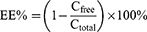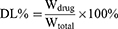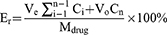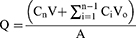Back to Journals » International Journal of Nanomedicine » Volume 19
Fructus Xanthii and Magnolia liliiflora Volatile Oils Liposomes-Loaded Thermosensitive in situ Gel for Allergic Rhinitis Management
Authors Jing Z , Li W, Liao W, Lv Y , Liu Y, Jiang H, Feng Y
Received 23 October 2023
Accepted for publication 1 February 2024
Published 19 February 2024 Volume 2024:19 Pages 1557—1570
DOI https://doi.org/10.2147/IJN.S445240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Zhongxu Jing,* Wenqing Li,* Wei Liao, Ying Lv, Yuwei Liu, Haibo Jiang, Yufei Feng
Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Ministry of Education, Harbin, 150040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yufei Feng, Key Laboratory of Basic and Application Research of Beiyao (Heilongjiang University of Chinese Medicine), Harbin, 150040, People’s Republic of China, Tel +86-18503653988, Email [email protected]
Purpose: The aim of the present study was to fabricate a Fructus Xanthii and Magnolia liliiflora volatile oils liposomes-loaded thermosensitive in situ gel (gel/LIP/volatile oil) for effectively treating allergic rhinitis via intranasal administration.
Patients and Methods: Particle size, polymer dispersity index (PDI), entrapment effectiveness, and cumulative drug permeation of the developed liposomes were assessed. Then, a thermoreversible in situ gel was created using the liposomes loaded with volatile oils of Fructus Xanthii and Magnolia liliiflora. The effectiveness of this treatment for allergic rhinitis was confirmed by evaluating nasal symptoms, and hematological results, after injecting the formulation into the ovalbumin (OVA)-sensitized mice, we conducted hematoxylin-eosin staining (HE) and immunohistochemistry to evaluate the outcomes. The effects of the gel/LIP/volatile oil formulation for nasal delivery of volatile oil in the treatment of rhinitis were then assessed.
Results: The average particle size was 95.1 ± 3.6 nm, and the encapsulation efficiencies of Fructus Xanthii and Magnolia liliiflora volatile oils were 70.42 ± 5.41% and 67.10 ± 6.08%, respectively. Drug loadings of Fructus Xanthii and Magnolia liliiflora volatile oils were 9.10 ± 0.98% and 16.10 ± 1.03%, respectively. The binary formulation produced a gel rapidly in the nasal cavity with a strong mucosal adherence at a temperature of delivering volatile oil to the nasal mucosa steadily and continuously. After nasal administration, the gel/LIP/volatile oil sustained the volatile oil delivery into the mucosa. In comparison to the monolithic formulations, the gel/LIP/volatile oil binary formulation exhibited superior performance in terms of drug delivery capability and pharmacodynamic effects.
Conclusion: This binary preparation displayed the ability to deliver drugs to the nasal mucosa and exhibited positive pharmacodynamic effects in treating OVA-induced rhinitis in mice. As a result, it has the potential to serve as a delivery platform for Traditional Chinese medicine in the treatment of allergic rhinitis.
Keywords: volatile oil of Magnolia liliiflora, volatile oil of Fructus Xanthii, liposomes, thermosensitive gel, allergic rhinitis
Introduction
Allergic rhinitis (AR) is a highly prevalent chronic condition that impacts 400 million individuals globally and 100 million people in Europe.1,2 Intranasal glucocorticoids, antihistamines, and disodium cromoglycate are all viable pharmaceutical therapy choices. Antihistamine sedation, disodium cromoglycate nasal mucosal irritation, and candidiasis are a few of the moderate side effects of these corticosteroid drugs. Traditional Chinese medicine (TCM) for treating AR has several clear benefits. Recently, some studies have demonstrated effectiveness in managing the AR and immunological functions of the body.3–5 Some AR patients favor TCM treatments.6 Fructus Xanthii powder is recorded in “Ji Sheng Fang” and is composed of Fructus Xanthii, Magnolia liliiflora, Angelica dahurica, and mint. It was considered by Chinese doctors of all dynasties to be the main prescription formulation for treating nasal diseases, and Fructus Xanthii was the “monarch’s medicine”, while Magnolia liliiflora was the minister’s medicine in “Ji Sheng Fang”.7 Fructus Xanthii is a dried and ripe fruit with the involucre of Xanthium sibiricum Patr., Magnolia liliiflora consists of volatile oils and sesquiterpene lactones. It is included in the 20th edition of Chinese Pharmacopoeia, that volatile oils are the main pharmacodynamic components in Fructus Xanthii and Magnolia liliiflora.8,9 Studies have confirmed that both TCMS have anti-inflammatory, anti-allergic, and antihistamine effects. At the same time, their compatibility can reduce Fructus Xanthii toxicity and enhance its anti-inflammatory effects, have the effect of rapidly relieving nasal congestion symptoms, and treat AR with multi-target and multi-pathway regulation.10,11
Nasal medication delivery methods have the potential to bypass the first-pass effect and prevent the gastrointestinal tract’s elimination. In addition, based on the AR mechanism of action and the lesion position, the best method for treating AR clinically is to use medication directly on the nasal mucosa to focus its distribution, ensure maximum effectiveness, and reduce systemic pharmacological side effects. Based on the characteristics of a high absorption rate in nasal administration, a specific drug’s therapeutic benefit may be achieved with a lower dose.12,13 Intranasal drug delivery offers all of the previously mentioned benefits, has a large absorptive surface area and high vascularity,14–16 and is regarded as equivalent to the intravenous administration route.
