Back to Journals » Drug Design, Development and Therapy » Volume 16
Gemcitabine Combined with Cisplatin Has a Better Effect in the Treatment of Recurrent/Metastatic Advanced Nasopharyngeal Carcinoma
Authors Yang Q, Nie YH, Cai MB, Li ZM, Zhu HB, Tan YR
Received 14 December 2021
Accepted for publication 8 March 2022
Published 26 April 2022 Volume 2022:16 Pages 1191—1198
DOI https://doi.org/10.2147/DDDT.S353898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Qiao Yang,1 Yue Hua Nie,1 Man Bo Cai,1 Zhi Min Li,1 Hong Bo Zhu,2 Ye Ru Tan2
1Department of Oncology Radiotherapy, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, 421001, People’s Republic of China; 2Department of Medical Oncology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, 421001, People’s Republic of China
Correspondence: Ye Ru Tan, Department of Medical Oncology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, 421001, People’s Republic of China, Tel +86 0734-8578759, Email [email protected]
Objective: To explore the efficacy and safety of gemcitabine (GEM) combined with cisplatin (DDP) in the treatment of recurrent/metastatic nasopharyngeal carcinoma (NPC).
Methods: A total of 100 patients with recurrent/metastatic NPC treated in the First Affiliated Hospital, Hengyang Medical School, University of South China from January 2018 to March 2020 were retrospectively enrolled. Based on different chemotherapy schemes, they were assigned to an observation (Obs) group (DDP + GEM, n = 55) and a control (Con) group [DDP + FU (fluorouracil), n = 45]. The two groups were compared regarding the following items: therapeutic efficacy; serum levels of platelet-derived growth factor-BB (PDGF-BB), soluble epithelial cadherin (SE-CAD), and inflammation-related factors before and after treatment; toxic and side effects; 1-year survival rate; and quality of life (QOL) 6 months after treatment.
Results: The Obs group outperformed the Con group in therapeutic efficacy (P < 0.05). There were no significant differences in the levels of PDGF-BB, SE-CAD, interleukin (IL)-6, IL-10 and tumor necrosis factor (TNF)-α between the two groups before treatment (P > 0.05). After treatment, better improvements in PDGF-BB, SE-CAD and inflammatory factors were observed in the Obs group (P < 0.05). The toxic and side effects were significantly lower and the 1-year survival rate and patients’ QOL after 6 months of treatment were significantly higher in the Obs group compared with the Con group (P < 0.05).
Conclusion: GEM combined with DDP can provide more clinical benefits for patients with recurrent/metastatic advanced NPC, with less side effects, high tolerance and significant efficacy, which can be further promoted in clinical use.
Keywords: gemcitabine, cisplatin, recurrent/metastatic nasopharyngeal carcinoma, efficacy, safety
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignancy mostly found in the anterior wall of the nasopharyngeal roof and the pharyngeal recess. It is one of the most common clinical tumors, especially in Southern China.1,2 Due to the complicated anatomical structure of the nasopharynx and the deep location of the tumor, a comprehensive treatment regimen based on radiotherapy is often adopted. Although good efficacy can be achieved, approximately 20% of NPC patients still face treatment failure due to distant metastasis or local recurrence as most patients are already in the advanced stage when diagnosed.3,4 Once recurrence or metastasis occurs, the prognosis of patients is extremely poor, with an overall survival of only 20 months.5 Therefore, the treatment of recurrent/metastatic NPC carries huge implications for prolonging the survival of patients.
Currently, there is no uniform standard for induction chemotherapy of NPC, and fluorouracil (FU) combined with platinum or gemcitabine (GEM) combined with platinum is widely used.6 However, there are few comparative studies between the two regimens, especially in the treatment of recurrent NPC. Although FU + cisplatin (DDP) is a commonly used chemotherapy regimen at home and abroad, the response rate of this regimen is 40%-65%, with short effective duration, frequent side effects, and high intolerance.7 GEM is a nucleotide analogue that produces anti-tumor effects by inhibiting DNA synthesis and is often used in breast carcinoma, pancreatic cancer and non-small cell lung cancer.8 Recently reported clinical trial results demonstrate that GEM can be used to treat NPC with remarkable curative effect and can tolerate adverse reactions.9 DDP is a DNA-destroying chemotherapeutic agent that exerts cytotoxicity and/or induces apoptosis by forming DNA adducts or targeting therapy-associated cancer signaling pathways. At present, DDP-based chemotherapy is widely applied in clinical practice. A previous study has pointed out that GEM + DDP has a synergistic effect,10 but there are still relatively few studies comparing DDP + GEM with other chemotherapy regimens.
