Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Gender Differences in the Incidence of Nephropathy and Changes in Renal Function in Patients with Type 2 Diabetes Mellitus: A Retrospective Cohort Study
Authors Zhang F , Han Y, Zheng G, Li W
Received 5 December 2023
Accepted for publication 19 February 2024
Published 26 February 2024 Volume 2024:17 Pages 943—957
DOI https://doi.org/10.2147/DMSO.S451628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Fan Zhang,1,2 Yan Han,1,2 Guojun Zheng,3 Wenjian Li4
1Department of Endocrinology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 2Department of Clinical Nutrition, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 3Clinical Laboratory, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China; 4Department of Urology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, Changzhou, Jiangsu, People’s Republic of China
Correspondence: Wenjian Li, Department of Urology, Changzhou Third People’s Hospital, Changzhou Medical Center, Nanjing Medical University, 300 Lanling North Road, Changzhou, Jiangsu, 213001, People’s Republic of China, Tel +86-0519-82009011, Email [email protected]
Purpose: This research aims to examine and scrutinize gender variations in the incidence of diabetic nephropathy (DN) and the trajectory of renal function in type 2 diabetes mellitus (T2DM) patients.
Patients and Methods: We conducted a retrospective cohort study that enrolled 1549 patients diagnosed with T2DM from May 2015 to July 2023. We separately compared the clinical characteristics of male and female participants with and without DN. We utilized the Kaplan-Meier method to examine the cumulative incidence of DN among T2DM patients of varying genders. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using univariable and multivariable Cox proportional hazards regression analysis to evaluate the correlation between various factors and the risk of DN incidence. Multiple linear regression was utilized to investigate the relationship between ΔeGFR% and each factor. Logistic regression with cubic spline function and smooth curve fitting was employed to analyze the nonlinear link between ΔeGFR% and the risk of DN among participants of different genders.
Results: The prevalence of DN was higher in female participants (17.31%) than in male participants (12.62%), with a significant cumulative risk ratio (1.33 [1.02– 1.73], P = 0.034). Multiple linear regression analysis revealed that creatinine, female gender, blood urea nitrogen, alkaline phosphatase, and total cholesterol had a significant impact on ΔeGFR% in T2DM patients, with standardized β coefficients of − 0.325, − 0.219, − 0.164, − 0.084, and 0.071, respectively. The restricted cubic spline analysis demonstrated a strong negative association between ΔeGFR% and the risk of developing DN (P < 0.001).
Conclusion: Both male and female patients with T2DM had a higher prevalence of DN over the 5-year follow-up period. However, women had a greater risk of developing DN and a faster decline in renal function compared to men.
Keywords: gender differences, type 2 diabetes mellitus, nephropathy, renal function, glomerular filtration rate estimates
Introduction
Diabetes is a significant worldwide health threat, with 529 million people living with the disease globally in 2021. Based on an analysis conducted for the Global Burden of Disease Study (GBD Study 2021), the age-standardized prevalence of diabetes is 6.1%, with 96.0% of cases being type 2 diabetes mellitus (T2DM). It is estimated that the global population of individuals with diabetes will increase to 1.31 billion by 2050.1 The increasing prevalence of diabetes is expected to lead to complications associated with the disease. T2DM increases the risk of both macrovascular and microvascular diseases.2
Diabetic nephropathy (DN) is a frequently occurring complication of diabetes, and diabetes is a significant risk factor in the advancement of kidney disease. People diagnosed with diabetes are at an extremely high risk of developing chronic kidney disease (CKD).3,4 The incidence of DN increases as the prevalence of diabetes rises.5,6 Research suggests that approximately 20% to 40% of diabetic patients develop DN.7–9 DN’s primary clinical features are a reduced glomerular filtration rate and persistent albuminuria.10 Increased albuminuria and decreased glomerular filtration rate may lead to end-stage renal disease. Presently, DN is a significant contributor to CKD, renal failure, and end-stage kidney disease (ESKD).11–15 Additionally, DN increases the risk of developing cardiovascular disease.16 There was a trend for increasing risk of cardiovascular death with increasing nephropathy.17 Progression of DN is associated with a significant reduction in life expectancy and quality of life for patients. All patients with T2DM and CKD should be treated with a comprehensive plan.18
Evidence suggests a notable gender disparity in both the prevalence and progression of T2DM. Worldwide, rates of T2DM are rising in both men and women, although men have a higher prevalence of the condition in 140 countries.1 There are variations in complications of T2DM among men and women. Women with T2DM have a greater relative risk of cardiovascular disease and mortality as compared to men.19–22 Studies on the differences in microvascular disease between the genders are limited and inconclusive. Specifically, the association between gender and the development of nephropathy in patients with T2DM has been inadequately researched, and the presence of gender differences is yet to be determined. Men with T2DM have a higher risk of nephropathy compared to normoglycemic men. This risk is not apparent in women.23 However, women have a higher risk of kidney failure and renal insufficiency in T2DM.24 It has been reported that women have a higher risk of diabetic end-stage renal disease than men.25 In addition, another study found that men with newly diagnosed diabetes and pre-diabetes are at an increased risk of developing chronic kidney disease.26 Existing studies suggest inconclusive results, indicating an urgent need for further research.