The majority of nasal preparations that are presently commercially accessible are sprayed. Their therapeutic efficacy is somewhat impacted by the nasal cilia’s scavenging function, which results in a brief drug residence duration (15–30 min) on the human nasal mucosal surface.17 The creation of therapeutically effective and secure nasal preparations is an important research area with significant clinical application potential. A mucoadhesive system may be used to slow down a rapid mucociliary clearance and increase bioavailability. Drug residence is increased by these systems adhering to the mucus. Moreover, they facilitate the interaction between the nasal mucosa and the medication, resulting in improved drug absorption. As a result, the drug becomes more readily available in the body.18 Polymers in a fluid or semisolid state at the administration site are used to create an in situ gel, which reacts to outside stimuli. Additionally, these gels include conformations that can be changed in a unidirectional manner to create a semisolid or solid preparation.19 Various types of in situ gel options are available, including chemical material-sensitive, thermo-sensitive, ion-activated, electric-sensitive, magnetic field-sensitive, and ultrasonic-sensitive gels. The in situ gel that responds to temperature changes, also known as a temperature-sensitive gel, in situ gel is a widely used and highly advanced type of gel in current research. When the temperature changes from the normal room temperature to the temperature of the human body, a temperature-responsive in situ gel that exists in a liquid or semi-solid state at room temperature solidifies into a gel, providing good adhesion and gradual release effects.20,21 The gel offers the features of good biocompatibility, easy usage, simple production, excellent tissue affinity for the mucosa, and a long period of time due to its three-dimensional hydrophilic network structure.
Poloxamer has good surface activity, is water soluble, and has a high level of safety. Additionally, poloxamer has the ability to thermally reverse gel formation, which makes it a liquid at low temperatures and a gel at higher temperatures.22–24 In the present study, temperature-sensitive in situ gels containing poloxamer as their primary constituent were employed. Nanoparticles show potential as medication delivery devices in colloidal systems because of their higher stability and simplicity in production. Methods of combining nanoparticles with colloids are employed for regulated and targeted drug delivery, as well as for increasing the bioavailability of hydrophobic medicines.25–27
In order to avoid the low bioavailability of hydrophobic medicines during nasal administration, the current study focused on developing a nanoparticle system for nasal delivery. As a result, a thermally activated mucoadhesive in situ nasal gel was created. In this gel, the drug (in nano form) is evenly dispersed within a polymer-based thermosensitive gel. This delivery method is anticipated to overcome the shortcomings of rapid mucociliary clearance and combine the benefits of mucoadhesion with nasal delivery (Scheme 1). Because of its good water solubility, in addition to having low levels of toxicity and irritation, it is also compatible with most of the recommended ingredients for the formulation, poloxamer 407 was chosen as a thermosensitive gelling polymer.28 Poloxamer 188 can be used together with poloxamer 407 to adjust the transition temperature of gel formation to obtain a suitable temperature-sensitive gel, which is a representative excipient in this type of matrix.29
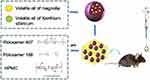 |
Scheme 1 The gel/LIP/volatile oil temperature-sensitive hydrogel steadily delivers volatile oil into the Nasal mucosa. |
Materials and Methods
Materials
From China’s Shanghai Jizhi Biochemical Co., Ltd., we purchased soy lecithin (SPC). Additionally, we bought cholesterol from the Chinese company Shanghai Aladdin Biochemical Co., Ltd. The volatile oil of Fructus Xanthii and volatile oil of Magnolia liliiflora were purchased from Ji’an Huaxin Natural Plant Essential Oil Co. (Jiangxi, China); methyl linoleate reference substance and camphor reference substance were provided by Chengdu Zhibiao Chemical Pure Biotechnology Co., Ltd. (Chengdu, China); chromatographic-grade methanol was purchased from Beijing Decema Co., Ltd., China; We bought poloxamer 407 and poloxamer 188 from BASF Co., Ltd. (German, Ludwigshafen); hydroxypropyl methylcellulose was obtained from Shanghai Maclean Biochemical Co., Ltd., China; mitochondrial stripping, we purchased IL-4, IL-13, TNF-α, IFN-γ, IgE, and histamine kits from RD Systems Inc. The C57BL/6J mice were obtained from Liaoning Changsheng Experimental Animal Technology Co., Ltd. in Liaoning, China. The Laboratory Animal Management Committee at Heilongjiang University of Traditional Chinese Medicine, the location of the study, gave its approval for animal trials (No. 2019112101). Number for an animal license (SYXK (Liao) 2020-0001). Every animal experiment was conducted in Heilongjiang University of Chinese Medicine’s Laboratory Animal Safety Evaluation Center’s SPF animal room. When doing research on animals, we adhere to the “3R” and “5F” principles, which call for the use of reduction, refinement, and replacement techniques. Important guidelines for preserving the well-being of experimental animals include freedom from thirst and hunger, freedom from discomfort, freedom from pain, damage, and sickness, freedom from the manifestation of one’s true character, and freedom from fear and worry. Additionally, we adhere to the International Council for Laboratory Animal Science (ICLAS) to ensure the welfare of laboratory animals.
Preparation of Fructus Xanthii and Magnolia liliiflora Volatile Oil Nanoliposomes
Thin-film hydration technique was used to prepare Fructus Xanthii and Magnolia liliiflora volatile oil nanoliposomes. A round-bottom flask was used to dissolve the volatile oils of Fructus Xanthii and Magnolia liliiflora, SPC, and cholesterol (mass ratio, 1:2:6:1). After evaporating the organic solvent with a rotary evaporator, a uniform coating was formed on the bottom round-bottom flask, the film was dried overnight under vacuum after which it was rehydrated using either phosphate-buffered saline solution (PBS, pH 7.4), and 4.2 mg/mL of phospholipids should be attained. To prepare the volatile oil-loaded liposomes, they were subjected to sonication in a 37 °C water bath for 10 minutes while being probed at 40 W for 100 seconds.
Determination of Encapsulation Efficiency and Drug Loading
Three mL of Fructus Xanthii and Magnolia liliiflora volatile liposome solution were placed into a 5-mL centrifuge tube. Following 30 minutes of high-speed centrifugation at a rate of 10,000 revolutions per minute, 2 mL of the liquid lying above the sediment was taken out. Following this, 2 mL of a solution containing 0.5 mole per liter of potassium hydroxide in methanol were introduced and the mixture was subjected to heating in a water bath set at a temperature of 50 °C for 10 minutes while being agitated. The solution was then removed from the heat and cooled, after that, deionized water was added in 5 mL. The supernatant was then centrifuged through a 0.22-µm membrane to obtain a fresh filtrate. At the same time, 2 mL of not ultrafiltered liposomes were aspirated, added to 4 mL of methanol, and sonicated for 5 min for demulsification. After that, 2 milliliters of a solution containing 0.5 moles per liter of potassium hydroxide dissolved in methanol was added. The mixture was heated in a water bath at a temperature of 50 °C for a duration of 10 minutes while being shaken. After that, the mixture was cooled down, deionized water was added in 5 mL, and the supernatant was obtained by centrifugation. After passing through a 0.22-µm membrane, the filtered liquid was examined using a gas chromatography-mass spectrometer (GC-MS) to determine the levels of unbound camphor and methyl linoleate, as well as the overall levels of camphor and methyl linoleate in the liposomes. GC-MS conditions are shown in supporting Information and a chromatogram of different samples is shown in Figure S1. The encapsulation efficiency and drug loading formulas are as follows:
Here, Cfree represents the concentration of free camphor or methyl linoleate, Cfree represents the concentration of total camphor and methyl linoleate in liposomes, Wdrug represents the mass of Fructus Xanthii and Magnolia liliiflora volatile components (calculated using the amount of methyl linoleate or camphor), Wtotal represents the total weight of SPC, cholesterol, and Fructus Xanthii and Magnolia liliiflora volatile oils.
