Back to Journals » Infection and Drug Resistance » Volume 16
Gastric Syphilis Mimicking Lymphoma: A Case Report
Authors Cao J , Zhu J , Xiang Y, Peng P, Liu Q , Fu H, Huang Y
Received 30 March 2023
Accepted for publication 5 July 2023
Published 11 July 2023 Volume 2023:16 Pages 4539—4544
DOI https://doi.org/10.2147/IDR.S414976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jiafei Cao,1,2,* Jian Zhu,3,* Yining Xiang,4 Pailan Peng,1,2 Qi Liu,1,2 Hua Fu,1,2 Yan Huang1,2
1Department of Gastroenterology, the Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China; 2Department of Gastroenterology, The Guizhou Hospital of the First Affiliated Hospital, Sun Yat-sen University, Guiyang, Guizhou Province, People’s Republic of China; 3Department of Gastroenterology, the Second People’s Hospital of Guiyang, the Affiliated Jinyang Hospital of Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China; 4Department of Pathology, the Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Huang, Department of Gastroenterology, the Affiliated Hospital of Guizhou Medical University, No. 28 Guiyi Street, Guiyang, Guizhou Province, 550004, People’s Republic of China, Tel +8618108509475, Email [email protected]
Abstract: Gastric syphilis is a rare manifestation of syphilis that occurs in about 1% of cases and is often overlooked due to its non-specific signs and symptoms. We report a case of a 28-year-old Chinese woman who presented with epigastric pain, nausea, vomiting, hematemesis, alopecia, and weight loss. She tested positive for Helicobacter pylori (H. pylori), with rapid plasma reagin (RPR) and Treponema pallidum particle assay (TPPA) tests showing titers of 1:128 and 1:320, respectively. CT imaging revealed thickening of the gastric wall, exudation around the antrum, and multiple lymphadenopathies. Gastroscopy showed multiple irregular ulcers, which resembled lymphoma. However, biopsy results did not support the presence of lymphoma, but immunohistochemistry showed an abundance of syphilis spirochetes in the mucosal lamina propria and glands. This led to a diagnosis of gastric syphilis. The patient received standard treatment for syphilis as well as anti-H. pylori therapy, and her symptoms and endoscopic findings gradually improved and eventually resolved. We hope that this case report can provide valuable insights into the diagnosis and management of gastric syphilis, which can mimic other diseases like lymphoma.
Keywords: gastric syphilis, Treponema pallidum, lymphoma, endoscopy, case report
Introduction
Syphilis is an infectious disease caused by the spirochete Treponema pallidum (T. pallidum), which is spread mainly via sexual contact or blood transfusion. First reports of syphilis as a sexually transmitted disease occurred in the 15th century.1 In the early 20th century, syphilis was a leading cause of neurologic and cardiovascular disease and a significant public health concern.2 Introduction of penicillin and efforts targeted at improving public health in the 1950s led to a decline in syphilis carriers. However, an increase in reported cases was observed in the 1980s after the emergence of acquired immunodeficiency syndrome.2 At present, infection with syphilis is relatively common in low- and middle-income countries. Conversely, in Western Europe and the Americas, syphilis rates tend to fluctuate periodically.3 Syphilis can be divided into three clinical stages that guide the treatment of patients with syphilis. Depending on the stage of the disease and organs involved, clinical presentation of syphilis can vary greatly, which renders diagnosis challenging. Specifically, in the gastrointestinal tract, syphilis can lead to hepatitis, gastritis, colitis, proctitis, and gastroparesis.4–6 Herein, we describe a rare case of gastric syphilis that resembled lymphoma on gastroscopy.
Case Presentation
A 28-year-old married Chinese woman presented to the hospital with epigastralgia, nausea, emesis, alopecia, and weight loss of five kilograms without apparent cause for more than 1 week, which was aggravated by hematemesis for 4 days. The patient had a history of two cesarean deliveries but denied any other previous medical record including gastrointestinal disease or significant medical illness. The patient was not taking any medications, such as non-steroidal anti-inflammatory drugs. The patient had communicated that she had only had sex with her husband since their marriage, but that her husband seemed promiscuous. A physical examination revealed tenderness in the epigastrium; no skin, oropharyngeal, or genital lesions were observed.
