Back to Journals » Journal of Inflammation Research » Volume 15
From Anti-HER-2 to Anti-HER-2-CAR-T Cells: An Evolutionary Immunotherapy Approach for Gastric Cancer
Authors Sun J, Li X, Chen P, Gao Y
Received 26 March 2022
Accepted for publication 29 June 2022
Published 17 July 2022 Volume 2022:15 Pages 4061—4085
DOI https://doi.org/10.2147/JIR.S368138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zili You
Jiangang Sun,1,* Xiaojing Li,2,* Peng Chen,1 Yongshun Gao1
1Department of Gastrointestinal Surgery, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China; 2Department of Pharmacy, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peng Chen; Yongshun Gao, Department of Gastrointestinal Surgery, the First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe East Road, Zhengzhou, Henan, 450052, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Current Therapeutic modalities provide no survival advantage to gastric cancer (GC) patients. Targeting the human epidermal growth factor receptor-2 (HER-2) is a viable therapeutic strategy against advanced HER-2 positive GC. Antibody-drug conjugates, small-molecule tyrosine kinase inhibitors (TKIs), and bispecific antibodies are emerging as novel drug forms that may abrogate the resistance to HER-2-specific drugs and monoclonal antibodies. Chimeric antigen receptor-modified T cells (CAR-T) targeting HER-2 have shown considerable therapeutic potential in GC and other solid tumors. However, due to the high heterogeneity along with the complex tumor microenvironment (TME) of GC that often leads to immune escape, the immunological treatment of GC still faces many challenges. Here, we reviewed and discussed the current progress in the research of anti-HER-2-CAR-T cell immunotherapy against GC.
Keywords: CAR-T, HER-2, gastric cancer, immunotherapy, target
Introduction
Gastric cancer (GC) ranks fifth in incidence and fourth in mortality among all malignancies worldwide, which was equal to more than 1 million new cases and 769 thousand deaths in 2020.1 Given the considerable tumor heterogeneity, the five-year survival rate of advanced GC is reported to be less than 30%.2,3 At present, the treatment of GC mainly includes surgical resection,4,5 chemotherapy,6,7 traditional Chinese medicine (TCM) therapy,8 targeted therapy9,10 and immunotherapy11,12 (Figure 1).
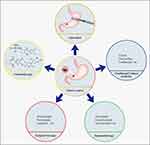 |
Figure 1 Treatment strategies for gastric cancer. Surgical resection, chemotherapy, traditional Chinese medicine, targeted therapy and immunotherapy. |
Based on the results of CLASS0113 and CLASS0214 clinical trials, laparoscopic total gastrectomy is a potentially safe alternative to open total gastrectomy for both advanced and early stage (I) GC patients. Recent studies have also reported high efficacy and low toxicity of TCM-based treatment of GC,8 although the molecular mechanisms are still unclear. Furthermore, perioperative chemotherapy for GC has reached a consensus based on the results of CLASSIC, MAGIC, RESOLVE and other randomized controlled trials conducted over the past decade.15 Despite advances in the molecular typing of GC and the development of targeted and immunogenic drugs, their clinical applications remain limited,16 especially for the human epidermal growth factor receptor type 2 (HER-2) positive,17 microsatellite instability-high18 and Epstein–Barr virus-associated19 subtypes. Moreover, studies have increasingly shown that conventional chemotherapy is not the optimum choice for perioperative treatment, and the outcomes of the patients depend significantly on the specific tumor stage and mutation status.
HER-2 is a member of the epidermal growth factor receptor (EGFR) family,20 and is overexpressed in many solid tumors including breast cancer (BC), stomach cancer, colon cancer and ovarian cancer.21,22 The Phase 3 ToGA trial established trastuzumab as a first-line treatment for advanced HER-2 positive GC.23 However, lapatinib, trastuzumab emtansine (T-DM1) and pertuzumab have not shown encouraging results after first-line treatment progression.24 Immunotherapy and targeted therapy are now indispensable for GC treatment. The development of immune inhibitors against advanced GC cells has been one of the most significant improvements in recent years.25 Chimeric antigen receptor T cell therapy (CAR-T) is a promising treatment strategy against cancers.26 Two CAR-T cell-based therapies have been approved by the Food and Drug Administration (FDA) to treat refractory leukemia and lymphoma.27 However, the efficacy of CAR-T cells against sarcomas and other solid tumors is limited due to the immunosuppressive tumor microenvironment (TME).28,29 Compared to conventional therapies, CAR-T cells can directly recognize antigens on the surface of tumor cells and kill tumor cells, thereby reducing the rejection response.30 New-generation cellular immunotherapies, such as combined immune checkpoint inhibitors, cytokine-induced lymphocyte and T-cell targeted killing, are promising strategies against solid tumors31 but are still at the stage of clinical trials for GC.
Nevertheless, EGFR or CAR-T targeting alone cannot achieve ideal efficacy against GC due to the heterogeneity of tumor cells, immunosuppressive TME and antigen migration. Here, we reviewed and discussed the various immunotherapeutic strategies that have been developed so far to target HER-2 in GC.
Targeted HER-2 Therapy
Structure and Function of HER-2
The first EGFR was discovered in the 1970s, and since then four members of the family, namely EGFR/HER-1/ErbB1, HER-2/ErbB2, HER-3/ErbB3 and HER-4/ErbB4,32,33 have been characterized. The HER-2 and ErbB2 oncogenes were initially identified in rodents and humans, respectively, but were later found to be homologous to each other.34–36 All the members of HER family have the same extracellular domains, lipophilic transmembrane regions, intracellular domains containing tyrosine kinases, and carboxy-terminal regions.35,37 Binding of ligands to the extracellular domains of HER proteins leads to dimerization and transphosphorylation of their intracellular domains.38 However, ErbB2 has no direct ligand,39 and the crystal structure of its extracellular region indicates an extended configuration with four domains arranged in a manner similar to that seen in the EGFR dimer. Thus, ErbB2 has a ligand-independent active conformation.40,41 This is consistent with the fact that ErB2 homodimers are spontaneously formed in cells overexpressing ErbB2, which is the preferred dimer partner of other ErbB receptors.42 Activation of HER-2 and EGFR leads to the phosphorylation of the ErbB dimer, which stimulates the downstream RAS/MEK, PI3K/AKT, Src kinases and STAT pathways.43 HER-2 initiates GC development in the form of EGFR, HER-2 dimers, and HER-2/HER-3 dimers.
EGFR in GC
The EGFR family is highly expressed in 40–60% of GC tumors.44 Anti-EGFR drugs block the downstream signal transduction pathway in cancer cells45 by targeting the extracellular, transmembrane and intracellular regions of EGFR.46 EGFR-specific ligands, such as EGF, bind to their extracellular region and mediate homo/heterodimerization, resulting in autophosphoacylation of the receptor47 and activation of a series of downstream signal transduction pathways in GC cells48,49 including VAV2-RhoA,50 STAT5,51 PI3K/AKT/mTOR,52 etc. (Figure 2). The pathways culminate in the activation of transcription factors, leading to tumor cells’ proliferation, infiltration, and metastasis, inhibiting tumor cells’ apoptosis, and enhancing tumor angiogenesis.
HER-2/HER-2 Dimer in GC
The HER receptor exists as a monomer or as a homo/heterodimer,53 and HER-2 preferentially binds to the dimeric form.53,54 The HER-2 pathway is altered during GC development, either due to aberrant changes in HER-2 structure, dysregulation of downstream effectors of HER-2, or interaction of HER-2 with other membrane receptors.48 As shown in Figure 2, dimerization of HER-2/HER-2 activates the SRC-FAK,55 GRB2/SOS/JAK256 and RAS-MEK-MAPK signaling pathways in GC cells,57 and promotes cell adhesion, migration, growth, proliferation, and metastasis.