This study analyzed gender disparities in the prevalence of DN and renal function trends among T2DM patients. It offers proof for diabetes care customization, implementing gender-specific prevention methodologies and management guidance.
Materials and Methods
Participants
This retrospective cohort study included participants diagnosed with T2DM who received care at Changzhou Third People’s Hospital from May 2015 to July 2023. Patients under 18 years of age, with a history of malignancy, acute or chronic nephritis, IgA nephropathy, or other primary renal diseases, those who underwent renal occupancy surgery, pregnant patients, had less than five years of follow-up, or had incomplete data were excluded. The study enrolled 1549 participants and was conducted in accordance with the Declaration of Helsinki. It was approved by the Ethics Committee of Changzhou Third People’s Hospital. Prior to the study, all participants completed an informed consent form.
Definition of diseases
T2DM was defined as either (1) a prior diagnosis by a medical professional, (2) a fasting blood glucose level of ≥7.0 mmol/L, (3) a glycosylated hemoglobin (HbA1c) level of ≥6.5%, or (4) the use of diabetic medications. Obesity was defined as a body mass index (BMI) of ≥ 28.0 kg/m2. Hypertension was defined as systolic blood pressure (SBP) of ≥ 140 mmHg and/or diastolic blood pressure (DBP) of ≥ 90 mmHg. Dyslipidemia was defined based on abnormal levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG), or a history of treatment for dyslipidemia. The study utilized the urinary albumin/creatinine ratio (UACR) to calculate kidney function. The estimated glomerular filtration rate (eGFR) was determined through the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using measures of serum creatinine and serum cystatin C. Blood samples were taken in the fasting state to measure eGFR.27 DN was diagnosed based on the criteria of UACR values greater than or equal to 30 mg/g and/or eGFR less than 60 mL/min/1.73m2.3 Annual reassessment was conducted for DN diagnosis during the follow-up period.
Measured Values and Variables
Demographic characteristics, socioeconomic status, lifestyle factors, disease history, and medication use were obtained from each participant’s medical records. Regular exercise was defined as participating in physical activity for at least 30 minutes at least three times per week. Education attainment was categorized as high school or above versus less than high school. Postmenopausal status was defined as a most recent menstrual period more than 12 months before the assessment. Participants’ height and weight were measured regularly, and the BMI was determined by dividing weight by height squared (kg/m2). The waist circumference (WC) was measured at the midpoint between the rib edge and the ilium’s upper edge at the expiration’s end. The hip circumference (HC) was measured at the most prominent buttock point. The waist-to-hip ratio (WHR) was calculated by dividing WC (cm) by HC (cm). Participants were instructed to fast for 8–10 hours before providing early morning fasting venous blood samples. The blood samples were analyzed for levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), TC, TG, HDL-c, and LDL-c. Fasting plasma glucose (FPG) was assessed using a standardized clinical auto-analyzer, along with HbA1c, blood urea nitrogen (BUN), creatinine, uric acid (UA), and eGFR. The difference in eGFR from baseline at the fifth year of subject follow-up was expressed as ΔeGFR, and ΔeGFR% represents the percentage of the above difference from baseline eGFR. Visceral fat area (VFA) was measured using the InBody770 (Biospace, Seoul, Korea).
