Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Genetic Polymorphism of Lipoprotein-Associated Phospholipase A2 Influences Susceptibility to Gestational Diabetes Mellitus in Chinese Population
Authors Qin L, Ma Q, Zhang C, Lu Z, Liu L, Huang Z
Received 26 August 2023
Accepted for publication 13 October 2023
Published 20 October 2023 Volume 2023:16 Pages 3285—3294
DOI https://doi.org/10.2147/DMSO.S430352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Lingyan Qin,* Qingwei Ma,* Chunrong Zhang, Zuojie Lu, Luchao Liu, Zhihu Huang
Department of Clinical Laboratory, Minzu Hospital of Guangxi Zhuang Autonomous Region, Affiliated Minzu Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhihu Huang, Department of Clinical Laboratory, Minzu Hospital of Guangxi Zhuang Autonomous Region, Affiliated Minzu Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530001, People’s Republic of China, Tel +86-0771-3112210, Fax +86-0771-863112210, Email [email protected]
Purpose: This paper aims to study the relationship between lipoprotein-associated phospholipase A2 (Lp-PLA2) and GDM (gestational diabetes mellitus) by detecting Lp-PLA2 level and its gene polymorphism.
Patients and Methods: From January to June 2022, 82 GDM patients treated in our hospital were included as an experimental group, and 89 healthy pregnant women during the same period were selected as the control group. Lp-PLA2 concentration and TG, TC, HDL-C, and LDL-C levels were tested with specialized instruments in clinical laboratories. The PLA2G7 gene polymorphisms (rs1805017, rs1805018, and rs76863441) were detected by fluorescent probe method and sequencing.
Results: Lp-PLA2 concentration was significantly higher in GDM group than control group (P< 0.05). Among three polymorphism loci of PLA2G7 gene (rs1805017, rs1805018, and rs76863441) the significant associations were only found in GT genotype of rs76863441 loci (P< 0.05).
Conclusion: Pregnant women with high levels of Lp-PLA2 concentration are more likely to develop GDM, especially those with PLA2G7 rs76863441 polymorphism. Lp-PLA2 concentration and PLA2G7 rs1805017 polymorphism may be a novel marker for GDM diagnosis and prediction.
Keywords: gestational diabetes mellitus, PLA2G7, Lp-PLA2, gene polymorphism
Introduction
Gestational diabetes mellitus (GDM) is a general term for abnormal glucose tolerance, abnormal fasting blood glucose, and diabetes detected or developed during pregnancy, excluding diabetes that already existed before pregnancy. According to the International Diabetes Federation, the global prevalence of GDM in 2021 was approximately 16.7% and has become one of the major public health problems. The prevalence of GDM is 20.9% in Asia and 14.8% in China,1–4 making gestational diabetes mellitus the most common complication of pregnancy. Current studies have shown that age, obesity, race, poor reproductive history, and family history of diabetes are the main factors affecting gestational diabetes. In general, the prevalence of GDM increases in Hispanics, African Americans, native Americans, and Asian or Pacific Islanders.3,4
Lipoprotein-related phospholipase A2 (Lp-PLA2), a member of the PLA2 superfamily, is a new inflammatory marker that has been studied intensively in recent years.5 Encoded by the PLA2G7 gene located in chromosome 6p12-21.1,6 the protein is secreted mainly by macrophages, endothelial cells, and mast cells in the vascular intima and binds to many lipoproteins. When it binds to low-density lipoprotein cholesterol (LDL-C), the production of LysoPC and free oxidized fatty acids (oxFA) can induce the production of a variety of pro-inflammatory cytokines, chemokines, and platelet-active substances, mediating the inflammatory cascade effect.7 According to inflammation theory, chronic low-grade inflammation plays an important role in the occurrence and development of T2DM and GDM, involving various pathophysiological processes of obesity and insulin resistance, and suggests that chronic low-grade inflammation may be an important initiating and expanding factor of insulin resistance. High expression of Lp-PLA2 can cause vascular wall damage, resulting in production of pro-inflammatory factors, increased expression of adhesion factors in vivo, and induced activation of inflammatory factors. Most of them bind to LDL-C and a small part binds to high-density lipoprotein cholesterol (HDL-C), which induces a chronic inflammatory response through hydrolysis of phospholipid oxide to produce LysoPC and oxFA. This process is closely related to atherosclerosis, hypercholesterolemia, and diabetes.8
Vascular endothelial injury and various complications are common in GDM patients. Therefore, we should pay attention to the early diagnosis of GDM. Currently, it has been documented that Lp-PLA2 levels are significantly higher than normal in GDM patients and are closely related to adverse pregnancy outcomes, but the research results are inconsistent, and there are also studies that suggest that the concentration of Lp-PLA2 was not associated with diabetes, but its enzyme activity levels were significantly elevated in diabetic patients.9 The pathophysiological mechanism of GDM is similar to that of T2DM, showing obvious regional and ethnic correlation. Therefore, there is a need to verify the levels of Lp-PLA2 in GDM populations of different races and regions.