Laboratory evaluation revealed hemoglobin at 110.00 g/L, peripheral blood glucose at 13.28 mmol/L, glycated hemoglobin at 10.19%, human chorionic gonadotropin at < 0.10 mIU/mL, erythrocyte sedimentation rate of 47 mm/h, C-reactive protein at 27.00 mg/L, and positivity for urine ketone. The patient was positive for Helicobacter pylori (H. pylori), with disintegrations per minute of 145 as detected by a 14C-urea breath test. Rapid plasma reagin (RPR) test and Treponema pallidum particle assay (TPPA) showed high titers of 1:128 and 1:320, respectively. The result of human immunodeficiency virus (HIV) antibody screening was non-reactive. Other laboratory parameters were within normal ranges.
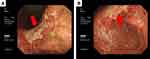
Abdominal contrast-enhanced computed tomography showed thickening of the gastric body and antrum wall, exudation around the gastric antrum, and multiple lymphadenopathies in the abdominal cavity and retroperitoneum (Figure 1). Color Doppler ultrasound showed no abnormalities in the thyroid gland, but numerous lymph nodes were observed bilaterally in the II and V areas of the neck. Gastroscopy revealed multiple geographically irregular, partially fused ulcers extending from the gastric body to the pylorus, and lymphoma could not be ruled out (Figure 2).
 |
Figure 1 Abdominal contrast-enhanced computed tomography showing thickening of the gastric body and antrum wall and exudation around the gastric antrum (Red arrow: lesion). |
Gastric biopsy specimens stained using hematoxylin and eosin (HE) exhibited severe chronic and active inflammation of the gastric mucosa with ulceration. In addition, a large number of plasma cells and minor neutrophil infiltration were observed in the intrinsic mucosa, blood vessels were dilated and congested, and spacing between the glands was increased. As shown in Figure 3, neutrophils had infiltrated and destroyed the glands, resulting in cryptitis. Immunolabeling and lymphoma gene rearrangement assays did not indicate the presence of lymphoma or tumor. However, T. pallidum immunohistochemistry (IHC) showed an abundance of syphilis spirochetes in the mucosal lamina propria and glands (Figure 4).
Based on the patient’s medical history, clinical presentation, serology, imaging, endoscopy, histopathology, and immunohistochemistry revealing the presence of abundant spirochetes, the patient was ultimately diagnosed with gastric syphilis. Additionally, the patient had a concurrent H. pylori infection. Initially, a 14-day quadruple therapy regimen was administered, including twice-daily oral administration of esomeprazole 20 mg, potassium bismuth citrate 220 mg, amoxicillin 1.0 g, and furazolidone 100 mg. However, despite this treatment, the patient’s clinical symptoms did not resolve. As a next step, we administered a single intramuscular dose of 2.4 million units of penicillin G benzathine once a week for 4 weeks. Following this treatment, the patient’s clinical symptoms and endoscopic manifestations gradually improved. We continued to monitor the patient for 6 months until her symptoms completely disappeared. Subsequent RPR testing for syphilis and detection of H. pylori both yielded negative results.
Discussion
Gastric syphilis, which is caused by the invasion of the stomach wall by T. pallidum, was first described in the 19th century.7 Clinical, serologic, and radiologic evidence from early studies performed in the 20th century suggested a high frequency of syphilitic gastritis.7,8 However, autopsy statistics indicated a low incidence of the disease, highlighting the importance of histologic confirmation of syphilis diagnosis.9,10 Gastric syphilis is a rare presentation that is observed in 1% of cases, usually developing during secondary syphilis.11 Because of its rarity and non-specific symptoms and signs, gastric syphilis is not well recognized by many physicians.5,12
Diagnosis can be challenging because most patients do not report a previous history of syphilis, and other clinical features of secondary syphilis are often absent.5,12 The most common symptoms of gastric syphilis are epigastric pain, fullness, nausea, vomiting, early satiety, and weight loss.5 Notably, two-thirds of patients lack syphilis-related symptoms, such as genital ulcers, inguinal lymphadenopathy, and rash.5 Physical examination does not usually contribute to the diagnosis, but epigastric tenderness is the most common sign of gastric syphilis.5 In the present case, the patient’s clinical manifestations, including epigastric pain, nausea, vomiting, hematemesis, and weight loss, were consistent with those reported in the literature. We did not find any other syphilis-related symptoms or signs. Interestingly, the patient also presented with alopecia, which had not been reported previously.