Triglyceride-glucose (TyG) index was calculated using the following formula: Ln [TG (mg/dL) × fasting plasma glucose (mg/dL)/2].28
Lipid accumulation product (LAP) was calculated as: LAP = (WC (cm) - 65) x TG(mmol/L) for males, and LAP = (WC (cm) - 65) x TG(mmol/L) for females.29
Statistical Analysis
Continuous variables were described using mean ± standard deviation (SD) or median (25th and 75th percentile), depending on normal distribution, assessed by the Shapiro–Wilk test. Categorical variables were presented as frequencies and percentages. Differences in continuously distributed variables were compared using independent samples t-test, while non-normally distributed variables were analyzed using the Mann–Whitney U-test. Categorical variables were compared between groups using the chi-square test. Gender-stratified cumulative risk curves for DN were depicted using the Kaplan-Meier method at baseline and after five years of follow-up. A comparison of male and female groups was conducted using the Log rank test. We conducted univariate and multivariate Cox proportional risk regression analysis to estimate hazard ratios (HRs) and associated 95% confidence intervals (CIs) for each factor’s association with DN incidence in different genders. We used multiple linear regression analysis to investigate the relationship between each factor and ΔeGFR%. After adjusting for age, we used cubic spline functions and smooth curve-fitted logistic regression to investigate the nonlinear relationship between ΔeGFR% and the risk of DN in participants of different genders. We calculated inflection points using a recursive algorithm if a nonlinear relationship was observed. P values were considered significant (two-tailed) if they were below 0.05. All statistical analyses were performed using SPSS version 23.0 (SPSS, IBM, Corp., Armonk, NY, USA). We used GraphPad Prism v.9.0 (GraphPad Software, USA) to plot the figures.
Results
Research Subgroups
Initially, this study included 14,236 patients diagnosed with T2DM who received medical care at the Changzhou Third People’s Hospital from May 2015 to July 2023. After excluding 12,373 patients who were under 18 years old, those with a history of malignancy, those with acute or chronic nephritis, IgA nephropathy, or other primary renal diseases, or those who had undergone renal occupancy surgery, those with less than five years of follow-up, and those with incomplete data, 1863 subjects met the study criteria. An additional 314 subjects with a diagnosis of DN at baseline were further excluded, leaving a final enrollment of 1549 participants. Out of the sample size, 1006 participants were male, and 543 were female. Among the female participants, the majority (73.5%) were postmenopausal. Only a small percentage of females (1.8%) reported using oestrogen-containing medication. To conduct the study, male and female participants were classified into two groups: one with DN based on its presence during the follow-up period and one without DN (non-DN) (Figure 1).
 |
Figure 1 Study flowchart. Abbreviations: T2DM, Type 2 diabetes mellitus; DN, Diabetic nephropathy. |
Comparison of Clinical Characteristics Between Male and Female Participants
In this study, female participants exhibited lower values of Height, Weight, BMI, WC, FPG, ALT, GGT, TG, BUN, creatinine, UA, eGFR, ΔeGFR, ΔeGFR%, and TyG. On the other hand, they showed higher values of AGE, VFA, TC, HDL-c, and LAP compared to male participants (all P < 0.05). It was found that female participants had lower levels of education and a smaller percentage of smoking and drinking histories compared to male participants. However, female participants had a higher proportion of regular physical activity. Additionally, female participants had a higher prevalence of dyslipidemia and a higher rate of anti-hypertensive and lipid-modifying medications but a lower rate of insulin use. Notably, the prevalence of nephropathy among female participants was higher (17.31%) than that among male participants (12.62%) (P = 0.012) (Table 1).
 |
Table 1 Clinical Characteristics of Overall Participants and Separated by Gender |
Comparison of Clinical Characteristics Between Male and Female Participants with and without DN
Among male participants, compared to those without DN, males with DN had higher FPG, AST, ALP, GGT, BUN, Creatinine, UA, and TyG values, while eGFR, ΔeGFR, and ΔeGFR% were lower (all P < 0.05). Male participant with DN were also observed to be older. Participants with DN had a significantly higher prevalence of hypertension compared to those without DN. Additionally, DN participants had lower levels of education and a higher percentage of smoking history. Participants with DN were also more likely to take oral hypoglycemic medications, insulin, and anti-hypertensive medications than those without DN.
