Back to Journals » Journal of Pain Research » Volume 15
Full Recovery after Multiple Treatments with Injectable Ice Slurry
Authors Moradi Tuchayi S, Wang Y, Pence IJ, Fast A, Stemmer-Rachamimov A, Evans CL, Anderson RR, Garibyan L
Received 17 May 2022
Accepted for publication 20 August 2022
Published 14 September 2022 Volume 2022:15 Pages 2905—2910
DOI https://doi.org/10.2147/JPR.S373421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Sara Moradi Tuchayi,1 Ying Wang,1 Isaac J Pence,1 Alex Fast,1 Anat Stemmer-Rachamimov,2 Conor L Evans,1 R Rox Anderson,1 Lilit Garibyan1
1Wellman Center for Photomedicine, Massachusetts General Hospital, Department of Dermatology, Harvard Medical School, Boston, MA, USA; 2Massachusetts General Hospital and Department of Pathology, Harvard Medical School, Boston, MA, USA
Correspondence: Lilit Garibyan, Wellman Center for Photomedicine, Massachusetts General Hospital, 50 Blossom Street-Thier 2, Boston, MA, 02114, USA, Tel +1 617-726-2005, Email [email protected]
Background: Cryoneurolysis uses tissue cooling as an opioid-sparing, long-lasting treatment for peripheral nerve pain. A nerve-selective method for cryoneurolysis by local injection of ice-slurry was developed to allow cryoneurolysis to be performed with a standard needle and syringe, similar to peripheral nerve blocks. Since the treatment of patients with chronic pain may require repeated injections, we investigated the safety and tolerance of repeated treatments in a rat model.
Methods: Three repeated ice-slurry treatments, given 6 weeks apart were performed around the rat sciatic nerve. Nerve and surrounding tissues were collected up to 4 months after the third treatment for analysis. Coherent anti-Stokes Raman scattering (CARS) microscopy was used to study effects on myelin sheaths and axon structure. Immunofluorescence (IF) staining was used to study effects on axon density. Hematoxylin and Eosin (H&E) staining was used to examine histologic effects on sciatic nerve and surrounding tissue.
Results: Histologic and CARS image analysis of nerve tissue collected months after three injections demonstrated recovery of nerve structure, myelin organization and axon density to baseline levels, without any residual inflammation, scarring or neuroma formation. No inflammation or scarring was detected in surrounding skin and muscle tissues.
Conclusion: Repeated ice-slurry injections cause temporary, nerve-selective and reversible changes in the peripheral nerve. There was no histologic damage to surrounding skin and muscle tissues. Repeated treatments with injectable ice-slurry for cryoneurolysis appear to be safe and well tolerated. Clinical studies for patients with chronic pain are warranted.
Keywords: pain treatment, slurry, cooling, multiple treatments
Introduction
Cryoneurolysis is an opioid-sparing therapy for long-lasting reduction of peripheral nerve pain. In recent years, cryoneurolysis has re-emerged as an addition to multimodal analgesic regimens for pain control, as it is able to achieve long-lasting analgesia.1,2 Cryoneurolysis is currently being used in some treatment centers to control post-operative pain, peripheral neuropathic pain, chronic refractory neuropathic pain, post-herniorrhaphy pain, knee osteoarthritis pain and other pain syndromes.3–8 In its current form, cryoneurolysis requires direct nerve-contact and freezing with an extremely cold cryoprobe (−60℃ and below) to provide long-lasting analgesia.3–9 However, the extreme cold temperature of these cryoprobes is not nerve-selective and can damage any tissue near the target nerve.10 Damage to surrounding tissue like muscle (myonecrosis), blood vessels (bleeding, hematoma), and skin (hyper- and hypopigmentation, ulceration, scar formation, alopecia) has been reported with cryoprobe cooling.10–12 In addition, these techniques are highly dependent on the skill of a trained operator able to visualize the nerve location, and are relatively time-consuming, thus limiting their use as a practical pain management modality.1,2,9 Also, capital equipment and expensive probes for each freezing cycle are needed, further limiting their use in pain management. The limitations of current cryoneurolysis methods have caused them to fall into disuse as means of producing long-lasting pain relief.
