of the three included studies has a fair-quality risk of bias, we still include this study due to the limited number of studies.
Conclusion
The genetic etiology of idiopathic CTEV cases has long been suggested and attempted to be proven, whether through varying study approaches, yet the genes involved and possibly interacting with other intrinsic and extrinsic factors have not been uncovered. As research regarding pathophysiology of congenital anomaly such as clubfoot are continually discovering molecular and genetic involvement, their interactions with other extrinsic factors are ever so important. HOXC family genes deletion and COL family and fibrous formation-related gene (HIF 1A) high expression seems related to recurrent or relapse CTEV. Cases where clubfoot recurs, regardless of brace or casting compliance, put forward the possibility of another cause of relapse through genetic involvement.
The rarity of literature exploring this matter has been reflected in this systematic review which gives opportunities for future research. Findings of genetic changes linked to idiopathic CTEV incidence from past studies can be the foundation for conducting upcoming research on ICTEV recurrency. The importance of pinpointing the genetic cause of relapse could help in screening treatment-resistant cases, and, hopefully, more aggressive treatment could be done to prevent future relapses.
Funding
This study receives no external funding.
Disclosure
The authors report no competing interests in this work.
References
1. Ballantyne JA, Macnicol MF. Congenital talipes equinovarus (clubfoot): an overview of the aetiology and treatment. Curr Orthop. 2002;16(2):85–95. doi:10.1054/cuor.2002.0251
2. Novotny T, Eckhardt A, Doubkova M, et al. The possible role of hypoxia in the affected tissue of relapsed clubfoot. Sci Rep. 2022;12:4462. doi:10.1038/s41598-022-08519-z
3. Haft GF, Walker CG, Crawford HA. Early clubfoot recurrence after use of Ponseti method in a New Zealand population. J Bone Joint Surg Am. 2007;89(3):487–493. doi:10.2106/JBJS.F.00169
4. Sheik-Ali S, Navarro SM, Keil E, Lavy C. The role of clubfoot training programmes in low and middle-income countries: a systematic review. Trop Doct. 2020;50(4):291–299. doi:10.1177/0049475520931343
5. O’shea RM, Sabatini CS. What is new in idiopathic clubfoot. Curr Rev Musculoskelet Med. 2016;9(4):470–477. doi:10.1007/s12178-016-9375-2
6. Uglow MG, Senbaga N, Pickard R, Clarke NMP. Relapse rates following staged surgery in the treatment of recalcitrant talipes equinovarus: 9- to 16-year outcome study. J Child Orthop. 2007;1(2):115–119. doi:10.1007/s11832-007-0024-6
7. Thomas HM, Sangiorgio SN, Ebramzadeh E, Zionts LE. Relapse rates in patients with clubfoot treated using the Ponseti method increase with time. JBJS Rev. 2019;7(5):e6. doi:10.2106/JBJS.RVW.18.00124
8. Goriainov V, Judd J, Uglow M. Does the Pirani score predict relapse in clubfoot? J Child Orthop. 2010;4(5):439–444. doi:10.1007/s11832-010-0287-1
9. Limpaphayom N, Sailohit P. Factors related to early recurrence of idiopathic clubfoot post the Ponseti method. Malays Orthop J. 2019;13(3):28–33. doi:10.5704/MOJ.1911.005
10. Honein MA. Family history, maternal smoking, and clubfoot: an indication of a gene-environment interaction. Am J Epidemiol. 2000;152(7):658–665. doi:10.1093/aje/152.7.658
11. Khan PS, John B, Bhatty S. Efficacy of Ponseti technique in virgin and relapsed clubfeet a comparative study. J Foot Ankle Surg. 2018;57(6):1110–1114. doi:10.1053/j.jfas.2018.05.004
12. Yong BC, Xun FX, Zhao LJ, Deng HW, Xu HW. Systematic review of association studies of common variants associated with ICTEV in humans. Springerplus. 2016;5(1):896. doi:10.1186/s40064-016-2353-8
13. Pavone V, Chisari E, Vescio A, Lucenti L, Sessa G, Testa G. The etiology of idiopathic congenital talipes equinovarus: a systematic review. J Orthop Surg Res. 2018;13(1):206. doi:10.1186/s13018-018-0913-z
14. Alvarado DM, McCall K, Hecht JT, Dobbs MB, Gurnett CA. Deletions of 5’ HOXC genes are associated with lower extremity malformations, including clubfoot and vertical talus. J Med Genet. 2016;53(4):250–255. doi:10.1136/jmedgenet-2015-103505
15. Weymouth KS, Blanton SH, Bamshad MJ, et al. Variants in genes that encode muscle contractile proteins influence risk for isolated clubfoot. Am J Med Genet A. 2011;155(9):2170–2179. doi:10.1002/ajmg.a.34167
16. Gurnett CA, Keppel C, Bick J, Bowcock AM, Dobbs MB. Absence of HOXD10 mutations in idiopathic clubfoot and sporadic vertical talus. Clin Orthop Relat Res. 2007;462:27–31. doi:10.1097/BLO.0b013e31805d8649
17. Ošťádal M, Eckhardt A, Herget J, et al. Proteomic analysis of the extracellular matrix in idiopathic pes equinovarus. Mol Cell Biochem. 2015;401:133–139. doi:10.1007/s11010-014-2300-3
18. Eckhardt A, Novotny T, Doubkova M, et al. Novel contribution to clubfoot pathogenesis: the possible role of extracellular matrix proteins). J Orthop Res. 2019;37(3):769–778. doi:10.1002/jor.24211
19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. IJS. 2021;88:105906. doi:10.1016/j.ijsu.2021.105906
20. Barker S, Chesney D, Miedzybrodzka Maffulli N. Genetics and epidemiology of idiopathic congenital talipes equinovarus. J Pediatr Orthop. 2003;23:265–272.
21. Dobbs MB, Gurnett CA. Genetics of clubfoot. J Pediatr Orthop B. 2012;21(1):7–9. doi:10.1097/BPB.0b013e328349927c
22. Gurnett CA, Alaee F, Kruse LM, et al. Asymmetric lower-limb malformations in individuals with homeobox PITX1 gene mutation. Am J Hum Genet. 2008;83(5):616–622. doi:10.1016/j.ajhg.2008.10.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.