Among female participants, compared to those without DN, those with DN were older and exhibited higher levels of HbA1c, AST, ALP, GGT, BUN, creatinine, and UA values. At the same time, BMI, TC, eGFR, ΔeGFR, and ΔeGFR% were lower than those observed in female participants without DN (all P < 0.05). We also observed comparable trends and some distinctive characteristics. Participants with DN had a longer duration of diabetes compared to those without DN. Regarding comorbidities, the prevalence of both obesity and hypertension was significantly higher in participants with DN than in those without DN. Similar to male participants, female participants with DN had lower educational attainment and a higher rate of smoking history. Regarding medication use, female participants with DN not only had higher rates of taking oral hypoglycemic medications and using insulin but also had significantly higher rates of using anti-hypertensive and lipid-modifying medications compared to those who did not have DN (Table 2).
 |
Table 2 Clinical Characteristics of the Study Participants Classified by the Presence of Different Gender and Incidence of Diabetic Nephropathy |
Cumulative Incidence of DN
During the follow-up period, DN was diagnosed in 94 females (17.31%) and 127 males (12.62%), with incidence rates of 3.46 and 2.52 per 100 patients per year in females and males, respectively. The cumulative incidence risk for DN in females was significantly higher than in males, with a hazard ratio of 1.33 (95% CI 1.02–1.73), log-rank P = 0.034 (Figure 2).
Study on the Correlation of Factors in the Development of DN by Gender
Univariate and multivariate Cox proportional risk regression analyses were used to explore the association of factors with the incidence of DN in different genders. In males, AGE, Creatinine, UA, ΔeGFR%, TyG, and hypertension were significantly associated with the incidence of DN (all P < 0.05) as per Table 3. As per Table 4, AGE, BUN, Creatinine, ΔeGFR%, and diabetes duration were significantly associated with the incidence of DN in females (all P < 0.05).
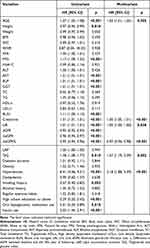 |
Table 3 Univariate and Multivariate Cox Proportional Hazards Regression Analyses for the Association Between Various Factors and Incident Diabetic Nephropathy in Male |
 |
Table 4 Univariate and Multivariate Cox Proportional Hazards Regression Analyses for the Association Between Various Factors and Incident Diabetic Nephropathy in Female |
Analysis of Factors Associated with Declining Renal Function
Multiple linear regression analysis was utilized to examine the correlation between ΔeGFR% and each associated factor in patients with T2DM. Results revealed that creatinine, female sex, BUN, ALP, and TC were the factors that influenced ΔeGFR%. The standardized β coefficients for these factors were −0.325, −0.219, −0.164, −0.084 and 0.071, respectively (Table 5).
 |
Table 5 Multivariate Linear Regression Analysis Between Δegfr% and Various Factors |
Correlation Between Reduced Renal Function and DN Lesions
Age is a significant factor in DN lesions in male and female populations. Therefore, we examined the nonlinear correlation between ΔeGFR% and the risk of DN in gender-specific participants. We employed logistic regression with a cubic spline function and a smoothed curve fitting, adjusting for age to ensure objectivity and obtain precise results. RCS analysis indicated a negative correlation between ΔeGFR% and the risk of DN incidence in both male and female T2DM patients (P < 0.001). This means a smaller ΔeGFR% corresponds to a more significant decline in renal function and a higher incidence of DN. The risk of DN was also found to be negatively correlated with ΔeGFR%(P < 0.001), as indicated in Figure 3. The incidence of DN increased significantly in male and female patients with T2DM when their ΔeGFR% was less than −2.30 and −4.28, respectively. These findings demonstrate a close correlation between ΔeGFR% and the risk of DN in the range of closely associated.
Discussion
In this study, gender differences in the prevalence of nephropathy and changes in renal function were examined among patients with T2DM after a 5-year follow-up period. Results indicated a higher prevalence of DN among women, evidencing gender distinctions in the prevalence of nephropathy in T2DM patients. Furthermore, it was observed that as the length of follow-up increased, women exhibited a more notable decline in renal function. Upon further study, we discovered that age, insulin resistance, and hypertension significantly correlated with DN’s prevalence in male T2DM patients. In female patients, age and duration of diabetes were correlated. These findings reveal the differential influence of gender on the development of DN in patients with T2DM, providing new perspectives for clinical treatment and prevention.