In contrast, injection of local anesthetics induces selective nerve block for effective pain relief, but they are short acting. The longest injectable anesthetic works only for 48–72 hours, compared to months of pain relief achieved by cryoneurolysis.13 Advantages of anesthesia by local injection include nerve-selectivity, and being a common and practical procedure familiar to most physicians. If a nerve-selective, injectable form of cryoneurolysis existed, the procedure could be as familiar and practical as injection anesthesia for nerve block, with the added advantages of very long duration and complete recovery of nerve.
We developed a novel injectable and nerve-selective method of cryoneurolysis that overcomes the limitations of currently available techniques.14 A biocompatible ice-slurry is locally injected, consisting of a homogeneous suspension of small ice particles in an electrolyte solution. The high latent heat of fusion of water (334 J/g) extracts large amounts of tissue heat during ice melting, while clamping temperature just below 0℃. The advantage of performing cryoneurolysis by injection is more than a convenience. Being a fluid suspension, ice slurry can infiltrate by flowing around the target nerve, unlike the extremely cold cryoprobe devices now held in direct contact with nerves. In addition, use of a standard hypodermic needle and syringe, routinely used by pain physicians, can make ice-slurry treatment more familiar and easily performed at potentially lower cost.
We have used the sciatic nerve model to demonstrate the feasibility, safety, and effectiveness of ice slurry injection in selectively targeting peripheral nerves via cryoneurolysis after a single injection.14 However, many patients with chronic pain are likely to require multiple treatments over time to maintain pain reduction. Therefore, we studied the selectivity and recovery after repeated treatments with ice-slurry injection.
Methods
Adult male Sprague-Dawley rats 200–250g and 7–8 weeks old were purchased from the Charles River Laboratories (Wilmington, MA). Animals were housed under pathogen-free conditions in an animal facility at the Massachusetts General Hospital in accordance with animal care regulations. Total of 13 animals were used in this study. Animal studies were approved by Massachusetts General Hospital IACUC, and followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Slurry composed of normal saline (0.9% sodium chloride) and 10% glycerol was produced using a sterilized commercial slurry maker (Vollrath Frozen Beverage Dispenser; Vollrath Co, LLC, Sheboygan, WI) and a sterilized commercial blender (HGB150, Waring Commercial, Torrington, CT) as described previously.14 Briefly, room temperature sterile normal saline with 10% glycerol solution was added into the sterile slurry maker which was cooled with constant mixing to produce the ice slurry of about 50% ice-content. The slurry mixture was then blended in the sterile blender to create an injectable ice slurry with 40% ice-content. Ice-particle size was controlled by blending to produce slurry with ice-particle size that was consistently injectable through a 15-gauge needle. Slurry temperature was measured with a thermocouple (Omega, USA). Rats were injected with 15mL of −5°C ice slurry with 40% ice content or the identical composition and volume at room temperature (control solution), around the sciatic nerve using 15-gauge needle as previously described.14 Total of 3 injections were performed, 6-weeks apart. Coherent Anti-Stokes Raman Scattering (CARS) microscopy was used to assess myelin and nerve structure as previously described.14,15 Briefly, the CARS microscope used a confocal microscope (Olympus FV1000, Center Valley, PA) modified with a dual output femtosecond pulsed laser system (Spectra-Physics Insight DeepSee, Santa Clara, CA) to generate Raman signal from myelin sheath lipids via CH bond vibrational modes. Depth stacks were acquired from the surface of the nerve to 20µm within the tissue at 1-µm intervals, resulting in 21-frame image stacks. The volumetric image sets were projected to 2D images, using false color to denote depth within the stack: cyan features are most superficial, while red features are deeper within the tissue. A series of three such images were acquired from each sample. The corrected correlation parameter (CCP) was extracted to assess