HER-2/HER-3 Dimer in GC
The HER-2/HER-3 heterodimer is the most mitogenic of all ErbB receptors,58,59 and is constitutively active in GC cells overexpressing the HER-2 gene.60,61 Recent studies have showed that the HER-2-HER-3 dimer is related to the occurrence, growth, metastasis and drug resistance of tumors. The HER-2/HER-3 dimer signals through the RAS-MEK-MAPK and PI3K-AKT pathways (Figure 2) upon EGF binding.62 Activation of the PI3K/AKT pathway can lead to tumor drug resistance, and preclinical trials of PI3K inhibitors have indicated that this pathway is a suitable target for tumor therapy.63 In addition, some studies have shown that inhibition of PI3K or MEK alone, or in combination with anti-HER-2 therapy, might be a reformative treatment scheme for some patients with HER-2 positive GC.64 Approximately 34–59% of the patients with HER-2 positive GC also overexpress HER-3 and are resistant to trastuzumab,65 which can be attributed to the negative feedback regulation of HER-3 mediated by the HER-2-dependent P13K-AKT pathway, making trastuzumab unresponsive to ligand-dependent dimerization of HER-2/HER-3.66
Drugs Targeting HER-2 in the Treatment of GC
Currently, drugs targeting HER-2 in the treatment of GC can be divided into four categories: first-generation HER-2 monoclonal antibody, second-generation HER-2 monoclonal antibody, small-molecule tyrosine kinase inhibitors (TKIs), antibody-drug conjugates (ADCs) and bispecific antibodies. The latest research progress on these drugs is detailed in Table 1.
 |
Table 1 Drugs Targeting HER-2 in the Treatment of Gastric Cancer |
First-Generation HER-2 Monoclonal Antibody
Trastuzumab was the first monoclonal antibody approved by FDA to treat HER-2 positive GC.81 The TOGA trial demonstrated for the first time that the combination of trastuzumab and fluorouracil was superior to chemotherapy for the treatment of HER-2 positive advanced GC,82 and significantly prolonged overall survival (OS) of patients.82 Since then, several studies have confirmed the efficacy and safety of trastuzumab against advanced HER-2 positive GC.83,84 However, acquired resistance to trastuzumab has been a major challenge and has a genetic basis in some patients, which eventually limits its therapeutic efficacy.85 Early clinical studies had also reported cardiac side effects of trastuzumab, such as left-heart insufficiency and congestive heart failure.86
Second-Generation HER-2 Monoclonal Antibody
The second generation of HER-2 targeted drugs has been developed to counteract the emergence of trastuzumab resistance. Pertuzumab binds to the extracellular domain II of the HER-2, blocking ligand-induced heterodimerization of HER-2 and downstream signaling.87 It has been proved to significantly improve the outcomes in patients with advanced HER-2 positive BC compared to the combination of chemotherapy and trastuzumab.88 Another study found that pertuzumab extended the median progression-free survival (PFS) of patients with BC by 7.7 months compared to that of the placebo arm.89 However, the JACOB trial showed that the combination of pertuzumab, trastuzumab and chemotherapy did not significantly improve the survival of HER-2 positive patients with GC or gastroesophageal junction cancer (GEJC) compared to the placebo.68 Therefore, more studies are needed to further determine the efficacy of pertuzumab in stomach and other cancers.
Small-Molecule TKIs
Small-molecule TKIs can also be used to target HER-2. For instance, lapatinib is an oral TKI specific for both EGFR and HER-2.90 It blocks HER-1 and HER-2 by reversibly binding to the cytoplasmic ATP binding sites in the tyrosine kinase domain.90,91 A Phase II trial using lapatinib as a first-line monotherapy for patients with HER2-positive GC failed to achieve the desired results, showing an overall response rate (ORR) of 11% and a median OS of 4.8 months.69 Besides, one study showed that lapatinib is not superior to trastuzumab as the first- and second-line treatment for advanced GC.70 However, evidence showed that the combination of lapatinib and capecitabine could effectively treat HER2-positive GC with bone and meningeal metastasis in patients who were unresponsive to trastuzumab and chemotherapy.92 This can be attributed to the fact that lapatinib can cross the blood–brain barrier unlike larger antibodies.93 Furthermore, lapatinib is also a more suitable option than trastuzumab for patients at risk of cardiac events.93 Nevertheless, it is still at the stage of clinical trials. Afatinib and neratinib are other potential TKIs,72,73 although there are no clinical studies related to GC.
Antibody-Drug Conjugates
The combination of anti-HER-2 antibodies with effective drugs or cellular immunotherapy can effectively ablate HER-2-overexpressing tumors. T-DM1 or T-DM1 is a HER-2-targeting ADC that consists of a stable thioether linker between trastuzumab and the cytotoxic agent maytansine, and is currently in phase III development for HER-2 positive cancer.94 The efficacy and toxicity of T-DM1 were established in patients with HER-2 mutant lung adenocarcinoma,95 and a subsequent study in patients with GC indicated stronger anti-cancer activity compared to trastuzumab.96 However, the randomized, open-label, adaptive Phase 2/3 GATSBY trial reported a similar efficacy of T-DM1 and taxane in previously treated patients with HER-2 positive advanced GC.74 Furthermore, most patients with HER2-positive BC or GC exhibited primary or acquired resistance to T-DM1.20,97 XMT-1522 is another HER-2 ADC that was found to be effective against T-DM1 resistant HER-2 positive BC and GC cell lines, as well as xenograft models.98
DS-8201a is an ADC specific to HER-2 that consists of a human monoclonal antibody connected to a topoisomerase I inhibitor through a cleavable peptide-based linker.98 The most recently developed HER-2-targeting ADCs include SUYD985 and ARX788. SYD985 couples a duocarmycin payload with trastuzumab,99 and ARX788 is a proprietary version of the monomethyl auristatin F payload connected via a non-cleavable linker.77 SYD985 has not been studied in GC, while ARX788 has shown antitumor effects in preclinical models of T-DM1 resistant HER-2 positive GC.77,100 Currently, more anti-HER-2 ADCs have been developed that can potentially overcome drug resistance and improve therapeutic outcomes in patients with GC.
Bispecific Antibodies
The fusion of two recombinant antibodies into bispecific antibodies (BsAbs) can achieve dual-targeting function.101 ZW25 (azymetric) is a BsAb specific for two HER-2 epitopes, the trastuzumab-binding ECD4 and pertuzumab-binding ECD2, and is effective and well tolerated in patients with various HER-2 positive cancers.78 However, its role in GC needs to be further explored. MCLA-128 is a full-length humanized IgG1 BsAb with enhanced antibody-dependent cell-mediated cytotoxicity (ADCC), targeting HER-2 and HER-3.102 It has been shown to be effective against HER-2 positive GC and GEJC.79,103 The BsAb Mm-111 targets HER-2 and HER-3, and its binding to HER-3 blocks protein binding and inhibits modulin-activated HER-3 signaling.104 McDonagh et al showed that the combination of Mm-111 with trastuzumab or lapatinib improved antitumor activity, and may supplement existing HER-2 targeted therapies against drug-resistant or recurrent tumors.105 Triad or quadruple antibodies against tumor-specific antigens are also being developed to benefit more patients.