Gender differences were also noted in the prevalence of T2DM. Overall, diabetes is more prevalent among men worldwide, but women experience a higher T2DM rate.30 This gender gap in diabetes prevalence is contrary to reproductive life stages - while young and middle-aged populations show a higher occurrence of T2DM in men,31 postmenopausal and older women are more likely to have the condition.32 This difference between genders is caused by genetic and hormonal influences on pathophysiology, clinical manifestations, diagnosis, and response to treatment.33,34 Regarding macrovascular complications of T2DM, gender differences are more clearly defined. Women with T2DM have a higher relative risk of cardiovascular disease and death than men.19–22 However, the incidence of microvascular complications, particularly DN, has been reported less frequently, and the results have been inconsistent. An observational study of gender differences in target organ damage in insulin-resistant patients revealed a higher prevalence of vascular and renal damage, a more tremendous increase in intima-media thickness, and a more significant number of vascular plaques in females with T2DM compared to males.35 Another study indicated a greater risk of DN in women with T2DM than in men.36 Studies have reported that men play an essential role in the decline of renal function in patients with T2DM and that the overall prevalence of DN is higher in men than in women.37 The inconsistent results regarding gender differences may be attributed to various risk factors for diabetes prevalence and delayed diagnosis of diabetes.38 In the current study, our findings indicate that the cumulative incidence of DN was significantly greater in women diagnosed with T2DM than in their male counterparts (HR = 1.33 [95% CI 1.02 −1.73]), corroborating previous research results.
Advanced age, smoking, hypertension, obesity, and poor glycemic and lipid control are recognized risk factors for developing DN.39 Age is an independent risk factor for both T2DM and DN.40 The incidence of chronic renal failure increases with age in both men and women. With the aging population continuing to grow, the prevalence of older adults with chronic kidney disease is expected to rise.41 Our study found a significant association between older age and DN prevalence in male and female T2DM patients. In a prospective cohort study of 6513 patients with T2DM, smoking was identified as an independent risk factor for the development of microalbuminuria after data analysis.42 This finding emphasizes the negative role of smoking in diabetic complications. Furthermore, a comprehensive meta-analysis has provided compelling evidence supporting smoking as a significant causative factor for DN, with an OR of 1.70, indicating that smokers are at a significantly higher risk of developing DN.43 Our study found that the proportion of male and female patients diagnosed with DN who had a smoking history was considerably higher than those without DN. This finding emphasizes the prevalence and importance of smoking in the development of diabetic nephropathy. It suggests that attention to the renal health risks of smoking is necessary for all diabetic patients, regardless of gender. In the UKPDS trial, patients with T2DM were assigned a target blood pressure of 150/85 mmHg, and the study followed them for a median of 15 years. The results demonstrated a significant 37% reduction in the incidence of microvascular complications compared to patients with a target blood pressure of 180/105 mmHg.24 Our findings indicate a significantly higher prevalence of hypertension in both male and female patients with DN. Hypertension was confirmed as a significant influencing factor in the development of DN in male patients, according to a multivariate COX regression analysis. Obesity promotes the process of focal and segmental glomerulosclerosis, leading to the development of hyperproteinuria.44 In a cohort study, obese individuals, especially those with excess central adiposity, were more likely to develop DN.45 The prevalence of obesity was significantly higher in female patients with DN than in those without DN. Hyperglycemia is a recognized major underlying factor for the development and progression of nephropathy in diabetic patients. Oxidative stress induced by hyperglycemia activates pathways associated with inflammation and fibrosis, ultimately leading to kidney injury and dysfunction.46,47 In this study, male and female patients with DN were more likely to use oral hypoglycemic medication and insulin and had poorer glycemic control than patients without DN. It is worth noting that among female patients, those with DN had a significantly longer duration of diabetes. Furthermore, multivariate COX regression analysis revealed an association between the duration of diabetes and the development of DN. Insulin resistance was found to be associated with the development of DN in male patients. These findings are essential for understanding the pathogenesis of DN and developing targeted treatment programs. Dyslipidemia, comprising elevated triglycerides, low-density lipoproteins, apolipoprotein B, and decreased high-density lipoproteins, are all independently associated with the emergence of DN in a cohort of T2DM.48 Our investigation found no evidence linking lipid management to the development of DN. This could be due to the participants receiving more consistent lipid-control therapies while under observation at the hospital and receiving regular follow-ups. Serum creatinine levels and eGFRs are commonly utilized to determine renal function. An elevated serum creatinine indicates a reduced glomerular filtration rate, while an eGFR below 60 mL/min/1.73m² indicates chronic kidney disease.49 Consistent monitoring of alterations in renal function is critical for optimizing outcomes and reducing the effect of kidney disease on the overall health and quality