Determination of in vitro Release Rate of Fructus Xanthii and Magnolia liliiflora Volatile Oil Nanoliposomes
The in vitro release of Fructus Xanthii and Magnolia liliiflora volatile oil nanoliposomes was determined by dialysis.30 Specifically, 0.5 mL of volatile oil nanoliposomes and free volatile oil solution (camphor content: 35 ng/mL, linoleic acid content: 42 ng/mL) were added to a dialysis bag (6000–8000 Da) and placed in 30 mL of release medium. The given specimen was subjected to centrifugation at a speed of 50 revolutions per minute and a temperature of 37 °C. The medium used for releasing the substances was a solution of 0.1% Tween 80 in PBS, with a pH value of 7.4. At specific time intervals (0.5, 1, 2, 4, 6, 8, 12, 24, 36, and 48 hours), 0.5 mL of the release medium was collected. To maintain the volume, an equal amount of fresh-release medium was added to the drug-release medium. The sample’s quantities of camphor and linoleic acid were measured, and each time’s cumulative release was determined using the prescribed formula.
The equation represents the cumulative release of a drug from a liposome. Er is the cumulative drug release, Ve is the displacement volume, Ci is the concentration of the drug released during the ith replacement sampling, Vo is the volume of the release medium, and Cn is the concentration of the release medium in the nth sampling. The drug concentration Mdrug represents the amount of drug in the liposome. A release curve was subsequently generated.
The Process of Creating a Thermosensitive in situ Gel
Poloxamer 407 and Poloxamer 188 were utilized in the formulation of composite hydrogels, the procedure for preparing these hydrogels has been detailed in prior publications.31 Briefly, gel/LIP/volatile oil was prepared using the cryolytic method. Poloxamer 407 and Poloxamer 188 and hydroxypropyl methylcellulose (HPMC) were dissolved in the volatile oil of Magnolia liliiflora and Fructus Xanthii containing 50 mL of the liposome solution at concentrations of 14.015%, 7.681%, and 0.500%, respectively. The preparation was continuously stirred to disperse the components and refrigerated for 24 h until completely swollen to obtain a clear, evenly dispersed, lump-free liquid.
Determination of Gelation Temperature, Gelation Time, Gel Strength, and Mucosal Adhesion
The gelation temperature was measured using the inversion method with some modification.32 A total of 50 mL of the prepared non-coagulated gel solution was placed into a 100-mL beaker and a thermometer was inserted 2 cm below the gel liquid level. The temperature was kept rising at the rate of (0.5–1.0) °C·min−1 in a constant-temperature water bath. The solution change was observed each time the temperature rose. The temperature when the solution stopped flowing after the beaker was inverted was recorded as the gelling temperature. Each individual sample underwent three parallel measurements, and the resulting gelling temperature was determined by calculating the average value. After intranasal administration, the gel preparation may be diluted due to the presence of nasal secretions. To investigate how dilution impacts the gelation temperature of the thermosensitive gel, we added phosphate buffer (0.25 mL/g gel) to 4 g of the prepared gel in a small beaker, following the method described by Soliman et al.33 We then measured the gelation temperature after the dilution.
The rod-stop method was modified to determine the gelation time.34 Five mL of the mixture was put in a 20 mL beaker that was set on a heated magnetic stirrer. At 34°C, the liquid was continuously swirled (30 rpm). The time was noted when gelation caused the magnetic rod to stop moving. The outcomes represented the average of the measurements from three independent experiments that were run concurrently.
The amount of time needed for a mass to insert 5 cm into the gel was used to gauge gel strength. Each sample (50 g) was placed into a 100-mL measuring cylinder to gel at 34°C in a water bath that was maintained at a constant temperature. The gel solution was then covered with a 35 g weight, which was permitted to insert 5 cm into the gel. The resulting penetration time was recorded.35
A two-arm balancing method was used to determine the mucosal adhesion gel strength.36 A sample of pig nose mucosa was positioned on a glass vial filled with PBS at a temperature of 34 °C and a pH of 7.4. The glass vial was securely attached to the center of a phosphate buffer-containing beaker with a pH of 7.4 and a temperature of 34 °C. The medication, which had been fixed in a gel form, was bonded to the rubber stopper’s underside. The strength of adhesion between the mucosa and the gel was determined by calculating the amount of stress required to detach the gel from the mucosal surface per unit area. The procedure for calculating the duration of adhesion between the gel preparation and the mucosa was based on the method described by Wang et al37 but with a few minor modifications, to the sample was added a pregelatinized sample (2 g) containing 0.1% methylene blue, which was then applied to the mucosa of a pig nose at 34 °C.
The experiment involved rinsing the sample with a synthetic fluid that mimics the patient’s nasal fluid. The rinsing was done at a consistent rate of 5 mL/min and a pH of 7.4. The experiment was conducted in a controlled environment with a constant temperature of 34 °C and at an angle of 40°. The length of time needed to completely wash off the formulation was calculated based on the color shift. The average of four separate measurements was presented as the outcome for each of the aforementioned tests.