CAR-T Cell Immunotherapy for GC
CAR-T cell immunotherapy uses genetically engineered T cells to eliminate tumor cells expressing specific antigens.106 CAR-T cells were developed two decades ago and have since been divided into four generations based on the structure of intracellular signal transduction regions. Gross et al107 first proposed the concept of CAR-T therapy in 1989 and successfully constructed the first-generation CAR by combining the single-chain fragment variable (scFv) monoclonal antibody with immunoreceptor tyrosine-based activation motifs (ITAMs) like CD3ζ and FcεRIγ.108 The second-generation CAR was constructed by Finney et al and consists of a costimulatory domain that can overcome the poor T cell amplification and cytokine production of first-generation CARs.109 The third-generation CAR was generated by combining two tandem costimulatory molecules to further enhance the effector function and in vivo persistence of the T cells.110 Fourth-generation CAR-T cells were engineered to secrete a large number of cytokines into the tumor site to activate the innate immune response and enhance the antitumor effect.111 The current status of CAR-T cell therapy against GC has been summarized in Figure 3A.
CAR-T Targets in GC
Several clinical trials are ongoing worldwide on first-, second-, and third-generation CAR-T cells112 targeting CD19, B7-1/B7-2, CD155, CEA, CLDN 18.2, EGFR, EpCAM, FOLR1, HER-2, HVEM, ICAM-1, LSECtin, MSLN, MUC1, NKG2D, PD-L1, PSCA and so on. Details are summarized in Table 2. The GC-related targets for CAR-T cell therapy include CLDN 18.2, FOLR1, HER-2, ICAM-1, MSLN, NKG2D, PD-L1 and PSCA (Figure 3B), and have been discussed in greater detail in the following sections. However, most clinical trials on CAR-T cell therapy have been on lymphoid leukemia, a considerable number of which have reported that CD19-targeting CAR-T cells can alleviate or even cure refractory and relapsed B-cell malignancies with a complete response (CR) rate of >80%.113 In recent years, CAR-T cells against hematoma antigens such as CD22,114 CD30115 and CD123116 have also been studied in clinical trials. For other solid tumors, tumor-associated antigens (TAAs) rather than tumor-specific antigens are the preferred targets for CAR-T cell therapy. The clinical studies on CAR-T cell therapy against solid tumors are listed in Table 3.
 |
Table 2 Tumor-Associated Receptors of CAR-T Cell Target |
 |
Table 3 CAR-T Related Clinical Studies in Solid Tumors |
CLDN 18.2
CLDN 18, a member of the CLAUDIN (CLDN) family, is encoded by the CLDN 18 gene and is expressed in the epithelium.189 CLDN 18.2, the second isotype of Claudine 18, is located in the extracellular membranes.190 It is usually expressed in primary GC tumors but may also be present in differentiated gastric mucosal epithelial cells.190 CLDN 18.2 is expressed in 70% of the primary and metastatic gastric adenocarcinomas, and therefore is considered as a potential therapeutic target in GC.191 Hua Jiang et al found that CLDN18.2-CAR-T cells are effective against CLDN18.2 positive tumors, including GC.134 Besides, Guoyun Zhu et al indicated that targeting CLDN 18.2 through ADCs or BsAbs may be effective against GC and pancreatic cancer.136
FOLR1
FOLR1 (folic acid receptor 1), also known as folic acid receptor α and folate-binding protein, is a glycosylphosphatidylinositol junction protein192 that is closely related to tumor progression and cell proliferation.193,194 It is overexpressed in the tumors of ovarian, breast, colorectal, kidney, lung, and other solid tumors, and is present at low levels in normal cells.195,196 As reported, FOLR1 is highly expressed in about one-third of patients with GC, and FOLR1-CAR-T cells have exhibited high anti-cancer activity in preclinical studies.147
ICAM-1
ICAM-1 (intercellular cell adhesion molecule-1) belongs to the immunoglobulin superfamily of glycoproteins,197 and mediates cell–cell and cell-matrix adhesion.198 It is overexpressed in various cancers, including GC, and is associated with poor survival.199 Recently, Min IM et al reported encouraging results with anti-ICAM-1 CAR-T cells in thyroid tumor models.200 In addition, the strategy of anti-ICAM-1 CAR-T cells with or without chemotherapy has been found to be promising for the treatment of ICAM-1+ patients with advanced GC.161
MSLN
Mesothelin (MSLN) is a membrane protein (40 kDa) that is expressed in normal epithelial tissues and highly upregulated in breast, lung, pancreas, ovary, mesothelioma, and gastric tumor cells.201–203 MSLN-specific CAR-T cells have been engineered for solid cancers, including mesothelioma, pancreatic cancer, BC, lung cancer and GC.202,204–206 Jiang LV et al found that a peritumoral delivery strategy improved the infiltration of anti-MSLN CAR-T cells into a subcutaneous GC xenograft, which significantly inhibited tumor growth.202 Besides, Zhang Q et al discovered that MSLN-CAR-T cells reduced the growth of MSLN-positive tumor cells by significantly increasing the levels of T cells and cytokines.207 In addition, the growth of GC cells can also be inhibited by anti-MSLN-CAR-T cells,208 indicating its potential as a therapeutic option against GC.
NKG2D Receptor
Natural killer group 2 member D (NKG2D) receptor is a lectin-like transmembrane glycoprotein that is expressed primarily in natural killer (NK) cells, CD8+ T cells and auto-immunosuppressed CD4+ T cells.209 NKG2D is expressed at low levels or entirely absent in normal tissues or cells, although its expression increases rapidly in response to pathogens, genotoxic drugs, or malignant transformation of cells.210 Therefore, NKG2D is a potentially suitable target for CAR-T cell therapy. In addition, Spear et al found that NKG2D-specific CAR-T cells not only killed the tumor cells directly but also activated the host immune system.211 At present, NKG2D-targeting CAR-T cells have been proved to be effective against multiple myeloma,212 glioblastoma,213 and hepatocellular carcinoma.214 Furthermore, the up-regulation of NKG2D levels in GC cells can sensitize them to NKG2D-CAR-T cells-mediated cytotoxicity.215 The currently ongoing clinical trials of CAR-T cells targeting NKG2D, including those in patients with GC, are expected to be completed in 2021 (NCT04107142).
PD-L1
Programmed death ligand 1 (PD-L1) is a member of the B7 family and the ligand of PD-1.216,217 It is composed of 290 amino acids218 and is expressed on the surface of several tumor cells, including lung cancer,219 BC,220 and GC.221 Chimeric switch receptor PD-L1 can enhance the function of CAR-T cells in solid tumors.222,223 CAR-T cells targeting PD-L1 effectively suppressed the growth of GC patient-derived xenograft (PDX) in animal models.224 Further research revealed the killing effect of PD-L1 on GC, therefore improving the killing effect of CAR-T cells in GC.177
PSCA
Prostate stem cell antigen (PSCA) is a glycosyl-phosphatidylinositol cell immobilized by a face protein that belongs to the Thy-1/Ly-6 family.225 Existing evidence has indicated that PSCA-CAR-T cells are effective against metastatic prostate cancer and non-small cell lung cancer (NSCLC).178,226 In vivo experiments have shown that PSCA-CAR-T cells inhibited the growth of prostate cancer PDX and extended the survival of tumor-bearing mice.227 A Phase I clinical trial was initiated to evaluate PSCA-CAR-T cells in patients with relapsed and refractory metastatic prostate cancer.228 In addition, Di Wu et al have confirmed the feasibility of anti-PSCA-CAR-T cells against GC,179 suggesting a potential clinical application.