The biological function of Lp-PLA2, an independent risk factor for atherosclerosis, is controlled by PLA2G7 gene in the body. Mutations in the PLA2G7 gene affect the expression and activity formation of Lp-PLA2.10 Its cDNA was cloned in 1995 and contains 12 exons encoding 441 amino acids.11 El-Saed et al12 found that some of PLA2G7 gene loci are independent risk factors for coronary heart disease in Asian population, while there is no such correlation in European population. The polymorphism of PLA2G7 gene may have different mechanisms of action in different regions and races, which is controversial. At present, there are limited studies on Lp-PLA2 level and its gene polymorphism in GDM population, especially among the Chinese population, so it is necessary to carry out this study.
Materials and Methods
Study Subjects
GDM patients at 24–28 weeks of pregnancy treated in our hospital from January to June 2022 were included as an experimental group. There were 82 patients with GDM. In the same period, 89 healthy pregnant women without GDM were selected as control group. We have set extremely strict inclusion criteria and following inclusion criteria were assessed. According to the guidelines of International Association of the Diabetes and Pregnancy Study Groups (IADPSG, 2014), the diagnostic criteria of “one-step method” for GDM: a 2-h 75g-OGTT should be fasted for at least 8 h, and the blood glucose values of pregnant women should be measured on fasting and 1 h and 2 h after drinking glucose. GDM can be diagnosed if the blood glucose level reaches any of the following criteria: fasting blood glucose (FBG) ≥5.1 mmol/L, blood glucose ≥10.0 mmol/L at 1 h, and blood glucose ≥8.5 mmol/L at 2 h. Patients were aged 20–45 and have a single pregnancy. Women with pre-pregnancy diabetes, heart disease, or liver, kidney, or other related diseases were excluded. The study was approved by Minzu Hospital of Guangxi Zhuang Autonomous Region.
Detection Methods
Serum samples and EDTA-K2 anticoagulant samples of pregnant women at 24–28 weeks were collected according to inclusion and exclusion criteria, and they were divided into pregnant women with GDM (case group) and healthy pregnant women without GDM at the same time (control group) according to OGTT blood glucose results.
A total of 4mL of maternal peripheral venous blood was collected, and 2mL of which was put into a tube without anticoagulant. After centrifugation, serum was used to detect blood glucose, Lp-PLA2 concentration, HDL-C, LDL-C, and so on. The other 2mL was stored with EDTA-K2 anticoagulant and used for genomic DNA extraction of peripheral blood in strict accordance with the requirements of the kit. The extracted genomic DNA samples were stored at −80°C until detecting PLA2G7 gene polymorphism.
Wego AUTOLUMIS3000 and original reagent (Weihai City, China) to detect Lp-PLA2 concentration; Hitachi 7600 automatic biochemical analyser (Japan) and related reagents (Meikang Biotechnology Co., Ltd, Ningbo, China) to detect blood glucose, HDL-C, LDL-C, triglyceride (TG), and total cholesterol (TC); Fluorescence quantitative PCR instrument (AnalytikJena qTOWERE2.2 and Biometra EasyCycler Gradient, Germany) and direct DNA sequencing were used to detect PLA2G7 gene polymorphisms. The PCR primers and products are shown in Table 1. DNA extraction reagent was provided by Guangdong Kaipu Biotechnology Co., Ltd. The amplification reagent was provided by Takara Biotechnology (Dalian) Co., Ltd. and Jiangsu Kangwei Century Biotechnology Co., Ltd (China). The experiment was conducted according to the reagent manufacturer’s protocol or the operating rules of the instrument. PCR conditions were the following for rs1805017and rs1805018: initial steps at 95°C for 1 min, and then repeating the following 40 cycles, denaturing at 95°C for 10 s, annealing at 59°C for 10 s, and extending at 72°C for 20 s. PCR conditions were the following for rs76863441: initial steps at 95°C for 3 min, and then repeating the following 35 cycles, denaturing at 94°C for 30 s, annealing at 55°C for 30 s, and extending at 72°C for 30 s with a final extension of 5 min. PCR products were sequenced (Beijing Tsingke Biotech Co., Ltd.), shown in Figures 1–3.