In radiologic examinations, fibrotic constriction, rigidity of the gastric wall, hypertrophy, and irregular folds are found most frequently. In endoscopy, multiple ulcerations, nodular mucosa, and erosions are the most common lesions.5 The gastric antrum and mid-body to pylorus are the most commonly affected areas, while the upper body and whole stomach can be affected in rare cases.5,13 These findings indicate that gastric syphilis may resemble various etiologies such as lymphoma, infiltrating tumors, and Crohn’s disease. In the present case, the patient had thickening of the gastric body and antrum wall, exudation around the gastric antrum, and multiple lymphadenopathies in the abdominal cavity and retroperitoneum. Gastroscopy revealed multiple geographically irregular, partially fused ulcers extending from the gastric body to the pylorus. Combined with an H. pylori infection, which is a prominent risk factor for mucosa-associated lymphoid tissue lymphoma, the patient was initially suspected of having lymphoma.
Histologically, gastric syphilis is characterized by severe, chronic, and active inflammation of the gastric mucosa involving the submucosal layer, with visible crypt abscesses, venule inflammation, granuloma, thickened submucosa, lymphocyte and plasma cell infiltration, intramucosal blood vessel wall thickening, and surrounding infiltration with inflammatory cells.14 In addition to serologic assays, the diagnosis of gastric syphilis requires the detection of T. pallidum on biopsied specimens with Warthin–Starry silver staining, immunofluorescence microscopy, or polymerase chain reaction.15 In the present case, the patient’s endoscopic tissue biopsy did not support a diagnosis of lymphoma or tumor. HE staining of the gastric mucosa revealed severe inflammation and significant infiltration of plasma cells. These results indicated the possibility of a specific infection. Considering her spouse’s history of promiscuity, we suspected the possibility of sexually transmitted diseases. Consequently, we enhanced our screening protocol for sexually transmitted infections, including testing for HIV and syphilis. The results showed negative HIV antibodies, but positive syphilis antibodies. To further confirm the diagnosis, we conducted an RPR test and a TPPA test, which yielded high titers of 1:128 and 1:320, respectively. We highly suspected there to be a relationship between syphilis and gastric lesions. Therefore, T. pallidum IHC was performed, Surprisingly, the IHC analysis showed an abundance of syphilis spirochetes in the mucosal lamina propria and glands. We finally diagnosed this patient with gastric syphilis.
Standard treatment for syphilis often yields favorable results. Intramuscular injection with benzathine penicillin G is effective in treating gastric syphilis. In general, the duration of treatment should be determined by the stage of syphilis.13 Approximately 83% of confirmed patients receive penicillin, and their symptoms resolve rapidly. By contrast, 17% of patients are surgically treated due to complications or suspicion of infiltrating lymphoma or tumor.5 Our patient also had a concurrent H. pylori infection. Despite initially administering a 14-day quadruple therapy to eliminate H. pylori, the patient’s symptoms did not improve. As a next step, we administered intramuscular injections of 2.4 million units of benzathine penicillin G once a week for 4 weeks. Over time, the patient’s symptoms and endoscopic findings gradually improved. We followed up with the patient for 6 months until the patient’s symptoms disappeared completely and the tests for syphilis serology and H. pylori came back negative.