Ex vivo Nasal Mucosal Drug Penetration and Retention
The nasal mucosal penetration test was performed ex vivo, the Franz vertical diffusion cell approach was used.38 Briefly, an ex vivo nasal mucosa was first prepared. A newly killed pig’s nose was opened up, and the mucosa covering the turbinate bone and nasal septum was carefully peeled off with tweezers. After that, the area was rinsed with regular saline. The diffusion cell was fixed with the treated nasal mucosa (penetration area: 3.14 cm2). The supply tank was filled with 6 g of the test sample, which was then equally distributed over the nasal mucosa. The cell was then wrapped in plastic, placed in a receiving tank with 19 mL of normal saline (34 ± 0.2°C), and allowed to have direct contact with the lining of the nose. A 1-mL sample was obtained at 0, 0.5, 1, 2, 4, 6, 8, 12, 16, 24, 32, 40, and 48 h, by introducing the same volume of new media to the receiver compartment, the sink state was maintained, and the amount of penetrated volatile oil in various preparations was measured.
The diffusion apparatus was positioned in a temperature-controlled bath at a specific temperature of 34 ± 0.2 °C. It was then subjected to agitation using a magnetic stirrer rotating at a speed of 300 revolutions per minute. Time T was plotted on the x-axis, while the cumulative permeation per unit area Q (measured in µg/cm2) was plotted on the y-axis to create a graph illustrating the penetration of camphor and methyl linoleate into the mucosa. The cumulative volatile oil permeation of the gel was calculated using the formula below:
Where Cn is the drug concentration at the nth point, Ci is the drug concentration at the ith point, V and Vo are the volume of the receiving cell and the volume of the sampling solution, respectively, and A is the diffusion area of the diffusion cell. Linear regression of time was determined using Q (µg/cm2). A permeation curve was also generated.
After in vitro permeation studies lasting 48 hours, the nasal mucosal tissues were cleaned three times using a solution made of equal parts ethanol and water. The aim of this process was to remove any remaining medication residue that may have been on the tissue’s surface. The nasal mucosa was then chopped and put in a centrifuge tube, where it was sonicated with methanol for two more hours after being crushed with a tissue homogenizer. After that, the resultant supernatant was recovered by centrifuging for 30 minutes at 10,000 RPM. The volatile oil content was evaluated using the same methods.
Preparation of AR Model Mice and Intranasal Volatile Oil Administration
A total of 36 mice were separated into the following six groups at random: normal, AR model, naked volatile oil, AR + gel, AR + LIP, and AR + gel/LIP/volatile oil (Magnolia liliiflora volatile oil: 28×10−3 mg/mouse, camphor content in Magnolia liliiflora volatile oil: 5.01%, and Fructus Xanthii volatile oil: 14×10−3 mg/mouse, Fructus Xanthii volatile oil contains linoleic acid at a concentration of 2.98%.39 OVA sensitization was used to generate the AR model based on prior research.40 The initial sensitization process involved using a prepared mixture of allergen suspension that contained 0.04 mg of OVA and 0.45 mg of Al(OH)3 gel. This mixture was injected into the peritoneal cavity every second day, seven times in total. From day 15 to day 21, 20 µL of OVA (50 mg/mL) were injected once daily into each nostril using a micropipette. After the 29th day, the mice received this medication for an additional 14 days in a row. After being fixed for 10 minutes during the nasal drip, the mice were placed back in their cage. Approximately 10 µL of volatile oil, LIP/volatile oil, gel/volatile oil, and gel/LIP/volatile oil containing sterile saline were also given to the model group each time. Each mouse received a dose of 2.8×10−4 mg and 1.4×10−4 mg of Fructus Xanthii volatile oil. Utilizing a micropipette, the identical volume of regular saline and nasal drops was intraperitoneally administered to the mice in the normal group. The six-mouse groups were sacrificed under anesthetic on the 35th day, the mucosal tissues were extracted in order to conduct further investigations.
Assessment of Rhinitis Symptoms
We assessed all the mice for nasal symptoms using the methodology described by Wermeling et al.41 On the 35th day, each mouse was placed in an individual transparent cage. After a period of 30 minutes to adjust to the surroundings, we observed sneezing, nasal scratching, and nasal secretions for a duration of 10 minutes. None of the instances of sneezing or scratching received a score of 0, behaviors occurring between one and four times per minute received a score of 2, and behaviors that happened more than six times per minute were assigned a score of 3. No nasal secretions received a score of 0, one nostril secretion received a score of 1, two nostril secretions received a score of 2, and overflow liquids from two nostrils received a score of 3. These scores were used to compute and compare each mouse’s overall nasal symptom score.
Enzyme-Linked Immunosorbent Assay (ELISA)
We performed an ELISA assay to quantify the concentration of IL-4, IL-13, IFN-α, TNF-γ, IgE, and histamine in the serum samples. During the experiment, we adhered to the protocol outlined by the manufacturer. Using a microplate reader, the absorbance values at 450 nm were calculated. The average result was reported after each measurement was performed three times. IgE, IL-4, and TNF levels were recorded in pg/mL, whereas IL-13 values were recorded in ng/mL.
The Identification of Apoptotic Cells in the Nasal Mucosa
Nasal mucosa was examined using the TUNEL technique to identify cell apoptosis. Mouse nasal mucosa was embedded in paraffin and preserved with 10% neutral formaldehyde, dewaxed with standard xylene, and added to a gradient of ethanol in water. With a 400x light microscope (model ES18-TZLED), we randomly chose six distinct fields of view from each slice in order to calculate the cell apoptosis rate of each nasal mucosa group. Apoptotic cell nuclei were stained brown, while healthy cell nuclei were blue.
HE Staining
The maxillary skin was removed from the mice after they were sacrificed. We first detached the skin from the skull and then made an incision along the midline of the nose in order to visualize the nasal septum and both nasal cavities. The bilateral nasal mucosa tissues were promptly removed after continuous administration for a week, treated three times with PBS, immersed in a solution containing 4% paraformaldehyde to fix my tissues, and then, embedded in regular paraffin and cut into thin sections of approximately 5 µm. The sections were then stained with HE after undergoing a dewaxing process using xylene and being cleansed with a gradient of ethanol to water. Following the standard section dehydration, clearing, and resin mounting procedures, the nasal mucosal histological changes were examined under a microscope (CX43, Olympus, Tokyo, Japan).