myelin structure following the methodology described previously.14,15 Briefly, CCP corresponds to orderly structure of myelin sheaths. Samples of treated and untreated sciatic nerve, and of surrounding skin and muscle tissue were also processed and embedded in paraffin, and sectioned at 5μm. Sections obtained from sciatic nerve, skin and muscle samples were then deparaffinized, and stained with hematoxylin and eosin (H&E). For Immunofluorescence (IF) staining, sciatic nerve samples were deparaffinized, rehydrated and incubated in Cytomation Target Retrieval solution (DAKO, Carpinteria, CA) at 98°C for 30 minutes for antigen retrieval. Sections were permeabilized with 0.1% Triton X-100 in Tris-buffered saline for 15 minutes, blocked with a solution of 10% goat serum, 3% bovine serum albumin, 0.1% Tween-20 in Tris-buffered saline for 30 minutes, and treated overnight at 4°C with primary anti-rat antibodies (See Supplementary Table 1). Sections were washed in 0.01M PBS and fluorescent secondary antibodies were applied for 2 hours at room temperature. Sections were washed in 0.01M PO4− PBS, and VECTASHIELD Mounting Medium (Vector Labs, Burlingame, CA) was applied. An Olympus Fluoview FV1000 (Olympus, Center Valley, PA) laser scanning confocal microscope with IX81 inverted microscope base was used, with 40×0.80NA (UPLSAPO) objective lens for imaging. To determine the axon density, the number of NF200 positive axons was quantified in 10 random areas of 1000μm2 in each sample using ImageJ software. Statistical analysis was performed using Prism 9 (GraphPad Software, Inc., La Jolla, CA). Data were tested for normality using the D’Agostino & Pearson normality test, and non-parametric tests were used where an outcome measure did not follow the normal distribution. Ordinary one-way ANOVA followed by Dunnett’s multiple comparison test was used as the test of significance for immunofluorescence axon density. Kruskal–Wallis test followed by Dunn’s multiple comparison test was used as the test of significance for corrected correlation parameter (CCP) index. p < 0.05 was considered significant. All the bar graphs show mean + SD or median with interquartile range as noted.
Results
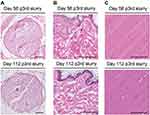
A total of 3 repeated ice-slurry treatments given 6-weeks apart were performed. The treatment group received 15mL ice-slurry injections with −5°C temperature, and control group received 15mL control solution injections with room temperature solution. The treated nerve, surrounding skin and muscle tissue from both groups, was collected at day 56 and day 112 post-third injection for analysis.
H&E stained sections from samples collected at days 56 and 112 post-injection showed normal nerve and other tissue. There was no inflammation, neuroma formation, fibrosis or scarring in the treated sciatic nerves (Figure 1A). No damage or scarring was detected in the surrounding skin, and muscle tissue (Figure 1B and C). After multiple treatments, ice-slurry treated tissues were indistinguishable from control tissues.
The effects on myelin morphology and nerve architecture were examined using CARS microscopy in treatment and control groups (Figure 2A). Corrected correlation parameter (CCP) index, which quantifies the degree of organization in the myelin and axon structure, did not show any difference between baseline, room temperature control and multiple ice-slurry treatment group rats at day 112 post-injection (0.89 [0.73 to 0.92], 0.84 [0.73 to 0.88], and 0.82 [0.77 to 0.87], respectively, p = 0.1048) (Figure 2B). There was slight reduction of CCP value at day 56 post-injection in the treatment group (0.72 [0.60 to 0.80]); however, the difference was not significant (p = 0.17). IF staining was used to examine the effect of repeated slurry injections on axon density (Figure 2C). The density of NF-200 positive axons quantified at day 56 and at day 112 post-injection, both showed levels indistinguishable from baseline and RT control groups (14.20 ± 2.30 at baseline, 14.45 ± 1.99 in slurry day 56, 14.45 ± 1.88 in slurry day 112, 14.70 ± 1.84 in room temperature group day 56, 13.60 ± 1.79 in room temperature group day 112, p = 0.4870) (Figure 2D). Thus, no difference from baseline or from RT control group was seen in treated rats with either CARS or IF imaging, indicating recovery of the nerve structure and axon density after 3 treatments.