Accordingly, the purpose of this study is to investigate the efficacy and safety of DDP + GEM in the treatment of recurrent/metastatic NPC, with a view to providing more clinical reference for the selection of treatment regimens for patients with the disease.
Materials and Methods
Patient Baseline Data
One hundred patients with recurrent/metastatic advanced NPC treated in The First Affiliated Hospital, Hengyang Medical School, University of South China between January 2018 and March 2020 were retrospectively enrolled, including 57 male patients and 43 female patients, with an average age of (64.32±8.18) years and a mean time of (18.31±3.62) months since the last treatment. Based on different chemotherapy schemes, they were divided into an observation (Obs) group (DDP + GEM, n=55) and a control (Con) group (DDP + FU, n=45). Inclusion criteria: (1) Patients who met the criteria of recurrent/metastatic NPC and were confirmed by pathological examination; (2) Patients with a Karnofsky Performance Scale (KPS) score ≥70. Exclusion criteria: (1) Time <12 months from the last chemotherapy; (2) Diseases associated with blood system; (3) Chemotherapy contraindications; (4) Severe liver and kidney dysfunction; (5) Communication disorder or mental illness. All patients sign a written informed consent form to give their consent for participation. This study, approved by the The First Affiliated Hospital, Hengyang Medical School, University of South China ethics committee, was conducted in compliance with the Declaration of Helsinki.
Chemotherapy Regimens
The Obs group was treated with DDP + GEM chemotherapy, and the specific scheme was as follows: GEM (Manufacturer: Qilu Pharmaceutical (Hainan) Co., Ltd., SFDA Approval No. H20113286) was injected intravenously on the 1st and 8th day of chemotherapy, and dripped within 30 min at a dose of 100 mg/m2; DDP (Manufacturer: Yunnan Phytopharmaceutical Co., Ltd., SFDA Approval No. H53021677) was injected intravenously within 3 h on the first day of chemotherapy at a dose of 80 mg/m2. Those with effective treatment completed 4 chemotherapy cycles with 21 days as a chemotherapy cycle.
The Con group received DDP + FU chemotherapy, and the specific scheme was as follows: DDP was injected intravenously on the first day of chemotherapy (about 3 h) at a dose of 80 mg/m2. After completion, intravenous FU (Manufacturer: Shanghai Xudong Haipu Pharmaceutical Co., Ltd., SFDA Approval No. H31020593) was continuously injected for 96 h at a dose of 4000 mg/m2.
The treatment lasted for a minimum of 4 courses and a maximum of 6 courses of 21 days each. Withdrawal was generally not considered if there was no disease progression or intolerable toxic and side effects.
Outcome Measures
(1) The therapeutic efficacy of the two groups were evaluated and compared. The efficacy evaluation criteria of this study were based on the Response Evaluation Criteria in Solid Tumors11 formulated by the World Health Organization (WHO). According to the medical examination and imaging data after treatment, the therapeutic efficacy was divided into complete response (CR): tumor disappearance; partial response (PR): tumor regression ≥50%; stable disease (SD): tumor growth <25% or regression by <50%, which lasted for more than 4 weeks, and; progressive disease (PD): tumor growth >25%. Overall response rate (ORR) = CR + PR. (2) Serum levels of platelet-derived growth factor-BB (PDGF-BB) and soluble epithelial cadherin (SE-CAD) before and after treatment were detected using the enzyme-linked immunosorbent assay (ELISA) with kits purchased from Abcam. All operations were strictly in accordance with the instructions. (3) Before and 4 weeks after treatment, ELISA was used to measure serum levels of interleukin (IL)-6, IL-10 and tumor necrosis factor (TNF)-α with kits all purchased from Abcam. (4) The toxic and side effects of the two groups during treatment were recorded and compared, including abnormal liver and kidney function, leukopenia, gastrointestinal reaction and thrombocytopenia. (5) The 1-year survival rate of the two groups was evaluated and compared, with the deadline of patient death or April 1, 2021, whichever came first. Follow-up of patient outcomes continued after the deadline. (6) After 6 months of treatment, the quality of life (QOL) of patients was assessed using the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30)12 from 5 domains of physical, role, emotional, cognitive, social functioning and total health score. A higher score indicates a better QOL.