Results and Discussion
Characterization of Volatile Lipid Plastids in Fructus Xanthii and Magnolia liliiflora
Three batches of Fructus Xanthii and Magnolia liliiflora volatile oil nano-liposomes were collected and their color was observed. The samples were diluted and placed onto a dedicated copper net. After staining with phosphotungstic acid, the mixture was allowed to dry naturally. We utilized a transmission electron microscope to conduct observations and capture images of the morphology of the particles (TEM; Figure 1A). The results showed that the prepared nanoliposomes exhibited light blue fluorescence. Liposome appearance was excellent under TEM, and their shape was spherical. The Fourier transform infrared spectroscopy (FTIR) spectrum of Fructus Xanthii and Magnolia liliiflora volatile oil nano-liposomes is shown in Figure 1B. The absorption peaks of the mixture were all from the volatile oil, phospholipids, and cholesterol. In the absorption peaks of the mixture and liposomes, 3344 cm−1 was the characteristic peak generated by the water vapor association when potassium bromide was compressed. Furthermore, 1743 cm−1, 1159 cm−1, and 1080 cm−1 were the characteristic absorption peaks of phospholipids, while 1379 cm−1, 1467 cm−1, and 1080 cm−1 were the characteristic absorption peaks of cholesterol.42,43 In the liposome, the absorption peak of the volatile oil at 1200–1350 cm−1 disappeared, indicating that the two volatile oils formed a new phase with the interaction of SPC and cholesterol, which demonstrated the success of the liposome preparation.
Three batches of Fructus Xanthii and Magnolia liliiflora volatile oil nano-liposomes were prepared. Their particle size and zeta potential were determined (Figure 1C, Table 1). The results showed that the liposome particle size was 95.1 ± 3.6 nm. The PDI was 0.174 ± 0.031 (Table 1), indicating that the PDI of each liposome was relatively uniform. The zeta potential was −17.2 ± 1.9 mV (Figure 1D, Table 1). The entrapment efficiency of Fructus Xanthii and Magnolia liliiflora volatile oils was 70.42 ± 5.41% and 67.10 ± 6.08%, respectively, and the highest drug loading was 9.10 ± 0.98% and 16.10 ± 1.03%, respectively (Table 1).
 |
Table 1 Physicochemical Characteristics of the LIPs (Mean±SD, n=3) |
Drug-loaded liposome release properties in the release media were also determined. The data presented in Figure 1E demonstrates that the combined amount of Fructus Xanthii volatile oil and Magnolia liliiflora volatile oil released from LIPs after 48 hours was 43.57% and 39.79% respectively, showing that it was continuously released over a sustained period of time. This gradual process helps to avoid abrupt carrier release during delivery.
Characterization of Thermosensitive Gel/LIP/Volatile Oil
The prescription was optimized using Design Expert 12.0.3 software (Stat-Ease, Minneapolis, MN, USA) software, the optimal prescription was 14.015% Poloxamer 407, 7.681% Poloxamer 188, and 0.5% HPMC. Three batches of samples were prepared using the optimal process for result verification and quality evaluation. Gel/LIP/volatile oil state before and after gelation is shown in Figure 1F. The value and range of the optimized gelling temperature and gelling temperature after dilution were determined (Table S1). The predicted gelling temperature of the prepared preparation was 34.0°C, and the predicted gelling temperature after dilution was 34.03°C. Typically, the nasal preparations effectively preserved the gel integrity for a duration of 20–50 seconds, while the gel/LIP/volatile oil did it for 43.6 ± 9.07 s. The mucosal adhesion strength of the gel/LIP/volatile oil was 30 ± 1.74 g/cm2. The gelation time of the gel/LIP/volatile oil was 190.3 ± 8.43 s, while the adhesion duration was 5.9 ± 1.12 h, ensuring that the gel stayed in the nasal cavity for a long time.
Efficiency of ex vivo Permeation and Retention of Volatile Oil in Nasal Mucosal Tissue
The Cumulative Volatile oil of Fructus Xanthii permeation from LIP/volatile oil, gel/volatile oil, and binary formulation ofgel/LIP/volatile oil were 22.53 ug/cm2, 4.81 ug/cm2, and 18.28 ug/cm2, respectively (Figure 2A). While Cumulative Volatile oil of Magnolia liliiflora permeation from gel/volatile, LIP/volatile oil, and gel/LIP/volatile oil were 11.05 ug/cm2, 50.79 ug/cm2, and 42.42 ug/cm2, respectively (Figure 2B). The flux (Jss) values were measured for various formulations of Fructus Xanthii and Magnolia liliiflora. For Fructus Xanthii, the Jss values were found to be 0.50 µg/cm2/hr, 0.10 µg/cm2/hr, and 0.42 µg/cm2/hr for the LIP/volatile oil, gel/volatile oil, and gel/LIP/volatile oil binary formulations, respectively. On the other hand, the Jss values for Magnolia liliiflora formulations were determined to be 1.05 µg/cm2/hr, 0.24 µg/cm2/hr, and 0.90 µg/cm2/hr for the LIP/volatile oil, gel/volatile oil, and gel/LIP/volatile oil binary formulations, respectively. These findings demonstrated that the hydrogel formulation had a stronger inhibition impact than delivery vehicles in preventing volatile oil from penetrating the mucosa. The volatile oil was removed to determine the effective dosage that remained in the inflamed mucosa following the topical application of various formulations to the mucosa 48 hours after administration and delivery. The formulation consisting of gel/LIP and volatile oil exhibited the highest rate of mucosal retention 20.41% of the initial dose of Fructus Xanthii volatile oil remained in the mucosa. In comparison, 27.81% of the Magnolia liliiflora volatile oil was retained. After 48 h, the mucosal retention of the Fructus Xanthii and Magnolia liliiflora gel volatile oils was 5.96% and 6.28%, respectively, while that of Fructus Xanthii and Magnolia liliiflora LIP/volatile oils was slightly lower at 9.32% and 10.56%, respectively (Figure 2C and D). Generally speaking, the gel/LIP/volatile oil binary formulation may be the best option for local nasal mucosal administration because it has the lowest drug penetration and maximum retained rates.