 |
Table 1 PCR Primers and Products for PLA2G7 Gene |
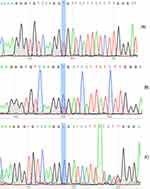 |
Figure 1 PCR products were sequenced for rs1805017 loci of PLA2G7 gene polymorphism (reverse sequencing). (A) rs1805017-A/A; (B) rs1805017-A/G; (C) rs1805017-G/G. |
 |
Figure 2 PCR products were sequenced for rs1805018 loci of PLA2G7 gene polymorphism. (A) rs1805018-T/T; (B) rs1805017-C/T; (C) rs1805017-C/C. |
 |
Figure 3 PCR products were sequenced for rs76863441 loci of PLA2G7 gene polymorphism (reverse sequencing). (A) rs76863441-G/G; (B) rs76863441-G/T; (C) rs76863441-T/T. |
Statistical Analysis
SPSS 24.0 analysis software was used for statistical research. Independent sample t-test was used for comparison between measurement data groups, and the mean ± standard deviation was calculated. The distribution differences between the genotype and allele of PLA2G7 locus were analyzed and compared between the case group and the healthy control group. Fisher’s exact test and x2 test were used to compare the genotype and allele frequencies between the two groups. Odds ratios and 95% confidence intervals were calculated to evaluate the risk of SNPs and GDM susceptibility. The p-value cutoff for significance was 0.05 (P<0.05 was considered to be statistically significant).
Results
General Information of Study Subjects
The information of study population is shown in Table 2, which consisted of age, TG, TC, HDL-C, and LDL-C. There is no statistically significant difference between the GDM group and control group (P>0.05).
 |
Table 2 The Participates Characteristics of Both GDM Group and Control Group (Mean ± SD) |
Lp-PLA2 Level and Gene Polymorphism
Lp-PLA2 concentration was significantly higher in GDM group than control group (P<0.05) (Figure 4). Among three polymorphism loci of PLA2G7 gene (rs1805017, rs1805018, and rs76863441), the significant associations were only found in GT genotype of rs76863441 loci (P<0.05). The detailed information is shown in Table 3.
 |
Table 3 Comparison of PLA2G7 Genotype and Allele Frequency Between GDM Group and Control Group |
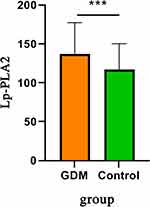 |
Figure 4 Lp-PLA2 concentration was significantly higher in GDM group than control group. ***P<0.05. |
Discussion
In recent decades, with the improvement of economic conditions, the change of lifestyle and the postponement of childbearing age, the prevalence of GDM is also increasing, and the incidence of GDM is on the rise in China and the world.2 If not treated in time, this disease is likely to lead to serious maternal and fetal short-term and long-term complications, including pyelonephritis and mastitis in pregnant women. It can even induce ketoacidosis. The main effects on the fetus are abortion or premature delivery, fetal growth and development restriction, macrosomia or large infant, deformed fetus, and even stillbirth. The main effects on neonates are increase in neonatal respiratory distress syndrome, hypoglycemia, erythrocytosis, and hyperbilirubinemia.
Most women were not screened for diabetes before pregnancy, and the clinical diagnosis of GDM was determined by an oral glucose tolerance test (OGTT) at 24–28 weeks of gestation. However, since glucose tolerance test was performed in late pregnancy, the health of mother and child had been affected to varying degrees before intervention, despite positive prediction later. Early gestation is a critical period of fetal growth and development, so early screening of GDM and effective identification of risk groups are very important. Currently, the OGTT test method is a little complicated, requiring fasting and multiple blood draws, which may lead to gastrointestinal discomfort and decreased compliance in pregnant women. At present, there is still a lack of specific biomarkers for the screening of early and mid-term GDM, and the sensitivity and specificity of traditional predictors are low. For example, FPG (fasting plasma glucose) test is prone to false positives due to dietary factors,13 causing psychological stress in patients. Hemoglobin A1c levels are associated with GDM and adverse pregnancy outcomes,14 but HbA1c is susceptible to interference by maternal iron and hemoglobin levels, and a specific reference range for HbA1c in pregnancy has not been established, and no guidelines currently recommend its use in the diagnosis of GDM. Therefore, the search for GDM biomarkers with simple detection, easy to repeat, high sensitivity, and high specificity has a long way to go.