Statistical Analysis
The data was analyzed by SPSS18. 0 (Beijing NDTimes Technology Co., Ltd.), and visualized into corresponding figures via GraphPad Prism 6. Counting data were analyzed using the Chi-square test. For measurement data, independent samples t-test was used for inter-group comparisons, and paired t-test was used for intra-group comparisons before and after treatment. Patient survival was analyzed by the Log rank test and visualized by the Kaplan-Meier method. Differences with P values <0.05 were considered statistically significant.
Results
Comparison of General Information
The general data of patients such as gender, age and smoking history were not significantly different between the two groups (P>0.05), suggesting comparability. Table 1
 |
Table 1 General Data [n (%)] |
Comparison of Therapeutic Efficacy
The number of patients with CR, PR, SD and PD were 0, 32, 18 and 5 in the Obs group, and 0, 20, 12 and 13 in the Con group, respectively. The ORR of the Obs group was 90.91%, which was significantly higher than that of 71.11% in the Con group (P<0.05). Table 2
 |
Table 2 Comparison of Therapeutic Efficacy Between the Two Groups [n (%)] |
Comparison of PDGF-BB and SE-CAD Levels Before and After Treatment
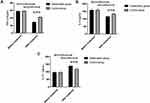
The PDGF-BB and SE-CAD levels, which showed no significant differences between the two groups before treatment (P>0.05), decreased in both groups after treatment, especially in the Obs group (P<0.05). Figure 1
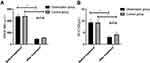 |
Figure 1 Comparison of PDGF-BB and SE-CAD between the two groups before and after treatment. (A) Comparison of PDGF-BB; (B) comparison of SE-CAD. Note: *P<0.05. |
Comparison of Inflammatory Factors Before and After Treatment
No significant difference was observed in serum TNF-α, IL-6, and IL-10 levels between the two groups before treatment (P>0.05). After treatment, serum TNF-α and IL-6 decreased significantly in both groups (P<0.05), with more evident reductions in the Obs group (P<0.05). After treatment, IL-10 was elevated both in the two groups whereas more obvious elevation of IL-10 was observed in the Obs group (P<0.05). Figure 2
Comparison of Toxic and Side Effects During Treatment
The incidence of adverse reactions was 10.91% in the Obs group and 33.33% in the Con group, with a significant difference between the two groups (P<0.05). Table 3
 |
Table 3 Comparison of the Incidence of Adverse Reactions Between the Two Groups [n (%)] |
Comparison of 1-Year Survival Rate
Without any lost follow-up, 17 patients in the Obs group died one year after treatment, with a 1-year survival rate of 69.09%; while 26 patients in the Con group died, with a 1-year survival rate of 42.22%. The 1-year survival rate was significantly higher in the Obs group compared with the Con group (P<0.05). Figure 3
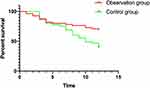 |
Figure 3 Comparison of 1-year survival rate between the two groups. |
Comparison of QOL After 6 Months of Treatment
The Obs group outperformed the Con group in the QOL score assessed form the physical, role, emotion, cognition, social functioning dimensions and total health score, with statistical significance (P<0.05). Table 4
 |
Table 4 Comparison of Quality of Life Between the Two Groups |
Discussion
NPC originates from nasopharyngeal epithelial tissue, with complex pathogenesis and insidious onset, so its early onset is easily overlooked.13 The 5-year survival rate can reach more than 80% in early NPC patients, but it decreases to about 30% in middle-advanced NPC patients with metastasis or recurrence. Therefore, reasonable treatment of advanced NPC is particularly critical to improve the prognosis of patients.14 Recurrent/metastatic NPC is sensitive to chemotherapy, but with short remission and poor prognosis.15 Therefore, it is of great significance to find effective chemotherapy regimens.