Impact of Administering Volatile Oil on the Behavioral Performance of Mice
In the experiment, we prepared mice models of AR. We then treated the mice with different combinations of AR + gel/volatile oil, AR + LIP/volatile oil, and AR + gel/LIP/volatile oil. We observed that the mice treated with AR + gel/volatile oil, AR + LIP/volatile oil, and AR + gel/LIP/volatile oil had a significant decrease in the number of nasal scratches and sneezing compared to the AR model mice. The group treated with AR + LIP/volatile oil showed the best results with the most noticeable improvement in symptoms. On the other hand, the mice in the volatile oil group showed only a slight improvement in AR symptoms, which was not very obvious (Figure 3). This could be due to the fact that the volatile oil was efficiently eliminated by the cilia in the nasal cavity.
 |
Figure 3 Nasal symptom score results. Each value represents the mean ± SD of the 6 rats in each group. ##p < 0.01 compared with the control group; **p < 0.01 compared with the allergic rhinitis (AR). |
Histamine and Inflammatory Cytokine Levels
We utilized ELISA to evaluate the levels of IL-4, IL-13, IFN-α, TNF-γ, IgE, and histamine, which play a crucial role in AR. The groups after the administration of volatile oil (AR + volatile oil, AR + gel/volatile oil, AR+LIP/volatile oil, and AR + gel/LIP/volatile oil) had lower levels of IL-4, IL-13, IFN-α, TNF-γ, IgE, and histamine compared to the AR model group (Figure 4). Overall, the pharmacodynamic impact was better in the AR + gel/LIP/volatile oil group. As a result, these findings revealed that AR + gel/LIP/volatile oil had the best control over inflammatory cytokines.
Effect of Volatile Oil Delivery on Mouse Nasal Mucosa Apoptosis
The effects of each preparation’s nasal injection of volatile oil on nasal mucosal cells were assessed using TUNEL labeling (Figure 5). After analyzing the nasal mucosa in each group, it was clear that the model group’s nasal mucosal synovial cells had more brown particles in their cytoplasm than the normal group did, indicating greater nasal mucosal cell death. The number of cells undergoing apoptosis in the nasal mucosa was significantly reduced in both the volatile oil delivery group and the volatile oil group versus the model group. The gel/LIP/volatile oil group had the fewest apoptotic cells among all the groups.
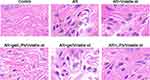 |
Figure 5 Histopathological changes in the nasal mucosa of rats were investigated by hematoxylin-eosin (HE) staining (400×). |
HE Staining
Changes in the nasal mucosal histological tissue also demonstrated the model’s effectiveness (Figure 6). The overall arrangement of the nasal mucosa in the control group appeared normal, and there were no signs of eosinophil or infiltration of inflammatory cells in the lamina propria. In contrast, the model group had significant inflammatory cell infiltration. The AR + gel/LIP/volatile oil group showed the best performance among the treatment groups. The HE results were consistent with the levels of histamine release and cytokines. In the groups treated with AR + gel/volatile oil and AR + LIP/volatile oil, the nasal mucosa’s general structure did not show significant changes, but there were changes in the individual lamina propria. There was sporadic infiltration of inflammatory cells in localized areas. Compared to the group treated with just the volatile oil.
The binary preparation, as well as the other two monophyletic formulations, significantly decreased degenerative changes in the nasal mucosa of the rhinitis model mouse. In summary, pathological findings demonstrated that the AR + gel/LIP/volatile oil group was able to significantly decrease the pathological alterations in the nasal mucosa. Furthermore, the nasal mucosa damage brought on by AR was decreased in the AR + LIP/volatile oil and AR + gel/volatile oil groups. While the AR + gel/volatile oil group had enhanced nasal mucosal cells, the AR+LIP/volatile oil group appeared to generate less inflammation.
Discussion
In the present study, a gel/LIP/volatile oil nasal drug delivery preparation was created against the background of poor patient compliance and short retention time of general nasal drug delivery preparations. Liposomes and temperature-sensitive gel were combined to form a binary preparation. Liposomes were used to wrap the drug to improve the poor water solubility of the volatile oil and at the same time increase the drug release performance. The high deformability of Liposomes can prevent the rupture of Liposomes vesicles when passing through the nasal mucosa. The sudden release of the thermosensitive gel alone was avoided, and the phase transformation of the thermosensitive gel in the nasal cavity was used to address the problem of liposomes as liquid preparations that are easily cleared by the nasal cilia, as well as their poor stability. The present study evaluated the gel/LIP/volatile oil and compared it to other preparations. The results demonstrated that it can significantly improve the in vitro mucosal permeability and nasal mucosal retention of the Fructus Xanthii and Magnolia liliiflora volatile oils. The in situ gel was prepared according to the optimal formula properties from a solution state to a semi-solid state at 34°C with a slow release effect. These results provide a good theoretical foundation for drug release in the treatment of AR diseases.
Several problems need to be solved when the in situ gel is used for the nasal drug delivery system. First, after the in situ gel is used in the nasal cavity, the gelation time is too long and the drug diffuses in the form of a solution, leading to its sudden release. Second, the excessive strength of the in situ gel may damage the nasal mucosa. Third, the in situ gel is not sticky enough and is easily removed by the cilia. Due to a unique reverse thermal gelling feature, poloxamer 407 remains liquid at low temperatures and solidifies at body temperature.44,45 In the present study, the in situ gel nasal delivery system with poloxamer 407 as the excipient was selected, and various factors were comprehensively considered to improve and optimize the prescription properties. The excipient HPMC and poloxamer 188 improved the gel’s strength and were added to poloxamer 407, which can adjust the transition temperature of gel formation to obtain a temperature-sensitive gel with appropriate viscosity.46
Liposomes have been widely developed in the past decades and have become promising tools, especially in the field of local therapeutic drug delivery. The drugs they produce can not only reduce the required dose and side effects and improve drug absorption, but also more easily transport the active ingredients to the target site through the biological barrier of the body, with the advantages of controlled release, targeting, high drug loading, biocompatibility, and biodegradability.34 Liposomes bind to the in situ gel. They can further improve the solubility and stability of the drug, reduce adverse reactions, and achieve a longer sustained release effect.47,48 In the present study, the volatile oils of Fructus Xanthii and Magnolia liliiflora were loaded with lipids and cholesterol, and the liposome formulation was optimized using the central composite design-response surface methodology. The experimental results showed that the best formula had a mass ratio of volatile oil (X1) between Fructus Xanthii and Magnolia liliiflora of 1:2.3, a mass ratio of lecithin to cholesterol (X2) of 6.2:1, and aqueous phase (X3) dosage of 12.2 mL. On this basis, the optimal gel/volatile oil formulation was obtained using the central composite design-response surface methodology, the optimal prescription was 14.015% Poloxamer 407, 7.681% Poloxamer 188, and 0.5% HPMC.