HER-2-Specific CAR-T Cells in the Treatment of GC
Construction of HER-2-Targeted CAR
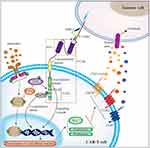
The CAR targeting HER-2 consists of an extracellular antigen-binding region, a transmembrane region, and an intracellular signal transduction region.229,230 The extracellular antigen-binding region is composed of a single-chain variable fragment (scFv) and the hinge region of the anti-HER-2monoclonal antibody.231 The variable weight chain and the variable weight chain constitute the scFv,232 which recognizes and binds to the TAAs on the surface of tumor cells.233 In addition, it determines the specificity of CAR antigens and can bind to multiple TAAs in an MHC-independent, non-restrictive manner.234,235 IL13Rα2 can also be combined with HER-2 on the surface of tumor cells by CAR-T cells, further enhancing their activation.236 The transmembrane region is involved in signal transduction, although it is unclear whether it also has an effect on the structure and biochemistry of CAR.237 Finally, CAR-T cells can also increase the immune response by releasing tumor cell killing factors. The details of the process are illustrated in Figure 4.
Advances in HER-2-Targeted CAR-T Cell Therapy for GC
Current immunotherapeutic strategies against GC include nonspecific immunoboosters, tumor vaccines, adoptive cell transfer, and monoclonal antibodies.238 The HER-2 signaling pathway is a key target of the adoptive immune cell therapy against solid tumors.156 Although several HER-2 targeted drugs have entered clinical trials for patients with GC, the FDA has approved only trastuzumab for first-line treatment of patients with advanced GC.239–241 In addition, HER-2-targeted CAR-T cell therapy for GC is increasingly gaining attention to avoid drug resistance and improve treatment outcomes.241,242 Song et al produced genetically modified human T cells that express HER-2-specific CAR consisting of CD137 and CD3ζ,156 which not only recognized and killed HER-2+ GC cells in vitro but also showed effective and persistent antitumor activity against HER-2+ GC xenografts in vivo.156 This suggested that HER-2-targeted CAR-T cells might be suitable for the treatment of advanced HER-2+ GC, although their toxicity and immunogenicity will have to be verified in future trials.156,243–245 Furthermore, the focus of future studies would be to improve the antitumor activity of HER-2 targeted CAR-T cells by improving their proliferation capacity, function and persistence.
Ahmed et al constructed the second generation of HER-2-targeted CAR composed of FRP5-CD28-CD3ζ, and found that CAR-T cells had high affinity for HER-2 monoclonal antibody and specifically recognized and killed HER-2+ glioblastoma cells.246 HER-2-specific T cells have also been found to be effective against HER-2+ osteosarcoma cells.247 Sun et al successfully constructed a novel humanized chA21-28z CAR consisting of a chA21 single-chain variable region and an intracellular signal transduction region containing CD28 and CD3ζ. The CD4+ and CD8+ CAR-T cells248 recognized and killed HER-2+ ovarian cancer cells in vitro and significantly inhibited the growth of xenografts in mice.248 Taken together, HER-2 targeted CAR-T cell immunotherapy for GC can be further improved.
Current Status of Clinical Research on HER-2-CAR-T Therapy
HER-2-targeted CAR-T cell therapy is currently in the preclinical stage for GC, while clinical trials are underway for other solid tumors (summarized in Table 4). Ahmed et al administered high-dose HER-2-CAR-T cells to 10 patients with recurrent or refractory HER-2 positive sarcomas (5 osteosarcomas, 3 rhabdomyosarcomas, and 1 synovial sarcomas) who had received myeloablative therapy (fludarabine or fludarabine plus cyclophosphamide) and found that the combination of HER-2-CAR-T cells with other immunomodulatory agents cleared the tumors.154 The efficacy of CAR-T-HER-2 immunotherapy has also been demonstrated against tumors of the central nervous system,139 rhabdomyosarcoma,138 biliary tract cancers and pancreatic cancer.188 In addition, results of a phase I clinical trial indicated that the EGFR-CAR-T cell therapy was feasible and safe for patients with EGFR positive advanced NSCLC.184 Similar results were observed in patients with pancreatic carcinoma.185 O’Rourke et al suggested that overcoming adaptive changes in the local TME and addressing antigenic heterogeneity might improve the efficacy of EGFR variant III (EGFRvIII)-targeted strategies against glioblastoma.249 At present, more than 20 clinical trials are being conducted for HER-2-CAR-T therapy (Table 5), of which 2 are related to GC.
 |
Table 4 HER Family-Related CAR-T Clinical Studies in Cancers |
 |
Table 5 Ongoing Clinical Trials of HER-2-CAR-T Therapy |
The Safety of HER-2-CAR-T
There are several concerns about HER-2-targeted CAR-T cell therapy. Side effects of CAR-T cell therapy include systemic toxicity associated with T cell activation and cytokine release, as well as local toxicity caused by the specific interaction between target antigens expressed by non-malignant cells and CAR-T cells.250,251
To avoid systemic toxicity while maintaining clinical efficacy, CAR-T cells should be injected at a threshold that activates cytokine secretion but not above the level that induces a cytokine storm.252 The degree of CAR-T cell activation is influenced by tumor burden, tissue distribution and antigen expression, affinity of the scFv to the antigen and the costimulatory elements included in the CAR.250,253 Therefore, tumor burden and antigen expression/distribution should be considered when designing CARs to reduce the risk of systemic toxicity. For instance, HER-2 is not a tumor-specific antigen and is also expressed in normal tissues.254,255 One study reported that patients with metastatic colon cancer developed acute respiratory distress and pulmonary edema 15 minutes after receiving HER-2-specific CAR-T cells, followed by multiple organ failure and even death, suggesting off-tumor effects caused by CAR-T cells that recognize HER-2 expressed in normal lung tissues.256 Differences in binding sites between various scFv and HER-2 might influence the antitumor and off-tumor effects of HER-2 blockade by CAR-T cell cells.257 Luo et al selected HER-2 and CD3-targeted CAR-T cells to reduce the damage to normal tissues.258 The route to administer CAR-T cells is another factor that affects toxicity. Katz et al found that the intraperitoneal rather than the intravenous injection of CAR-T cells had a stronger effect on peritoneal metastasis and ascites, along with less toxicity.259 Thus, the improvement of the safety level is a prerequisite for the clinical translation of HER-2-CAR-T cell therapy.
CAR-T cell therapy has been widely used to treat hematologic malignancies, but its use is limited in solid tumors due to factors, such as low penetration. Incorporation of the tumor-penetrating signal peptide iRGD can improve the penetration of HER-2-CAR-T cells and therefore improve their efficacy.260 The novel CAR design is also a viable direction for HER-2-specific CAR-T cell therapy.261 The HER-2 binding domain of HER-2-CAR-T cells is not limited to scFv; the designed ankyrin repeat protein (DARPin) has also been used to bind HER-2 in other tumors.262 Several novel DARPin molecules with high affinity to HER-2 receptor have been developed, including MP0274, DARPin 9.26, DARPin 9.29, etc.263,264 In addition, CAR-modified NK cells, cytokine-induced killer (CIK) cells, and γδ T cells are other promising cell-based options.265,266 CAR-NK and CAR-CIK cells targeting HER-2 have achieved good efficacy against BC and glioblastoma multiforme,266,267 and are expected to be introduced into the treatment of HER-2 positive GC.