of life of individuals with diabetes.50 At least annually, urinary albumin and eGFR should be assessed in patients with T2DM regardless of treatment.51 Previous studies have debated gender differences in renal dysfunction, with some reporting a greater predisposition for renal dysfunction in male patients with T2DM,37,52 and others suggesting a greater predisposition for renal dysfunction in female patients.24,36,53 It is widely acknowledged that men with DN experience faster progression and more frequent instances of dialysis.37 Conversely, women with T2DM and end-stage renal disease face a greater likelihood of mortality than men due to elevated inflammation and oxidative stress levels, as well as malleable gender-specific discrepancies in treatment modalities and accessibility.35,54–56 A recent meta-analysis identified a standardized mortality ratio from fatal kidney disease of 1.44 (95% CI 1.02–2.05) for females compared to males.57 Our study found significant elevations in serum creatinine levels and significant decreases in eGFR in both male and female T2DM patients with DN. Additionally, the Cox proportional risk regression analysis results demonstrated significant associations between Creatinine and ΔeGFR% and the prevalence of DN in both genders. Furthermore, RCS analyses indicated a negative correlation between ΔeGFR% and the risk of DN in both male and female T2DM patients. A lower decrease in estimated glomerular filtration rate (eGFR%) was associated with a higher incidence of diabetic nephropathy and a more significant decline in renal function. An analysis of factors related to changes in renal function uncovered gender disparities in the decreasing renal function of T2DM patients. Specifically, declines in renal function were more prominent in females than males (B = −7.346 [−8.906, −5.786]).
Sex hormones may contribute to explaining gender differences, and the hormone estrogen appears to have a protective effect on the kidneys.58 The protection offered by estrogen may come from either a direct impact on the kidneys or an indirect result of estrogen circulating throughout the body.59 Research has established that estrogen exhibits anti-inflammatory properties and that immune cells, such as antigen-presenting and T cells, contain estrogen receptors. These receptors modulate the immune response, potentially safeguarding the kidneys during hyperglycemia.60 However, this protective effect is significantly reduced in postmenopausal women displaying lower endogenous estradiol levels.24,61,62 Our study found that 73.5% of female participants were postmenopausal, which can decrease estrogen levels. Additionally, a small number of female participants (1.8%) reported using estrogenic medications, which may also result in low estrogen levels. These factors could potentially contribute to the high prevalence of DN in female T2DM patients. Furthermore, disparities in behavior and treatment between genders can also contribute to differences in outcomes of decreased kidney function. Research has shown that men receive more intense treatment for T2DM and cardiovascular disease, which could lead to earlier diagnosis and treatment of complications.63 Additional studies suggest that women have lower medication adherence than men in treating diabetes and dyslipidemia.64 They also indicate that women tend to have poorer outcomes for dyslipidemia.65 These differences can increase the incidence of DN in women. In this study, we observed a higher prevalence of dyslipidemia in female T2DM patients compared to males. It is important to consider the lower educational level of female patients, which may have contributed to their lesser adherence to treatment compared to men, ultimately affecting treatment outcomes. In summary, our and previous research shows that women with T2DM are at greater risk of developing renal dysfunction compared to men.
However, our study has some limitations. Firstly, this single-center study is not representative of the general population, thus impeding the provision of a comprehensive understanding of diabetic patients. To remedy this, future investigations should be multicenter and prospective, focusing on mechanisms. Secondly, our study participants were exclusively from hospitals and exhibited relatively poor glycemic control during their initial visits, potentially overestimating the prevalence of DN within the T2DM population. Generalization of these findings to other populations may be limited because all study participants were from eastern China, which may have different body composition than other regions. Additionally, the study could not standardize patient diet, exercise, and medication due to differences in treatment requirements, impeding our ability to consider these factors. Finally, it is essential to note that this study lacked specific information on participants’ sex hormones. This limitation weakened our interpretation and understanding of the study results. Obtaining more detailed data on sex hormones in future studies would enhance the scientific validity and rigor of the study, allowing for a more accurate exploration of the influence of relevant factors on the results.
Conclusion
In conclusion, male and female participants with T2DM exhibited a higher prevalence of DN over the 5-year follow-up. However, women had a greater risk of developing DN and a more accelerated decline in renal function than men. Future studies should investigate the underlying mechanisms of this correlation and employ the knowledge gained to devise personalized treatment strategies to avert nephropathic complications among patients with T2DM. Special consideration should be given to vulnerable populations in research and clinical settings. Women with T2DM should pay increased attention to changes in their renal function, implementing preventive or interventional measures early to enhance renal protection and minimize the risk of DN.