The findings of the study also demonstrated that while the release time of the volatile oil from the gel was extended, the effectiveness of retaining the two volatile oils in the nasal mucosa when delivered through a pure gel was not satisfactory. On the other hand, the gel/LIP/volatile oil group exhibited greater stability in laboratory conditions compared to the gel/volatile oil and LIP/volatile oil groups. This group also showed higher retention of volatile oil in the nasal mucosa and a lower amount of volatile oil that could permeate through. It is possible that the gel/LIP/volatile oil combination retains the volatile oil in a three-dimensional polymer matrix, allowing for extended contact time with the nasal mucosa and improved delivery to the irritated area. Liposomes containing volatile oil should have a low chance of being absorbed by non-nasal local absorption. They should also have the greatest therapeutic impact on the nasal mucosa. In vivo, results also demonstrated that the pharmacodynamic results of the AR + gel/LIP/volatile oil group were better than those of the AR + gel/volatile oil and LIP/volatile oil groups. As a result, gel/LIP/volatile oil is better for RA treatment.
Conclusion
This study evaluated the drug-containing liposomes encapsulated in temperature-sensitive gel in vivo and in vitro experiments, the results showed that drug containing liposomes encapsulated in temperature sensitive gel had ideal gel temperature, gel time and sustained release effect. The nasal delivery technique improves AR and helps medications stay longer in the nasal cavity. As a result, the sustained-release gel/LIP/volatile oil combination is a viable RA therapy approach that may be used as a model for creating novel formulations.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 81703944 and 82174232), the Heilongjiang Natural Science Foundation Project (YQ2019H031), the Postdoctoral Researchers Settled in Heilongjiang Scientific Research Startup Fund (2020), Excellent Scholar of the Qihuang Project of Heilongjiang University of Chinese medicine (2023), Heilongjiang Province Youth Qihuang Scholar Training Project (2023), and the Heilongjiang Touyan Innovation Team Program.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss MS. WAO White Book on Allergy. Vol 3. Milwaukee, WI: World Allergy Organization; 2011:156–157.
2. Badhe RV, Nipate SS. Nasal bioadhesive drug delivery systems and their applications. Bioadhes Drug Delivery. 2020;2020:259–305.
3. Abdeltawab H, Svirskis D, Sharma M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin Drug Deliv. 2020;17(4):495–509. doi:10.1080/17425247.2020.1731469
4. Boddu SHS, Kumari S. A short review on the intranasal delivery of diazepam for treating acute repetitive seizures. Pharm. 2020;12(12). doi:10.3390/pharmaceutics12121167
5. Bodratti AM, Alexandridis P. Formulation of poloxamers for drug delivery. J Funct Biomater. 2018;9(1). doi:10.3390/jfb9010011
6. Chaudhari P, Ghate VM, Lewis SA. Supramolecular cyclodextrin complex: diversity, safety, and applications in ocular therapeutics. Exp Eye Res. 2019;189:107829. doi:10.1016/j.exer.2019.107829
7. Huang J, Mensi M, Oveisi E, Mantella V, Buonsanti R. Structural sensitivities in bimetallic catalysts for electrochemical CO(2) reduction revealed by Ag-Cu nanodimers. J Am Chem Soc. 2019;141(6):2490–2499. doi:10.1021/jacs.8b12381
8. De Bernardi M, Mellerio G, Paternoster-Colombo M, Vidari G, Vita-Finzi P. Constituents of essential oil of Aframomum giganteum. Planta Med. 1981;41(4):359–365. doi:10.1055/s-2007-971727
9. Deshkar SS, Shirolkar SV, Patil AT. Vaginal bioadhesive drug delivery systems and their applications. Bioadhes Drug Delivery. 2020;2020:307–369.
10. Wang J, Liu W, Luo G, Li Z, Zhao C, Zhang H. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ Sci. 2018;11(12):3375–3379. doi:10.1039/C8EE02656D
11. Bergmann KC, Klimek L, Nehr S, Straff W, Werchan B. Allergenic pollen: is it also an indoor problem. Allergo J Int. 2021;1–2. doi:10.1007/s40629-020-00158-y
12. Gao W, Zhang Y, Zhang Q, Zhang L. Nanoparticle-hydrogel: a hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016;44(6):2049–2061. doi:10.1007/s10439-016-1583-9
13. Ghori MU, Mahdi MH, Smith AM, Conway BR. Nasal drug delivery systems: an overview. Am J Pharmacol Sci. 2015;3(5):110–119.
14. Marriott C, Martin GP. Relevance of mucus to advanced drug-delivery - preface. Am J Pharmacol Sci. 1993;11(3):R7–R7.
15. Flores ML, Stortz CA, Cerezo AS. Studies on the skeletal cell wall of the cystocarpic stage of the red seaweed Iridaea undulosa B. Part II. Fractionation of the cell wall and methylation analysis of the inner core-fibrillar polysaccharides. Int J Biol Macromol. 2000;27(1):21–27. doi:10.1016/s0141-8130(99)00116-6
16. Illum L. Nasal drug delivery--possibilities, problems and solutions. J Control Release. 2003;87(1–3):187–198. doi:10.1016/s0168-3659(02)00363-2
17. Patel HR, Patel RP, Patel MM. Poloxamers: a pharmaceutical excipients with therapeutic behaviors. Int J PharmTech Res. 2009;1(2):299–303.
18. Jain KK. An overview of drug delivery systems. Methods Mol Biol. 2020;2059:1–54. doi:10.1007/978-1-4939-9798-5_1
19. Qiao Y, Wan J, Zhou L, et al. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(1):e1527. doi:10.1002/wnan.1527
20. Kucukler S, Darendelioğlu E, Caglayan C, Ayna A, Yıldırım S, Kandemir FM. Zingerone attenuates vancomycin-induced hepatotoxicity in rats through regulation of oxidative stress, inflammation and apoptosis. Life Sci. 2020;259:118382. doi:10.1016/j.lfs.2020.118382
21. Kim E, Lee J, Kim D, et al. Solvent-responsive polymer nanocapsules with controlled permeability: encapsulation and release of a fluorescent dye by swelling and deswelling. Chem Commun. 2009;12(12):1472–1474. doi:10.1039/b823110a
22. Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci. 2013;38(3–4):672–701.