Conclusion
HER-2-targeted drugs were initially developed for BC and have since been extended to other HER-2-overexpressing tumors, such as stomach and gastroesophageal cancers.268 The first-generation HER-2 monoclonal antibody of trastuzumab is still the first-line treatment for GC, despite the high rate of drug resistance. The second generation of pertuzumab has not been extensively studied in GC patients.269,270 The conjugation of HER-2 antibodies to novel cytotoxic drugs such as T-DM1 was deemed promising for the treatment of HER-2 overexpressing tumors.94,271 However, studies showed that most patients with BC or GC exhibited primary or acquired resistance to T-DM1.97,272 Although the HER-2-targeting TKI lapatinib has achieved a good effect in BC, it has not been effective against GC.273 Bispecific antibodies with dual-targeting functions have also shown encouraging results,274 but further research is still needed. In short, these HER-2-targeted therapies may obviate the resistance to first-line drugs, reduce metastasis or prevent recurrence, and may also be used in combination with chemoradiotherapy and monoclonal antibodies to further improve first-line therapy in patients with GC.
CAR-T cells are a highly promising immunotherapeutic approach for ablating solid tumors. However, the efficacy of HER-2-targeted CAR-T therapy in GC141,156,188 needs to be supported by large-scale, multi-center and high-quality randomized clinical trials and evidence-based studies before full-scale clinical application. Given inherent heterogeneity, immunosuppressive TME and antigen migration, single target CAR-T cell immunotherapy cannot achieve ideal outcomes.275–277 Future researches on HER-2-CAR-T therapy in GC may focus on the following aspects: 1) upgrading the structural design of CARs to improve antitumor activity and migration capacity, as well as constructing CARs to target multiple antigens; 2) exploring more therapeutic subsets of T cells to reduce tumor immune escape; 3) reversing the immunosuppressive TME (for example, PD-L1/PD-L2 blockade) and enhancing CAR-T cell proliferation and cytokine production; 4) adjusting and optimizing treatment regimens to minimize CAR-T cell-induced adverse reactions. Therefore, with the continuous development of genetic engineering technology, HER-2-CAR-T cell therapy will become a safe and effective treatment for GC and other solid tumors in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Xu DH, Li Q, Hu H, et al. Transmembrane protein GRINA modulates aerobic glycolysis and promotes tumor progression in gastric cancer. J Exp Clin Cancer Res. 2018;37(1):308. doi:10.1186/s13046-018-0974-1
3. Zhang C, Chen Z, Chong X, et al. Clinical implications of plasma ctDNA features and dynamics in gastric cancer treated with HER2-targeted therapies. Clin Transl Med. 2020;10(8):e254. doi:10.1002/ctm2.254
4. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–279. doi:10.3322/caac.21657
5. van der Veen A, Brenkman HJF, Seesing MFJ, et al. Laparoscopic Versus Open Gastrectomy for Gastric Cancer (LOGICA): a Multicenter Randomized Clinical Trial. J Clin Oncol. 2021;39(9):978–989. doi:10.1200/JCO.20.01540
6. Griffith DM, Li H, Werrett MV, Andrews PC, Sun H. Medicinal chemistry and biomedical applications of bismuth-based compounds and nanoparticles. Chem Soc Rev. 2021. doi:10.1039/d0cs00031k
7. Kang YK, Yook JH, Park YK, et al. PRODIGY: a Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol. 2021;39(26):2903–2913. doi:10.1200/JCO.20.02914
8. Cui J, Cui H, Yang M, et al. Tongue coating microbiome as a potential biomarker for gastritis including precancerous cascade. Protein Cell. 2019;10(7):496–509. doi:10.1007/s13238-018-0596-6
9. Hindson J. Nivolumab plus chemotherapy for advanced gastric cancer and oesophageal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(8):523. doi:10.1038/s41575-021-00484-8
10. Meric-Bernstam F, Bahleda R, Hierro C, et al. Futibatinib, an irreversible FGFR1-4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 2021. doi:10.1158/2159-8290.CD-21-0697
11. Salas-Benito D, Perez-Gracia JL, Ponz-Sarvise M, et al. Paradigms on Immunotherapy Combinations with Chemotherapy. Cancer Discov. 2021;11(6):1353–1367. doi:10.1158/2159-8290.CD-20-1312
12. Tarantino P, Modi S, Tolaney SM, et al. Interstitial Lung Disease Induced by Anti-ERBB2 Antibody-Drug Conjugates: a Review. JAMA Oncol. 2021. doi:10.1001/jamaoncol.2021.3595
13. Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: the CLASS-01 Randomized Clinical Trial. JAMA. 2019;321(20):1983–1992. doi:10.1001/jama.2019.5359
14. Liu F, Huang C, Xu Z, et al. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: the CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6(10):1590–1597. doi:10.1001/jamaoncol.2020.3152
15. Pietrantonio F, Miceli R, Raimondi A, et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J Clin Oncol. 2019;37(35):3392–3400. doi:10.1200/JCO.19.01124
16. Yeoh KG, Tan P. Mapping the genomic diaspora of gastric cancer. Nat Rev Cancer. 2021. doi:10.1038/s41568-021-00412-7
17. Haffner I, Schierle K, Raimundez E, et al. HER2 Expression, Test Deviations, and Their Impact on Survival in Metastatic Gastric Cancer: results From the Prospective Multicenter VARIANZ Study. J Clin Oncol. 2021;39(13):1468–1478. doi:10.1200/JCO.20.02761
18. Roudko V, Bozkus CC, Orfanelli T, et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell. 2020;183(6):1634–1649 e1617. doi:10.1016/j.cell.2020.11.004
19. Chen ZH, Yan SM, Chen XX, et al. The genomic architecture of EBV and infected gastric tissue from precursor lesions to carcinoma. Genome Med. 2021;13(1):146. doi:10.1186/s13073-021-00963-2
20. Goutsouliak K, Veeraraghavan J, Sethunath V, et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat Rev Clin Oncol. 2020;17(4):233–250. doi:10.1038/s41571-019-0299-9
21. Sareyeldin RM, Gupta I, Al-Hashimi I, et al. Gene Expression and miRNAs Profiling: function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers. 2019;11:5. doi:10.3390/cancers11050646
22. Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341(6151):1192–1198. doi:10.1126/science.1241145
23. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
24. Mezni E, Vicier C, Guerin M, Sabatier R, Bertucci F, Goncalves A. New Therapeutics in HER2-Positive Advanced Breast Cancer: towards a Change in Clinical Practices?pi. Cancers. 2020;12:6. doi:10.3390/cancers12061573
25. Menyhart O, Pongor LS, Gyorffy B. Mutations Defining Patient Cohorts With Elevated PD-L1 Expression in Gastric Cancer. Front Pharmacol. 2018;9:1522. doi:10.3389/fphar.2018.01522