Data Sharing Statement
The data utilized and analyzed in the present study are accessible from the corresponding author upon justified request.
Institutional Review Board Statement
The research adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of Changzhou Third People’s Hospital (No. 02A-A20230023).
Acknowledgments
The authors express gratitude to all study participants for their valuable contribution.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Science and Technology Project of Changzhou [CJ20200059, CJ20220226], and Key Talents Project of Changzhou Third People’s Hospital.
Disclosure
The authors declare no conflicts of interest in this study.
References
1. Collaborators GBDD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–234.
2. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390.
3. American Diabetes A. 11. Microvascular Complications and Foot Care: standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S124–S138.
4. de Boer IH. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):24–30.
5. Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–377.
6. Cockwell P, Fisher LA. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664.
7. Bonner R, Albajrami O, Hudspeth J, Upadhyay A. Diabetic Kidney Disease. Prim Care. 2020;47(4):645–659.
8. Umanath K, Lewis JB. Update on Diabetic Nephropathy: core Curriculum 2018. Am J Kidney Dis. 2018;71(6):884–895.
9. Afkarian M, Zelnick LR, Hall YN, et al. Clinical Manifestations of Kidney Disease Among US Adults With Diabetes, 1988-2014. JAMA. 2016;316(6):602–610.
10. Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13(5):311–318.
11. Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3–15.
12. Li J, Albajrami O, Zhuo M, Hawley CE, Paik JM. Decision Algorithm for Prescribing SGLT2 Inhibitors and GLP-1 Receptor Agonists for Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2020;15(11):1678–1688.
13. Duru OK, Middleton T, Tewari MK, Norris K. The Landscape of Diabetic Kidney Disease in the United States. Curr Diab Rep. 2018;18(3):14.
14. Zou Y, Zhao L, Zhang J, et al. Development and internal validation of machine learning algorithms for end-stage renal disease risk prediction model of people with type 2 diabetes mellitus and diabetic kidney disease. Ren Fail. 2022;44(1):562–570.
15. Johansen KL, Chertow GM, Foley RN, et al. US Renal Data System 2020 Annual Data Report: epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2021;77(4 Suppl 1):67.
16. Andresdottir G, Jensen ML, Carstensen B, et al. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care. 2014;37(6):1660–1667.
17. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63(1):225–232.
18. de Boer IH, Khunti K, Sadusky T, et al. Diabetes Management in Chronic Kidney Disease: a Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–3090.
19. Harreiter J, Fadl H, Kautzky-Willer A, Simmons D. Do Women with Diabetes Need More Intensive Action for Cardiovascular Reduction than Men with Diabetes? Curr Diab Rep. 2020;20(11):61.
20. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78.
21. Prospective Studies C, Asia Pacific Cohort Studies C. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018;6(7):538–546.
22. Peters SAE, Woodward M. Sex Differences in the Burden and Complications of Diabetes. Curr Diab Rep. 2018;18(6):33.
23. de Ritter R, Sep SJS, van der Kallen CJH, et al. Sex differences in the association of prediabetes and type 2 diabetes with microvascular complications and function: the Maastricht Study. Cardiovasc Diabetol. 2021;20(1):102.
24. Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group US. Risk factors for renal dysfunction in type 2 diabetes: u.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–1839.
25. Shen Y, Cai R, Sun J, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine. 2017;55(1):66–76.
26. Parizadeh D, Rahimian N, Akbarpour S, Azizi F, Hadaegh F. Sex-specific clinical outcomes of impaired glucose status: a long follow-up from the Tehran Lipid and Glucose Study. Eur J Prev Cardiol. 2019;26(10):1080–1091.
27. Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302(11):1186–1194.
28. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351.
29. Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26.
30. Campesi I, Franconi F, Seghieri G, Meloni M. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol Res. 2017;119:195–207.
31. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119.
32. Molitch ME, DeFronzo RA, Franz MJ, et al. Diabetic nephropathy. Diabetes Care. 2003;26(Suppl 1):S94–98.
33. Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37(3):278–316.
34. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582.
35. Gomez-Marcos MA, Recio-Rodriguez JI, Gomez-Sanchez L, et al. Gender differences in the progression of target organ damage in patients with increased insulin resistance: the LOD-DIABETES study. Cardiovasc Diabetol. 2015;14:132.
36. Yu MK, Lyles CR, Bent-Shaw LA, Young BA, Pathways A. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol. 2012;36(3):245–251.