Currently, many national and international guidelines recommend diagnostic and management strategies that focus on short-term risks during pregnancy and childbirth.15 This has led to increased direct and indirect costs of health care. Therefore, it is important to develop clear screening criteria for pregnant women and identify those at risk from an early and middle stage, which may lead to better management of gestational diabetes. In addition, we need to shift perceptions of gestational diabetes from a short-term condition that can increase the risk for babies to a long-term condition that can exacerbate obesity and cardiometabolic abnormalities in women and children in future generations. Therefore, the early diagnosis, prevention, and treatment of GDM and effective control of blood sugar are of great significance to the puerpera and neonates.
As far as we know, this is the first article that investigates Lp-PLA2 concentration and PLA2G7 rs76863441 polymorphism in pregnant women. Our study provides a good indicator for the development of GDM in healthy pregnant women. Several literature studies have reported the association between PLA2G7 gene polymorphism and disease risk, including coronary heart disease, ischemic stroke, hypertension, clinical atherosclerosis, Alzheimer’s disease, and acute pancreatitis.16–22 PLA2G7 rs76863441 polymorphism contributes to an increased risk of coronary heart disease, hypertension, and acute pancreatitis. PLA2G7 rs1805017 increases the risk of acute pancreatitis but decreases the risk of clinical atherosclerosis. PLA2G7 rs1805018 contributes no risk to Alzheimer’s disease.
However, the present article still has some limitations. Firstly, the number of enrolled pregnant women is not very large, and some confounding factors may cause bias to the final results. Secondly, bioinformatics and meta-analysis are very hot topics in genetic-association studies in recent years.23–40 Therefore, we should conduct more relevant studies about PLA2G7 gene in bioinformatics and meta-analysis, which may contribute to GDM diagnosis, prevention, and treatment.
Conclusion
Pregnant women with high levels of Lp-PLA2 concentration are more likely to develop GDM, especially those with PLA2G7 rs76863441 polymorphism. Lp-PLA2 concentration and PLA2G7 rs76863441 polymorphism may be a novel marker for GDM diagnosis and prediction.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval Statement
This study was conducted with approval from the Ethics Committee of Minzu Hospital of Guangxi Zhuang Autonomous Region, Affiliated Minzu Hospital of Guangxi Medical University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
Lingyan Qin and Qingwei Ma are co-first authors for this study. We acknowledge some students from Minzu Hospital of Guangxi Zhuang Autonomous Region for kind support.
Funding
The study was funded by self-funded research projects of Guangxi Zhuang Autonomous Region Health Commission (Grant No. Z20211292).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. 2019;10(1):154–162. doi:10.1111/jdi.12854
2. Li G, Wei T, Ni W, et al. Incidence and risk factors of gestational diabetes mellitus: a prospective cohort study in Qingdao, China. Front Endocrinol. 2020;11:636. doi:10.3389/fendo.2020.00636
3. Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–211. doi:10.1016/S0020-7292(15)30007-2
4. Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):494. doi:10.1186/s12884-018-2131-4
5. Blank ML, Lee T, Fitzgerald V, Snyder F. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid). J Biol Chem. 1981;256(1):175–178. doi:10.1016/S0021-9258(19)70115-X
6. Tjoelker LW, Wilder C, Eberhardt C, et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374(6522):549–553. doi:10.1038/374549a0
7. Jackisch L, Kumsaiyai W, Moore JD, et al. Differential expression of Lp-PLA2 in obesity and type 2 diabetes and the influence of lipids. Diabetologia. 2018;61(5):1155–1166. doi:10.1007/s00125-018-4558-6
8. Kim M, Yoo HJ, Lee D, Lee JH. Oxidized LDL induces procoagulant profiles by increasing lysophosphatidylcholine levels, lysophosphatidylethanolamine levels, and Lp-PLA(2) activity in borderline hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2020;30(7):1137–1146. doi:10.1016/j.numecd.2020.03.015
9. Nelson TL, Kamineni A, Psaty B, et al. Lipoprotein-associated phospholipase A(2) and future risk of subclinical disease and cardiovascular events in individuals with type 2 diabetes: the cardiovascular health study. Diabetologia. 2011;54(2):329–333. doi:10.1007/s00125-010-1969-4
10. Chi Y, Shi C, Zhang X, Xi Y. Interaction between nonsynonymous polymorphisms in PLA2G7 gene and smoking on the risk of coronary heart disease in a Chinese population. J Thromb Thrombolysis. 2018;46(1):125–130. doi:10.1007/s11239-018-1671-9
11. Stafforini DM, Satoh K, Atkinson DL, et al. Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest. 1996;97(12):2784–2791. doi:10.1172/JCI118733
12. El-Saed A, Sekikawa A, Zaky RW, et al. Ueshima H: association of lipoprotein-associated phospholipase A2 with coronary calcification among American and Japanese men. J Epidemiol. 2007;17(6):179–185. doi:10.2188/jea.17.179
13. Kansu-Celik H, Ozgu-Erdinc AS, Kisa-Karakaya B, Tasci Y, Erkaya S. Fasting and post-prandial plasma glucose screening for gestational diabetes mellitus. East Mediterr Health J. 2019;25(4):282–289. doi:10.26719/emhj.18.038
14. Immanuel J, Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr Diab Rep. 2017;17(11):115. doi:10.1007/s11892-017-0943-7
15. Saravanan P, Magee LA, Banerjee A. Diabetes in pregnancy working G, maternal medicine clinical study G, royal college of O, gynaecologists UK: gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793–800. doi:10.1016/S2213-8587(20)30161-3
16. Ma S, Ding L, Cai M, Chen L, Yan B, Yang J. Association Lp-PLA2 gene polymorphisms with coronary heart disease. Dis Markers. 2022;2022:9775699. doi:10.1155/2022/9775699
17. Wang Q, Hao Y, Mo X, et al. PLA2G7 gene polymorphisms and coronary heart disease risk: a meta-analysis. Thromb Res. 2010;126(6):498–503. doi:10.1016/j.thromres.2010.09.009
18. Liu X, Zhu RX, Tian YL, et al. Association of PLA2G7 gene polymorphisms with ischemic stroke in northern Chinese Han population. Clin Biochem. 2014;47(6):404–408. doi:10.1016/j.clinbiochem.2014.01.010
19. Kim M, Kim M, Yoo HJ, Jang HY, Lee SH, Lee JH. Effects of overweight and the PLA2G7 V279F polymorphism on the association of age with systolic blood pressure. PLoS One. 2017;12(3):e0173611. doi:10.1371/journal.pone.0173611
20. Santoso A, Maulana R, Alzahra F, Maghfirah I, Putrinarita AD, Heriansyah T. Associations between four types of single-nucleotide polymorphisms in PLA2G7 gene and clinical atherosclerosis: a meta-analysis. Am J Cardiovasc Dis. 2017;7(6):122–133.