23. Kumar GS, Kumar RA, Kumar PS, et al. Copper catalyzed oxidative coupling of amines with formamides: a new approach for the synthesis of unsymmetrical urea derivatives. Chem Commun. 2013;49(59):6686–6688. doi:10.1039/c3cc42381f
24. Laffleur F, Bauer B. Progress in nasal drug delivery systems. Int J Pharm. 2021;607:120994. doi:10.1016/j.ijpharm.2021.120994
25. Xiao MC, Liu SY, Zhang YF. Network pharmacological exploration of Fructus Xanthii combined with flos magnoliae in the treatment of allergic rhini-tis. Chin J Otorhinolaryngol Integr Med. 2021;29:341–346.
26. Paudwal G, Banjare N, Gupta PN. Nanovesicles for nasal drug delivery. In: Applications Nanovesicular Drug Delivery. Academic Press; 2022:81–101.
27. Pullen NA, Falanga YT, Morales JK, Ryan JJ. The Fyn-STAT5 pathway: a new frontier in IgE- and IgG-mediated mast cell signaling. Front Immunol. 2012;3:117. doi:10.3389/fimmu.2012.00117
28. Zhang MD, Si DH, Yi JD, Yin Q, Huang YB, Cao R. Conductive phthalocyanine-based metal-organic framework as a highly efficient electrocatalyst for carbon dioxide reduction reaction. Sci China Chem. 2021;64:1332–1339. doi:10.1007/s11426-021-1022-3
29. Ding Y, Chen YP, Zhang X, et al. Controlled intercalation and chemical exfoliation of layered metal-organic frameworks using a chemically labile intercalating agent. J Am Chem Soc. 2017;139(27):9136–9139. doi:10.1021/jacs.7b04829
30. Min W, Bo M, Bitao M, Iing W. Essential oil preparation of nano-liposomes and in vitro release study. Guiding J Tradit Chin Med Pharm. 2010;16(12):79–82. doi:10.13664/j.cnki.pcr.2009.03.027
31. Riese P, Sakthivel P, Trittel S, Guzmán CA. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv. 2014;11(10):1619–1634. doi:10.1517/17425247.2014.931936
32. Shelke S, Shahi S, Jalalpure S, Dhamecha D, Shengule S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J Drug Delivery Sci Technol. 2015;29:238–244. doi:10.1016/j.jddst.2015.08.003
33. Soliman KA, Ullah K, Shah A, Jones DS, Singh TRR. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov Today. 2019;24(8):1575–1586. doi:10.1016/j.drudis.2019.05.036
34. Yuan Y, Cui Y, Zhang L, et al. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int J Pharm. 2012;430(1–2):114–119. doi:10.1016/j.ijpharm.2012.03.054
35. Struß N, Badorrek P, Mattern C, Mattern U, Hohlfeld JM. The effect of a thixotropic nasal gel on nasal symptoms and inflammatory biomarkers in seasonal allergic rhinitis. Int Arch Allergy Immunol. 2020;181(5):385–394. doi:10.1159/000506129
36. Vanderstraeten MCM, Gutermuth J, Grosber M. Contact anaphylaxis to poloxamer 188 and 407 in a periodontal gel. Contact Dermatitis. 2021;85(2):253–255. doi:10.1111/cod.13834
37. Yu S, Bixi S, Xiaoshu G, Shuwen L, Rubin H, Bing H. Chitosan hydrogel doped with PEG-PLA nanoparticles for the local delivery of miRNA-146a to treat allergic rhinitis. Pharma. 2020;12:1–17. doi:10.3390/PHARMACEUTICS12100907
38. Wang S, Tang Q, Qian W, Fan Y. Meta-analysis of clinical trials on traditional Chinese herbal medicine for treatment of persistent allergic rhinitis. Allergy. 2012;67(5):583–592. doi:10.1111/j.1398-9995.2012.02806.x
39. He Z, Zhang Y, Han Y. Clinical research on allergic rhinitis treated with the modified xinyi cangerzi powerbined with cetirizcom. World J Integr Tradit West Med. 2018;13(07):953–956. doi:10.13935/j.cnki.sjzx.180717
40. Manuyakorn W, Benjaponpitak S, Kamchaisatian W, Vilaiyuk S, Sasisakulporn C, Jotikasthira W. Pediatric anaphylaxis: triggers, clinical features, and treatment in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2015;33(4):281–288. doi:10.12932/ap0610.33.4.2015
41. Wermeling DP, Miller JL, Archer SM, Manaligod JM, Rudy AC. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41(11):1225–1231. doi:10.1177/00912700122012779
42. Fan Y, Yi MM, Zhang BF. Spectroscopic properties of lycopene nanoliposomes. Food Sci. 2009;30(17):48–51.
43. An XW, Liu JT, Fan SD. Synthesis and antitumor activity of Schiff base oxovanadium complexes and drug loaded liposomes. J Northeast Normal Univ. 2010;42(02):91–96. doi:10.16163/j.cnki.22-1123/n.2010.02.025
44. Jin LY, Li J. Progress of poloxamer 407 in pharmaceutics. Pharm Clin Res. 2009;17(03):231–234. doi:10.13664/j.cnki.pcr.2009.03.027
45. Bateman RM, Sharpe MD, Jagger JE, et al. 36th international symposium on intensive care and emergency medicine: Brussels, Belgium. Crit Care. 2016;20(Suppl 2):94. doi:10.1186/s13054-016-1208-6
46. Wu Y, Liu Y, Li X, et al. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J Pharm Sci. 2019;14(1):1–15. doi:10.1016/j.ajps.2018.04.008
47. Attama AA, Schicke BC, Paepenmüller T, Müller-Goymann CC. Solid lipid nanodispersions containing mixed lipid core and a polar heterolipid: characterization. Eur J Pharm Biopharm. 2007;67(1):48–57. doi:10.1016/j.ejpb.2006.12.004
48. Attama AA, Müller-Goymann CC. Investigation of surface-modified solid lipid nanocontainers formulated with a heterolipid-templated homolipid. Int J Pharm. 2007;334(1–2):179–189. doi:10.1016/j.ijpharm.2006.10.032
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.