Discussion
In this study, we found that repeated treatments with ice-slurry did not cause permanent or long-term changes in the nerve structure and appeared to be safe to surrounding tissues. We have previously shown that a single injection of slurry around the rat sciatic nerve leads to significant decrease in mechanical pain for about 49 days, with full recovery of the nerve structure.14 In this study, we sought to investigate the safety of cryoneurolysis after multiple ice-slurry injections. Both CARS and IF imaging demonstrated recovery of nerve structural architecture and axon density to baseline levels and showed no difference compared to control solution treated rats. Samples of skin and muscle tissue collected near the target nerve where the injections were performed showed no inflammation, fibrosis or scar tissue formation. Thus, repeated cryoneurolysis with ice-slurry injections appears to be safe, producing nerve-selective effects that recover fully by 112 days from the last injection.
The results of this study are consistent with our previously published work demonstrating that single treatment with ice-slurry leads to cryoneurolysis, with complete structural nerve recovery and without damage to surrounding tissues.14 Others have reported that three repeated treatments of rat sciatic nerve with cryoprobes configured to deliver cooling temperature of about −60℃ did not result in long-term changes to nerve structure.16 Ice-slurry is cold, but far warmer than the cryoprobes used for cryoneurolysis. In addition, needles used for ice slurry injection are smaller than currently used cryoprobes, making the method less invasive than current cryoneurolysis. Although uncommon, neural ablation methods including chemical and surgical ablation may result in adverse effects such as neural damage due to neuritis, and neuroma formation. The risk of such adverse effects increases after repeated ablative procedures.17 Unlike these nerve ablation procedures, our histologic and CARS microscopy analysis of ice-slurry treated sciatic nerve showed no inflammation, neuroma formation, fibrosis or scarring after multiple ice-slurry treatments. Another advantage of the ice slurry method over the available neural ablative methods, such as radiofrequency, is that it is much easier to use. With radiofrequency treatment, the probe must be inserted tangentially to the nerves, which is technically much more complex. This does not apply to the ice slurry method as it is administered by a simple injection. Taken together, these results suggest that ice-slurry injection has a wide margin of safety compared with cryoprobe treatment or with any other nerve ablation treatment.
We hope that its efficacy for producing a prolonged analgesia, nerve-selective targeting, lack of adjacent tissue injury, complete recovery, and the familiarity of a local injection procedure will make ice-slurry injection for cryoneurolysis to be more accessible and easily performed. The findings of this study strongly suggest that multiple ice-slurry injections will be safe and well tolerated in patients with chronic pain, who will likely require repeated treatments. Clinical studies of ice-slurry injection for this application are warranted.
Conclusions
In this study, we found complete recovery after repeated ice-slurry injections in the rat sciatic nerve model, compared with baseline and control injections. There was no histologic damage to surrounding skin and muscle tissues. Myelin sheath and axons recovered completely to baseline levels. Multiple, repeated treatments with injectable ice-slurry for cryoneurolysis of peripheral nerves are nerve-selective, recover completely, and appear to be safe.
Study Approval
Animal studies were approved by Massachusetts General Hospital IACUC and followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Acknowledgments
Dr. Anderson is supported as the Lancer Endowed Chair in Dermatology.
Funding
This study was supported by the Henry M Jackson Foundation for the Advancement of Military Medicine, Inc., and Uniformed Service University Award (HU0001-17-2-0009). This work was partially supported by a P41 grant from the NIBIB (award number 2P41EB015871-31) to the Laser Biomedical Research Center.
Disclosure
R.R.A. and L.G. are inventors in patents related to this work, which are owned by the Massachusetts General Hospital. R.R.A., L.G., S.M.T., and Y.W. hold equity in Brixton Biosciences company recently founded to develop and commercialize this technology. R.R.A. and L.G. report Brixton Biosciences Company has licensed a patent related to ice slurry injection for pain control, from his employer, Mass General Brigham. R.R.A., L.G., S.M.T., and Y.W. also report equity in EyeCool Therapeutics, outside the submitted work. L.G. and R.R.A are members of the scientific advisory board of Brixton Biosciences Company. The company had no involvement in this study. C.L.E. is an inventor on patents related to coherent Raman imaging licensed by Harvard University to Leica Microsystems and Zeiss. The authors declare no other competing interests in this work.