Conclusion
Gastric syphilis is a relatively rare disease with nonspecific symptoms and endoscopic findings, often leading to its misdiagnosis as another gastrointestinal disorder. This can result in treatment delay and unnecessary surgeries. A high index of clinical suspicion is necessary for the diagnosis of gastric syphilis. Patients who are at risk for sexually transmitted diseases, and who present with abdominal complaints and atypical endoscopic lesions without other diagnoses regardless of the presence of H. pylori, should be evaluated for gastric syphilis in the differential diagnosis. An accurate diagnosis of gastric syphilis must combine a complete medical history (including that of sexually transmitted diseases), comprehensive physical examination, and radiologic, endoscopic, serologic, and histopathologic findings. Standard anti-syphilis therapy frequently yields favorable results.
Abbreviations
RPR, rapid plasma reagin; TPPA, Treponema pallidum particle assay; HE, hematoxylin and eosin; IHC, immunohistochemistry.
Ethics Approval and Consent to Participate
Written informed consent for the publication of this report was obtained from the patient and her husband. Details of the case can be published without institutional approval.
Acknowledgments
We would like to acknowledge the patient and her family, who provided their informed consent for this publication.
Funding
No funding was received from any public, commercial, or non-profit organization for this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tampa M, Sarbu I, Matei C, Benea V, Georgescu SR. Brief history of syphilis. J Med Life. 2014;7(1):4–10.
2. Hook EW, Marra CM. Acquired syphilis in adults. N Eng J Med. 1992;326(16):1060–1069. doi:10.1056/NEJM199204163261606
3. Hook EW. Syphilis. Lancet. 2017;389(10078):1550–1557. doi:10.1016/S0140-6736(16)32411-4
4. Ferzacca E, Barbieri A, Barakat L, Olave MC, Dunne D. Lower gastrointestinal syphilis: case series and literature review. Open Forum Infect Dis. 2021;8(6):ofab157. doi:10.1093/ofid/ofab157
5. Mylona EE, Baraboutis IG, Papastamopoulos V, et al. Gastric syphilis: a systematic review of published cases of the last 50 years. Sex Transm Dis. 2010;37(3):177–183. doi:10.1097/OLQ.0b013e3181c0d51f
6. Shinn B, Kistler C, Dhanekula RK, Civan J. Syphilis, The great mimicker, presents as a rare case of concurrent hepatitis and gastroparesis. ACG Case Rep J. 2019;6(6):e00067. doi:10.14309/crj.0000000000000067
7. Morton CB. Syphilis of the stomach: review of the literature and report of a case. Arch Surg. 1932;25(5):880–889. doi:10.1001/archsurg.1932.01160230063005
8. Sexton RL, Dunkley RE, Kreglow AF. Gastroscopic study of 100 cases of early syphilis. Trans Am Ther Soc. 1937;37:73–77.
9. Symmers D. Anatomic lesions in late acquired syphilis: a study of 314 cases based on the analysis of 4880 necropsies at Bellevue Hospital. JAMA. 1916;66:1457–1465. doi:10.1001/jama.1916.02580450023010
10. Meyer KA, Singer HA. Syphilis of the stomach with special reference to its incidence. Surg Gynecol Obst. 1929;1;23–29.
11. Atten MJ, Attar BM, Teopengco E, Nadimpalli V. Gastric syphilis: a disease with multiple manifestations. Am J Gastroenterol. 1994;89(12):2227–2229.
12. Hartwell JA. syphilis of the stomach: a critical review of reported cases from the pathological and clinical viewpoints. Ann Surg. 1925;81(4):767–790. doi:10.1097/00000658-192504000-00006
13. Yu HJ, Kim SJ, Oh HH, et al. Case report of gastric syphilis in Korea: clinical features, pathology, management, and prognosis. Medicine. 2021;100(50):e28212. doi:10.1097/MD.0000000000028212
14. Lan YM, Yang SW, Dai MG, Ye B, He FY. Gastric syphilis mimicking gastric cancer: a case report. WJCC. 2021;9(26):7798–7804. doi:10.12998/wjcc.v9.i26.7798
15. Chen CY, Chi KH, George RW, et al. Diagnosis of gastric syphilis by direct immunofluorescence staining and real-time PCR testing. J Clin Microbiol. 2006;44(9):3452–3456. doi:10.1128/JCM.00721-06
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.