37. de Hauteclocque A, Ragot S, Slaoui Y, et al. The influence of sex on renal function decline in people with Type 2 diabetes. Diabet Med. 2014;31(9):1121–1128.
38. Roche MM, Wang PP. Factors associated with a diabetes diagnosis and late diabetes diagnosis for males and females. J Clin Transl Endocrinol. 2014;1(3):77–84.
39. Radcliffe NJ, Seah JM, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2017;8(1):6–18.
40. Mu X, Wu A, Hu H, Zhou H, Yang M. Prediction of Diabetic Kidney Disease in Newly Diagnosed Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes. 2023;16:2061–2075.
41. Abdelhafiz AH. Diabetic Kidney Disease in Older People with Type 2 Diabetes Mellitus: improving Prevention and Treatment Options. Drugs Aging. 2020;37(8):567–584.
42. Cederholm J, Eliasson B, Nilsson PM, Weiss L, Gudbjörnsdottir S. Microalbuminuria and risk factors in type 1 and type 2 diabetic patients. Diabet Res Clin Pract. 2005;67(3):258–266.
43. Su S, Wang W, Sun T, et al. Smoking as a risk factor for diabetic nephropathy: a meta-analysis. Int Urol Nephrol. 2017;49(10):1801–1807.
44. Choung HG, Bomback AS, Stokes MB, et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int. 2019;95(3):647–654.
45. Chung HF, Al Mamun A, Huang MC, et al. Obesity, weight change, and chronic kidney disease in patients with type 2 diabetes mellitus: a longitudinal study in Taiwan. J Diabetes. 2017;9(11):983–993.
46. Kumar S, Khatri M, Memon RA, et al. Effects of testosterone therapy in adult males with hypogonadism and T2DM: a meta-analysis and systematic review. Diabetes Metab Syndr. 2022;16(8):102588.
47. Turkmen K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse. Int Urol Nephrol. 2017;49(5):837–844.
48. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018.
49. Thomas B. The Global Burden of Diabetic Kidney Disease: time Trends and Gender Gaps. Curr Diab Rep. 2019;19(4):18.
50. Kumari K, Kumar R, Memon A, et al. Treatment with Testosterone Therapy in Type 2 Diabetic Hypogonadal Adult Males: a Systematic Review and Meta-Analysis. Clin Pract. 2023;13(2):454–469.
51. Committee ADAPP. C11. Chronic Kidney Disease and Risk Management: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S175–s184.
52. Maric C, Sullivan S. Estrogens and the diabetic kidney. Gend Med. 2008;5 Suppl A(Suppl A):S103–113.
53. Coll-de-Tuero G, Mata-Cases M, Rodriguez-Poncelas A, et al. Chronic kidney disease in the type 2 diabetic patients: prevalence and associated variables in a random sample of 2642 patients of a Mediterranean area. BMC Nephrol. 2012;13:87.
54. Carrero JJ, de Mutsert R, Axelsson J, et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26(1):270–276.
55. Hecking M, Bieber BA, Ethier J, et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med. 2014;11(10).
56. Villar E, Chang SH, McDonald SP. Incidences, treatments, outcomes, and sex effect on survival in patients with end-stage renal disease by diabetes status in Australia and New Zealand. Diabetes Care. 2007;30(12):3070–3076.
57. Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(3):198–206.
58. Valdivielso JM, Jacobs-Cacha C, Soler MJ. Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(1):1–9.
59. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69.
60. Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44(1):3–15.
61. Earle KA, Ng L, White S, Zitouni K. Sex differences in vascular stiffness and relationship to the risk of renal functional decline in patients with type 2 diabetes. Diab Vasc Dis Res. 2017;14(4):304–309.
62. Maric C. Sex, diabetes and the kidney. Am J Physiol Renal Physiol. 2009;296(4).
63. Kramer HU, Raum E, Ruter G, et al. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: results from the DIANA study. Cardiovasc Diabetol. 2012;11:88.
64. Tran HV, Waring ME, McManus DD, et al. Underuse of Effective Cardiac Medications Among Women, Middle-Aged Adults, and Racial/Ethnic Minorities With Coronary Artery Disease (from the National Health and Nutrition Examination Survey 2005 to 2014). Am J Cardiol. 2017;120(8):1223–1229.
65. Gouni-Berthold I, Berthold HK, Mantzoros CS, Bohm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2008;31(7):1389–1391.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