References
1. Ilfeld BM, Preciado J, Trescot AM. Novel cryoneurolysis device for the treatment of sensory and motor peripheral nerves. Expert Rev Med Devices. 2016;13(8):713–725. doi:10.1080/17434440.2016.1204229
2. Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6(3):345–360. doi:10.36076/ppj.2003/6/345
3. Fanelli RD, DiSiena MR, Lui FY, Gersin KS. Cryoanalgesic ablation for the treatment of chronic postherniorrhaphy neuropathic pain. Surg Endosc. 2003;17(2):196–200. doi:10.1007/s00464-002-8840-8
4. Sepsas E, Misthos P, Anagnostopulu M, Toparlaki O, Voyagis G, Kakaris S. The role of intercostal cryoanalgesia in post-thoracotomy analgesia. Interact Cardiovasc Thorac Surg. 2013;16(6):814–818. doi:10.1093/icvts/ivs516
5. Dasa V, Lensing G, Parsons M, Harris J, Volaufova J, Bliss R. Percutaneous freezing of sensory nerves prior to total knee arthroplasty. Knee. 2016;23(3):523–528. doi:10.1016/j.knee.2016.01.011
6. Yoon JH, Grechushkin V, Chaudhry A, Bhattacharji P, Durkin B, Moore W. Cryoneurolysis in patients with refractory chronic peripheral neuropathic pain. J Vasc Interv Radiol. 2016;27(2):239–243. doi:10.1016/j.jvir.2015.11.027
7. Tanaka A, Al-Rstum Z, Leonard SD, et al. Intraoperative intercostal nerve cryoanalgesia improves pain control after descending and thoracoabdominal aortic aneurysm repairs. Ann Thorac Surg. 2020;109(1):249–254. doi:10.1016/j.athoracsur.2019.07.083
8. Radnovich R, Scott D, Patel AT, et al. Cryoneurolysis to treat the pain and symptoms of knee osteoarthritis: a multicenter, randomized, double-blind, sham-controlled trial. Osteoarthritis Cartilage. 2017;25(8):1247–1256. doi:10.1016/j.joca.2017.03.006
9. Bittman RW, Behbahani K, Gonzalez F, Prologo JD. Interventional cryoneurolysis: what is the same, what is different, what is new? Semin Intervent Radiol. 2019;36(5):374–380. doi:10.1055/s-0039-1696705
10. Law L, Derian A. Cryoanalgesia. StatPearls; 2020.
11. Hooshmand H, Hashmi M, Phillips EM. Cryotherapy can cause permanent nerve damage: a case report. AJPM. 2004;14(2):63–70.
12. Marsland AR, Ramamurthy S, Barnes J. Cryogenic damage to peripheral nerves and blood vessels in the rat. Br J Anaesth. 1983;55(6):555–558. doi:10.1093/bja/55.6.555
13. Prabhakar A, Ward CT, Watson M, et al. Liposomal bupivacaine and novel local anesthetic formulations. Best Pract Res Clin Anaesthesiol. 2019;33(4):425–432. doi:10.1016/j.bpa.2019.07.012
14. Garibyan L, Moradi Tuchayi S, Wang Y, et al. Neural selective cryoneurolysis with ice slurry injection in a rat model. Anesthesiology. 2020;133(1):185–194. doi:10.1097/ALN.0000000000003124
15. Bégin S, Bélanger E, Laffray S, et al. Local assessment of myelin health in a multiple sclerosis mouse model using a 2D Fourier transform approach. Biomed Opt Express. 2013;4(10):2003–2014. doi:10.1364/BOE.4.002003
16. Hsu M, Stevenson FF. Wallerian degeneration and recovery of motor nerves after multiple focused cold therapies. Muscle Nerve. 2015;51(2):268–275. doi:10.1002/mus.24306
17. Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. 2016;29(1):3–11. doi:10.3344/kjp.2016.29.1.3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.