In the present study, the efficacy and safety of FU + DDP and GEM + DDP in patients with recurrent/metastatic NPC were compared. FU + DDP is a commonly used chemotherapy regimen at home and abroad, but the response rate of this regimen is 40%-65%, with short reaction time, obvious gastrointestinal side effects, and intolerance in some patients.16 GEM, as a broad-spectrum nucleoside drug against solid tumors with a favorable clinical effect, has become the first-line anti-tumor drug today.17 Previous studies have evaluated the efficacy of GEM in the treatment of NPC, and the results revealed an ORR of 43.8% and a one-year survival rate of 67.0%, indicating that the anti-NPC efficacy of GEM is worthy of recognition.18 In recent years, there have been many studies on the combination therapy of GEM. For example, GEM + DDP was used in an early clinical trial for NPC, achieving an ORR of 42.7% and a 1-year survival rate of 67.0%.19 And reportedly, the total effective rate of GEM + oxaliplatin in the treatment of NPC was 52.0% and the one-year survival rate was 33.9%.20 All the preceding studies show that GEM alone or combination therapy yields good efficacy in the treatment of NPC. This study also revealed a significantly higher ORR in the Obs group, which indicated that GEM + DDP was superior to FU + DDP in treating NPC.
PDGF-BB, with elevated expression in tumor patients, is closely associated with the occurrence and development as well as angiogenesis of cancer cells, which is an important index for clinical evaluation of tumor growth and differentiation, as well as an independent factor for judging the prognosis of patients.21 SE-CAD, a polypeptide with high molecular weight, is closely correlated with the occurrence and invasion of cancer cells, which can assist in monitoring the status of cancer cells before and after radiotherapy.22 Our research results showed that PDGF-BB and SE-CAD were improved in both groups after treatment, and the improvement was more significant in the Obs group compared with the Con group, suggesting that DDP + GEM had better efficacy in the treatment of recurrent/metastatic NPC. The reason is that DDP, as a cell nonspecific drug, can inhibit DNA polymerase catalysis, thus preventing DNA replication and achieving the therapeutic purpose.23 While GEM, a pyrimidine anti-tumor drug with potent anti-tumor activity and affinity, is involved in DNA synthesis and can inhibit the progression of cells from G1 into S phase, resulting in cancer cell death.24 Therefore, under the co-action of the two drugs, PDGF-BB and SE-CAD are decreased and angiogenesis is reduced, thus inhibiting tumor growth. In addition, we compared the inflammation-related factors between the two groups, and the results identified better improvement in the Obs group. Moreover, a higher one-year survival rate was determined in the Obs group, which indicated that DDP + GEM was better than DDP + FU in the treatment of patients with metastatic/recurrent NPC.
For cancer patients, the toxic and side effects of chemotherapy also need to be taken seriously in addition to the chemotherapy efficacy.25 In this research, a significantly lower incidence of toxic and side effects was identified in the Obs group, which suggested that DDP + GEM had no serious adverse reactions and was safe in the treatment of recurrent/metastatic NPC. What’s more, the QOL assessment results revealed that the QOL score in the Obs group was significantly better than that of the Con group after 6 months of treatment. The reason is that in addition to the better efficacy of the Obs group, the lower toxicity is also an important factor affecting the QOL of patients.
To sum up, GEM + DDP has favorable efficacy in the treatment of advanced recurrent/metastatic NPC, with minor side effects, high tolerance, and more clinical benefits for patients, which can be further promoted in clinical practice.
Funding
Scientific research project of Hunan Provincial Health and Family Planning Commission (Subject number:20200863). Guiding Project of Hengyang City (Subject number:2020jh042757).
Disclosure
The authors declare no competing interests in this work.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
2. Guo R, Mao YP, Tang LL, Chen L, Sun Y, Ma J. The evolution of nasopharyngeal carcinoma staging. Br J Radiol. 2019;92(1102):20190244. doi:10.1259/bjr.20190244
3. Lee HM, Okuda KS, Gonzalez FE, Patel V. Current perspectives on nasopharyngeal carcinoma. Adv Exp Med Biol. 2019;1164:11–34.