21. Koshy B, Miyashita A, St Jean P, et al. Genetic deficiency of plasma lipoprotein-associated phospholipase A2 (PLA2G7 V297F null mutation) and risk of Alzheimer’s disease in Japan. J Alzheimers Dis. 2010;21(3):775–780. doi:10.3233/JAD-2010-100513
22. Ma M, Zhai CX, Sun CX. Correlations between LP-PLA2 gene polymorphisms and susceptibility and severity of acute pancreatitis in a Chinese population. Genet Test Mol Biomarkers. 2017;21(4):206–212. doi:10.1089/gtmb.2016.0243
23. Chen X, Wang Z, Yan Y, et al. XRCC3 C18067T polymorphism contributes a decreased risk to both basal cell carcinoma and squamous cell carcinoma: evidence from a meta-analysis. PLoS One. 2014;9(1):e84195. doi:10.1371/journal.pone.0084195
24. Qin LY, Chen X, Li P, Yang Z, Mo WN. Association between the XRCC3 Thr241Met polymorphism and cervical cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2014;14(11):6703–6707. doi:10.7314/APJCP.2013.14.11.6703
25. Wang Z, Chen X, Liu B, Li S, Liu M, Xue H. Quantitative assessment of the associations between DNA repair gene XRCC3 Thr241Met polymorphism and gastric cancer. Tumor Biol. 2014;35(2):1589–1598. doi:10.1007/s13277-013-1219-8
26. Yan Y, Chen X, Li T, Li M, Liang H. Association of OGG1 Ser326Cys polymorphism and pancreatic cancer susceptibility: evidence from a meta-analysis. Tumor Biol. 2014;35(3):2397–2402. doi:10.1007/s13277-013-1317-7
27. Chen X, Yan Y, Li P, Yang Z, Qin L, Mo W. Association of GSTP1 −313A/G polymorphisms and endometriosis risk: a meta-analysis of case-control studies. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):362–367. doi:10.1016/j.ejogrb.2013.10.005
28. Chen X, Zhang H, Li P, Yang Z, Qin L, Mo W. Gene expression of WWOX, FHIT and p73 in acute lymphoblastic leukemia. Oncol Lett. 2013;6(4):963–969. doi:10.3892/ol.2013.1514
29. Chen X, Mo W, Peng Q, Su X. Lack of association between Fas rs180082 polymorphism and risk of cervical cancer: an update by meta-analysis. BMC Med Genet. 2013;14(1):71. doi:10.1186/1471-2350-14-71
30. Chen X, Li P, Yang Z, Mo WN. Expression of fragile histidine triad (FHIT) and WW-domain oxidoreductase gene (WWOX) in nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2013;14(1):165–171. doi:10.7314/APJCP.2013.14.1.165
31. Chen X, Su X, Lin M, et al. Expression of miR-192-5p in colon cancer serum and its relationship with clinicopathologic features. Am J Transl Res. 2021;13(8):9371–9376.
32. Si D, Yao Y, Chen X, Qiu J. Ethnicity-stratified analysis of the association between P53 rs1042522 polymorphism and women HPV infection: a meta-analysis. Microb Pathog. 2021;161(Pt A):105099. doi:10.1016/j.micpath.2021.105099
33. Niu K, Chen X, Lu Y, Xu M. COL3A1 rs1800255 polymorphism is associated with pelvic organ prolapse susceptibility in caucasian individuals: evidence from a meta-analysis. PLoS One. 2021;16(4):e0250943. doi:10.1371/journal.pone.0250943
34. Chen X, Xu J, Zhu Q, Ren Y, Zhao L. Polymyxin B resistance rates in carbapenem-resistant Pseudomonas aeruginosa isolates and a comparison between Etest((R)) and broth microdilution methods of antimicrobial susceptibility testing. Exp Ther Med. 2020;20(2):762–769. doi:10.3892/etm.2020.8777
35. Jin X, Wu Y, Yin S, Chen X, Zhang Y. Association between the IL-10 and IL-6 polymorphisms and brucellosis susceptibility: a meta-analysis. BMC Med Genet. 2020;21(1):63. doi:10.1186/s12881-020-01006-0
36. Jin X, Yin S, Zhang Y, Chen X. Association between TLR2 Arg677Trp polymorphism and tuberculosis susceptibility: a meta-analysis. Microb Pathog. 2020;144:104173. doi:10.1016/j.micpath.2020.104173
37. Yuanyuan G, Xue Y, Yachao L, Xiao F, Xu C. Association between IL-18 −607 C/A polymorphism and the risk of prostate cancer: a meta-analysis of case-control studies. Asian Pac J Cancer Prev. 2019;20(6):1595–1602. doi:10.31557/APJCP.2019.20.6.1595
38. Jin X, Yin S, Zhang Y, Chen X. Quantitative assessment of the association between IL-10 −592 A/C polymorphism and Kawasaki disease risk in Chinese population: evidence from a meta-analysis. Cardiol Young. 2018;28(6):811–815. doi:10.1017/S1047951118000380
39. Jin X, Yin S, Zhang Y, Chen X. Association between TLR2 + 2477G/A polymorphism and bacterial meningitis: a meta-analysis. Epidemiol Infect. 2018;146(5):642–647. doi:10.1017/S0950268818000298
40. Chen X, Jiang M, Zhao RK, Gu GH. Quantitative assessment of the Association between ABC polymorphisms and osteosarcoma response: a meta-analysis. Asian Pac J Cancer Prev. 2015;16(11):4659–4664. doi:10.7314/APJCP.2015.16.11.4659
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