4. Guan S, Wei J, Huang L, Wu L. Chemotherapy and chemo-resistance in nasopharyngeal carcinoma. Eur J Med Chem. 2020;207:112758. doi:10.1016/j.ejmech.2020.112758
5. Xiao Z, Chen Z. Deciphering nasopharyngeal carcinoma pathogenesis via proteomics. Expert Rev Proteomics. 2019;16(6):475–485. doi:10.1080/14789450.2019.1615891
6. Munch S, Pigorsch SU, Devecka M, et al. Comparison of definite chemoradiation therapy with carboplatin/paclitaxel or cisplatin/5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat Oncol. 2018;13(1):139. doi:10.1186/s13014-018-1085-z
7. Jin Y, Shi YX, Cai XY, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138(10):1717–1725. doi:10.1007/s00432-012-1219-x
8. Ma BB, Tannock IF, Pond GR, Edmonds MR, Siu LL. Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer. 2002;95(12):2516–2523. doi:10.1002/cncr.10995
9. Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. doi:10.1016/S0140-6736(16)31388-5
10. Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995;22(4 Suppl 11):72–79.
11. Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36(9):850–858. doi:10.1200/JCO.2017.75.1644
12. Nolte S, Liegl G, Petersen MA, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the United States. Eur J Cancer. 2019;107:153–163. doi:10.1016/j.ejca.2018.11.024
13. Ng WT, Corry J, Langendijk JA, et al. Current management of stage IV nasopharyngeal carcinoma without distant metastasis. Cancer Treat Rev. 2020;85:101995. doi:10.1016/j.ctrv.2020.101995
14. Chen YP, Lv JW, Mao YP, et al. Unraveling tumour microenvironment heterogeneity in nasopharyngeal carcinoma identifies biologically distinct immune subtypes predicting prognosis and immunotherapy responses. Mol Cancer. 2021;20(1):14. doi:10.1186/s12943-020-01292-5
15. Kang Y, He W, Ren C, et al. Advances in targeted therapy mainly based on signal pathways for nasopharyngeal carcinoma. Signal Transduct Target Ther. 2020;5(1):245. doi:10.1038/s41392-020-00340-2
16. Nakamura K, Nakamura M, Kato H, Morita A. Low-dose 5-fluorouracil/cisplatin therapy as conversion chemotherapy for advanced extramammary Paget’s disease. Kaohsiung J Med Sci. 2020;36(4):287–288. doi:10.1002/kjm2.12168
17. Wei L, Wen JY, Chen J, et al. Oncogenic ADAM28 induces gemcitabine resistance and predicts a poor prognosis in pancreatic cancer. World J Gastroenterol. 2019;25(37):5590–5603. doi:10.3748/wjg.v25.i37.5590
18. Hu S, He X, Dong M, et al. [Efficacy and safety evaluation of gemcitabine combined with ifosfamide in patients with advanced nasopharyngeal carcinoma after failure of platinum-based chemotherapy]. Zhonghua Zhong Liu Za Zhi. 2015;37(8):632–636. Chinese.
19. Wang J, Li J, Hong X, et al. Retrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinoma. Oral Oncol. 2008;44(5):464–470. doi:10.1016/j.oraloncology.2007.06.004
20. Wang FH, Wei XL, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol. 2021;39(7):704–712. doi:10.1200/JCO.20.02712
21. Tang MKS, Yue PYK, Ip PP, et al. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9(1):2270. doi:10.1038/s41467-018-04695-7
22. Inanc M, Sirakaya HA, Karaman H, Bozkurt O. The prognostic importance of VEGF-A, PDGF-BB and c-MET in patients with metastatic colorectal cancer. J Oncol Pharm Pract. 2020;26(8):1878–1885. doi:10.1177/1078155220904151
23. Freyer DR, Brock PR, Chang KW, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. Lancet Child Adolesc Health. 2020;4(2):141–150. doi:10.1016/S2352-4642(19)30336-0
24. Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105(3):192–202. doi:10.1002/bjs.10776
25. Liu GF, Li GJ, Zhao H. Efficacy and toxicity of different chemotherapy regimens in the treatment of advanced or metastatic pancreatic cancer: a network meta-analysis. J Cell Biochem. 2018;119(1):511–523. doi:10.1002/jcb.26210
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.