26. Yu C, Liu X, Yang J, et al. Combination of Immunotherapy With Targeted Therapy: theory and Practice in Metastatic Melanoma. Front Immunol. 2019;10:990. doi:10.3389/fimmu.2019.00990
27. Xu Y, Jiang J, Wang Y, et al. Engineered T Cell Therapy for Gynecologic Malignancies: challenges and Opportunities. Front Immunol. 2021;12:725330. doi:10.3389/fimmu.2021.725330
28. Terry RL, Meyran D, Fleuren EDG, et al. Chimeric Antigen Receptor T cell Therapy and the Immunosuppressive Tumor Microenvironment in Pediatric Sarcoma. Cancers. 2021;13:18. doi:10.3390/cancers13184704
29. Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131. doi:10.1186/s12943-021-01428-1
30. Sun Y, Li F, Sonnemann H, et al. Evolution of CD8(+) T Cell Receptor (TCR) Engineered Therapies for the Treatment of Cancer. Cells. 2021;10:9. doi:10.3390/cells10092379
31. Pan MR, Wu CC, Kan JY, et al. Impact of FAK Expression on the Cytotoxic Effects of CIK Therapy in Triple-Negative Breast Cancer. Cancers. 2019;12:1. doi:10.3390/cancers12010094
32. Geng L, Wang Z, Yang X, et al. Structure-based Design of Peptides with High Affinity and Specificity to HER2 Positive Tumors. Theranostics. 2015;5(10):1154–1165. doi:10.7150/thno.12398
33. Cohen S. The epidermal growth factor (EGF). Cancer. 1983;51(10):1787–1791. doi:10.1002/1097-0142(19830515)51:10<1787::
34. Yu Y, Rishi AK, Turner JR, et al. Cloning of a novel EGFR-related peptide: a putative negative regulator of EGFR. Am J Physiol Cell Physiol. 2001;280(5):C1083–1089. doi:10.1152/ajpcell.2001.280.5.C1083
35. Huang F, Shi Q, Li Y, et al. HER2/EGFR-AKT Signaling Switches TGFbeta from Inhibiting Cell Proliferation to Promoting Cell Migration in Breast Cancer. Cancer Res. 2018;78(21):6073–6085. doi:10.1158/0008-5472.CAN-18-0136
36. Quijano-Rubio A, Yeh HW, Park J, et al. De novo design of modular and tunable protein biosensors. Nature. 2021;591(7850):482–487. doi:10.1038/s41586-021-03258-z
37. Kovacs E, Zorn JA, Huang Y, Barros T, Kuriyan J. A structural perspective on the regulation of the epidermal growth factor receptor. Annu Rev Biochem. 2015;84:739–764. doi:10.1146/annurev-biochem-060614-034402
38. Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26(10):1742–1751. doi:10.1200/JCO.2007.12.1178
39. Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–760. doi:10.1038/nature01392
40. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303. doi:10.1016/j.ccr.2014.02.025
41. Sliwkowski MX. Ready to partner. Nat Struct Biol. 2003;10(3):158–159. doi:10.1038/nsb0303-158
42. Roskoski R
43. Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12(8):553–563. doi:10.1038/nrc3309
44. Lieto E, Ferraraccio F, Orditura M, et al. Expression of vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) is an independent prognostic indicator of worse outcome in gastric cancer patients. Ann Surg Oncol. 2008;15(1):69–79. doi:10.1245/s10434-007-9596-0
45. Qi Z, Qiu Y, Wang Z, et al. A novel diphtheria toxin-based bivalent human EGF fusion toxin for treatment of head and neck squamous cell carcinoma. Mol Oncol. 2021;15(4):1054–1068. doi:10.1002/1878-0261.12919
46. Chi A, Remick S, Tse W. EGFR inhibition in non-small cell lung cancer: current evidence and future directions. Biomark Res. 2013;1(1):2. doi:10.1186/2050-7771-1-2
47. Arkhipov A, Shan Y, Kim ET, Dror RO, Shaw DE. Her2 activation mechanism reflects evolutionary preservation of asymmetric ectodomain dimers in the human EGFR family. Elife. 2013;2:e00708. doi:10.7554/eLife.00708
48. Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19(9):2075–2083. doi:10.1111/jcmm.12618
49. Katona BW, Rustgi AK. Gastric Cancer Genomics: advances and Future Directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):211–217. doi:10.1016/j.jcmgh.2017.01.003
50. Tegtmeyer N, Harrer A, Rottner K, Backert S. Helicobacter pylori CagA Induces Cortactin Y-470 Phosphorylation-Dependent Gastric Epithelial Cell Scattering via Abl, Vav2 and Rac1 Activation. Cancers. 2021;13(16):5498. doi:10.3390/cancers13164241
51. Wang M, Chen L, Chen Y, et al. Intracellular matrix Gla protein promotes tumor progression by activating JAK2/STAT5 signaling in gastric cancer. Mol Oncol. 2020;14(5):1045–1058. doi:10.1002/1878-0261.12652
52. Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10(11):643–655. doi:10.1038/nrclinonc.2013.170
53. Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12:S3–8. doi:10.1093/annonc/12.suppl_1.s3
54. Roskoski R
55. Nam HJ, Im SA, Oh DY, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. 2013;12(1):16–26. doi:10.1158/1535-7163.MCT-12-0109
56. Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135(10):1331–1339. doi:10.1007/s00432-009-0574-8
57. Su CC. Tanshinone IIA inhibits gastric carcinoma AGS cells by decreasing the protein expression of VEGFR and blocking Ras/Raf/MEK/ERK pathway. Int J Mol Med. 2018;41(4):2389–2396. doi:10.3892/ijmm.2018.3407
58. Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284(1):54–65. doi:10.1016/s0014-4827(02)00101-5
59. Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 2012;8(8):961–987. doi:10.2217/fon.12.95
60. Yokoyama H, Ikehara Y, Kodera Y, et al. Molecular basis for sensitivity and acquired resistance to gefitinib in HER2-overexpressing human gastric cancer cell lines derived from liver metastasis. Br J Cancer. 2006;95(11):1504–1513. doi:10.1038/sj.bjc.6603459
61. Jenke R, Holzhauser-Rein M, Mueller-Wilke S, Lordick F, Aigner A, Buch T. SATB1-Mediated Upregulation of the Oncogenic Receptor Tyrosine Kinase HER3 Antagonizes MET Inhibition in Gastric Cancer Cells. Int J Mol Sci. 2020;22(1):155. doi:10.3390/ijms22010082
62. Fukuda K, Funakoshi T. Metastatic Extramammary Paget’s Disease: pathogenesis and Novel Therapeutic Approach. Front Oncol. 2018;8:38. doi:10.3389/fonc.2018.00038
63. Jabbour E, Ottmann OG, Deininger M, Hochhaus A. Targeting the phosphoinositide 3-kinase pathway in hematologic malignancies. Haematologica. 2014;99(1):7–18. doi:10.3324/haematol.2013.087171
64. Mezynski MJ, Farrelly AM, Cremona M, et al. Targeting the PI3K and MAPK pathways to improve response to HER2-targeted therapies in HER2-positive gastric cancer. J Transl Med. 2021;19(1):184. doi:10.1186/s12967-021-02842-1
65. Han ME, Kim HJ, Shin DH, Hwang SH, Kang CD, Oh SO. Overexpression of NRG1 promotes progression of gastric cancer by regulating the self-renewal of cancer stem cells. J Gastroenterol. 2015;50(6):645–656. doi:10.1007/s00535-014-1008-1
66. Ishii K, Morii N, Yamashiro H. Pertuzumab in the treatment of HER2-positive breast cancer: an evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 2019;14:51–70. doi:10.2147/CE.S217848
67. Ryu MH, Yoo C, Kim JG, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51(4):482–488. doi:10.1016/j.ejca.2014.12.015
68. Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–1384. doi:10.1016/S1470-2045(18)30481-9
69. Iqbal S, Goldman B, Fenoglio-Preiser CM, et al. Southwest Oncology Group study S0413: a phase II trial of lapatinib (GW572016) as first-line therapy in patients with advanced or metastatic gastric cancer. Ann Oncol. 2011;22(12):2610–2615. doi:10.1093/annonc/mdr021
70. Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: tyTAN–a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–2049. doi:10.1200/JCO.2013.53.6136
71. Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC–A Randomized Phase III Trial. J Clin Oncol. 2016;34(5):443–451. doi:10.1200/JCO.2015.62.6598
72. De Pauw I, Wouters A, Van den Bossche J, et al. Preclinical and clinical studies on Afatinib in monotherapy and in combination regimens: potential impact in colorectal cancer. Pharmacol Ther. 2016;166:71–83. doi:10.1016/j.pharmthera.2016.06.014
73. Deeks ED. Neratinib: first Global Approval. Drugs. 2017;77(15):1695–1704. doi:10.1007/s40265-017-0811-4
74. Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–653. doi:10.1016/S1470-2045(17)30111-0
75. Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, Phase 1 study. Lancet Oncol. 2019;20(6):827–836. doi:10.1016/S1470-2045(19)30088-9
76. Banerji U, van Herpen CML, Saura C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–1135. doi:10.1016/S1470-2045(19)30328-6
77. Barok M, Le Joncour V, Martins A, et al. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Lett. 2020;473:156–163. doi:10.1016/j.canlet.2019.12.037
78. Meric-Bernstam F. ZW25 Effective in HER2-Positive Cancers. Cancer Discov. 2019;9(1):8. doi:10.1158/2159-8290.CD-NB2018-162
79. de Vries Schultink AHM, Bol K, Doornbos RP, et al. Population Pharmacokinetics of MCLA-128, a HER2/HER3 Bispecific Monoclonal Antibody, in Patients with Solid Tumors. Clin Pharmacokinet. 2020;59(7):875–884. doi:10.1007/s40262-020-00858-2
80. Kirouac DC, Du JY, Lahdenranta J, et al. Computational modeling of ERBB2-amplified breast cancer identifies combined ErbB2/3 blockade as superior to the combination of MEK and AKT inhibitors. Sci Signal. 2013;6(288):ra68. doi:10.1126/scisignal.2004008
81. Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5(1):229. doi:10.1038/s41392-020-00323-3
82. Van cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–484. doi:10.1007/s10120-014-0402-y
83. An E, Ock CY, Kim TY, et al. Quantitative proteomic analysis of HER2 expression in the selection of gastric cancer patients for trastuzumab treatment. Ann Oncol. 2017;28(1):110–115. doi:10.1093/annonc/mdw442
84. Wang DS, Liu ZX, Lu YX, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68(7):1152–1161. doi:10.1136/gutjnl-2018-316522
85. Okines AFC, Turner NC. Heterogeneous HER2 Amplification-a New Clinical Category of HER2-Positive Breast Cancer? Cancer Discov. 2021;11(10):2369–2371. doi:10.1158/2159-8290.CD-21-0936
86. Dokmanovic M, King KE, Mohan N, Endo Y, Wu WJ. Cardiotoxicity of ErbB2-targeted therapies and its impact on drug development, a spotlight on trastuzumab. Expert Opin Drug Metab Toxicol. 2017;13(7):755–766. doi:10.1080/17425255.2017.1337746
87. Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi:10.1016/s1535-6108(02)00097-1
88. Gleeson JP, Keegan NM, Morris PG. Adding Pertuzumab to Trastuzumab and Taxanes in HER2 positive breast cancer. Expert Opin Biol Ther. 2018;18(3):251–262. doi:10.1080/14712598.2018.1410132
89. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi:10.1056/NEJMoa1413513
90. Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7(2):98–107. doi:10.1038/nrclinonc.2009.216
91. Bilancia D, Rosati G, Dinota A, Germano D, Romano R, Manzione L. Lapatinib in breast cancer. Ann Oncol. 2007;18(Suppl 6):vi26–30. doi:10.1093/annonc/mdm220
92. Jiao XD, Ding C, Zang YS, Yu G. Rapid symptomatic relief of HER2-positive gastric cancer leptomeningeal carcinomatosis with lapatinib, trastuzumab and capecitabine: a case report. BMC Cancer. 2018;18(1):206. doi:10.1186/s12885-018-4116-0
93. Voigtlaender M, Schneider-Merck T, Trepel M. Lapatinib. Recent Results Cancer Res. 2018;211:19–44. doi:10.1007/978-3-319-91442-8_2
94. LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011;17(20):6437–6447. doi:10.1158/1078-0432.CCR-11-0762
95. Griguolo G, Braso-Maristany F, Gonzalez-Farre B, et al. ERBB2 mRNA Expression and Response to Ado-Trastuzumab Emtansine (T-DM1) in HER2-Positive Breast Cancer. Cancers. 2020;12:7. doi:10.3390/cancers12071902
96. Jhaveri KL, Wang XV, Makker V, et al. Ado-Trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. 2019;30(11):1821–1830. doi:10.1093/annonc/mdz291
97. Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48. doi:10.1038/s41571-019-0268-3
98. Le Joncour V, Martins A, Puhka M, et al. A Novel Anti-HER2 Antibody-Drug Conjugate XMT-1522 for HER2-Positive Breast and Gastric Cancers Resistant to Trastuzumab Emtansine. Mol Cancer Ther. 2019;18(10):1721–1730. doi:10.1158/1535-7163.MCT-19-0207
99. Menderes G, Bonazzoli E, Bellone S, et al. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine and Ovarian Carcinosarcoma with HER2/Neu Expression. Clin Cancer Res. 2017;23(19):5836–5845. doi:10.1158/1078-0432.CCR-16-2862
100. Skidmore L, Sakamuri S, Knudsen NA, et al. ARX788, a Site-specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-low and T-DM1-resistant Breast and Gastric Cancers. Mol Cancer Ther. 2020;19(9):1833–1843. doi:10.1158/1535-7163.MCT-19-1004
101. Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. doi:10.1038/s41573-019-0028-1
102. de Vries Schultink AHM, Doornbos RP, Bakker ABH, et al. Translational PK-PD modeling analysis of MCLA-128, a HER2/HER3 bispecific monoclonal antibody, to predict clinical efficacious exposure and dose. Invest New Drugs. 2018;36(6):1006–1015. doi:10.1007/s10637-018-0593-x
103. Meric-Bernstam F. MCLA-128 Fights NRG1 Fusion-Positive Cancers. Cancer Discov. 2019;9(12):1636. doi:10.1158/2159-8290.CD-NB2019-128
104. Yu S, Liu Q, Han X, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:31. doi:10.1186/s40164-017-0091-4
105. McDonagh CF, Huhalov A, Harms BD, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11(3):582–593. doi:10.1158/1535-7163.MCT-11-0820
106. Liu D, Zhao J, Song Y. Engineering switchable and programmable universal CARs for CAR T therapy. J Hematol Oncol. 2019;12(1):69. doi:10.1186/s13045-019-0763-0
107. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86(24):10024–10028. doi:10.1073/pnas.86.24.10024
108. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi:10.1073/pnas.90.2.720
109. Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–2797.
110. Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. doi:10.4049/jimmunol.172.1.104
111. Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. CAR T Cells in Solid Tumors: blueprints for Building Effective Therapies. Front Immunol. 2018;9:1740. doi:10.3389/fimmu.2018.01740
112. Im A, Pavletic SZ. Immunotherapy in hematologic malignancies: past, present, and future. J Hematol Oncol. 2017;10(1):94. doi:10.1186/s13045-017-0453-8
113. Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi:10.1016/S1470-2045(21)00375-2
114. Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol. 2020;38(17):1938–1950. doi:10.1200/JCO.19.03279
115. Ramos CA, Grover NS, Beaven AW, et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol. 2020;38(32):3794–3804. doi:10.1200/JCO.20.01342
116. Yao S, Jianlin C, Yarong L, et al. Donor-Derived CD123-Targeted CAR T Cell Serves as a RIC Regimen for Haploidentical Transplantation in a Patient With FUS-ERG+ AML. Front Oncol. 2019;9:1358. doi:10.3389/fonc.2019.01358
117. Lin S, Cheng L, Ye W, et al. Chimeric CTLA4-CD28-CD3z T Cells Potentiate Antitumor Activity Against CD80/CD86-Positive B Cell Malignancies. Front Immunol. 2021;12:642528. doi:10.3389/fimmu.2021.642528
118. Wenthe J, Naseri S, Labani-Motlagh A, et al. Boosting CAR T-cell responses in lymphoma by simultaneous targeting of CD40/4-1BB using oncolytic viral gene therapy. Cancer Immunol Immunother. 2021;70(10):2851–2865. doi:10.1007/s00262-021-02895-7
119. Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi:10.4161/2162402X.2014.994446
120. Cui J, Wang H, Medina R, et al. Inhibition of PP2A with LB-100 Enhances Efficacy of CAR-T Cell Therapy Against Glioblastoma. Cancers. 2020;12(1):87. doi:10.3390/cancers12010139
121. Li H, Ding J, Lu M, et al. CAIX-specific CAR-T Cells and Sunitinib Show Synergistic Effects Against Metastatic Renal Cancer Models. J Immunother. 2020;43(1):16–28. doi:10.1097/CJI.0000000000000301
122. Ghorashian S, Kramer AM, Onuoha S, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25(9):1408–1414. doi:10.1038/s41591-019-0549-5
123. Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL. J Clin Oncol. 2021:JCO2101405. doi:10.1200/JCO.21.01405
124. Meric-Bernstam F. CAR Linker Length Modulates CD22-Targeted CAR T-cell Efficacy in ALL. Cancer Discov. 2021;11(7):1611. doi:10.1158/2159-8290.CD-RW2021-065
125. Bueno C, Velasco-Hernandez T, Gutierrez-Aguera F, et al. CD133-directed CAR T-cells for MLL leukemia: on-target, off-tumor myeloablative toxicity. Leukemia. 2019;33(8):2090–2125. doi:10.1038/s41375-019-0418-8
126. Dai H, Tong C, Shi D, et al. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology. 2020;9(1):1846926. doi:10.1080/2162402X.2020.1846926
127. Han Y, Sun B, Cai H, Xuan Y. Simultaneously target of normal and stem cells-like gastric cancer cells via cisplatin and anti-CD133 CAR-T combination therapy. Cancer Immunol Immunother. 2021;70(10):2795–2803. doi:10.1007/s00262-021-02891-x
128. Vora P, Venugopal C, Salim SK, et al. The Rational Development of CD133-Targeting Immunotherapies for Glioblastoma. Cell Stem Cell. 2020;26(6):832–844 e836. doi:10.1016/j.stem.2020.04.008
129. Van Laethem F, Saba I, Lu J, et al. Novel MHC-Independent alphabetaTCRs Specific for CD48, CD102, and CD155 Self-Proteins and Their Selection in the Thymus. Front Immunol. 2020;11:1216. doi:10.3389/fimmu.2020.01216
130. Katz SC, Moody AE, Guha P, et al. HITM-SURE: hepatic immunotherapy for metastases phase Ib anti-CEA CAR-T study utilizing pressure enabled drug delivery. J Immunother Cancer. 2020;8(2):158. doi:10.1136/jitc-2020-001097
131. Kumar J, Kumar R, Kumar Singh A, et al. Deletion of Cbl-b inhibits CD8(+) T-cell exhaustion and promotes CAR T-cell function. J Immunother Cancer. 2021;9(1):215. doi:10.1136/jitc-2020-001688
132. Zhang C, Wang Z, Yang Z, et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol Ther. 2017;25(5):1248–1258. doi:10.1016/j.ymthe.2017.03.010
133. Zhang E, Yang P, Gu J, et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. J Hematol Oncol. 2018;11(1):102. doi:10.1186/s13045-018-0646-9
134. Jiang H, Shi Z, Wang P, et al. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer. J Natl Cancer Inst. 2019;111(4):409–418. doi:10.1093/jnci/djy134
135. Luo H, Su J, Sun R, et al. Coexpression of IL7 and CCL21 Increases Efficacy of CAR-T Cells in Solid Tumors without Requiring Preconditioned Lymphodepletion. Clin Cancer Res. 2020;26(20):5494–5505. doi:10.1158/1078-0432.CCR-20-0777
136. Zhu G, Foletti D, Liu X, et al. Targeting CLDN18.2 by CD3 Bispecific and ADC Modalities for the Treatments of Gastric and Pancreatic Cancer. Sci Rep. 2019;9(1):8420. doi:10.1038/s41598-019-44874-0
137. Klar AS, Gopinadh J, Kleber S, Wadle A, Renner C. Treatment with 5-Aza-2’-Deoxycytidine Induces Expression of NY-ESO-1 and Facilitates Cytotoxic T Lymphocyte-Mediated Tumor Cell Killing. PLoS One. 2015;10(10):e0139221. doi:10.1371/journal.pone.0139221
138. Hegde M, Joseph SK, Pashankar F, et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat Commun. 2020;11(1):3549. doi:10.1038/s41467-020-17175-8
139. Vitanza NA, Johnson AJ, Wilson AL, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med. 2021;27(9):1544–1552. doi:10.1038/s41591-021-01404-8
140. Xia L, Zheng Z, Liu JY, et al. Targeting Triple-Negative Breast Cancer with Combination Therapy of EGFR CAR T Cells and CDK7 Inhibition. Cancer Immunol Res. 2021;9(6):707–722. doi:10.1158/2326-6066.CIR-20-0405
141. Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188549. doi:10.1016/j.bbcan.2021.188549
142. Maggs L, Cattaneo G, Dal AE, Moghaddam AS, Ferrone S. CAR T Cell-Based Immunotherapy for the Treatment of Glioblastoma. Front Neurosci. 2021;15:662064. doi:10.3389/fnins.2021.662064
143. Huang SL, Wang YM, Wang QY, et al. Mechanisms and Clinical Trials of Hepatocellular Carcinoma Immunotherapy. Front Genet. 2021;12:691391. doi:10.3389/fgene.2021.691391
144. Maiti A, Daver NG. Lowering mTORC1 Drives CAR T-Cells Home in Acute Myeloid Leukemia. Clin Cancer Res. 2021;27(21):5739–5741. doi:10.1158/1078-0432.CCR-21-2574
145. Bughda R, Dimou P, D’Souza RR, Klampatsa A. Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: launching an Attack on Tumor Stroma. Immunotargets Ther. 2021;10:313–323. doi:10.2147/ITT.S291767
146. Lo A, Wang LS, Scholler J, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015;75(14):2800–2810. doi:10.1158/0008-5472.CAN-14-3041
147. Kim M, Pyo S, Kang CH, et al. Folate receptor 1 (FOLR1) targeted chimeric antigen receptor (CAR) T cells for the treatment of gastric cancer. PLoS One. 2018;13(6):e0198347. doi:10.1371/journal.pone.0198347
148. Kandalaft LE, Powell DJ
149. Song DG, Ye Q, Poussin M, Chacon JA, Figini M, Powell DJ
150. Moghimi B, Muthugounder S, Jambon S, et al. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun. 2021;12(1):511. doi:10.1038/s41467-020-20785-x
151. Yu J, Wu X, Yan J, et al. Anti-GD2/4-1BB chimeric antigen receptor T cell therapy for the treatment of Chinese melanoma patients. J Hematol Oncol. 2018;11(1):1. doi:10.1186/s13045-017-0548-2
152. Meng M, Wu YC. Combination of AAV-CCL19 and GPC3 CAR-T Cells in the Treatment of Hepatocellular Carcinoma. J Immunol Res. 2021;2021:1782728. doi:10.1155/2021/1782728
153. Pang N, Shi J, Qin L, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14(1):118. doi:10.1186/s13045-021-01128-9
154. Ahmed N, Brawley VS, Hegde M, et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi:10.1200/JCO.2014.58.0225
155. Nellan A, Rota C, Majzner R, et al. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer. 2018;6(1):30. doi:10.1186/s40425-018-0340-z
156. Song Y, Tong C, Wang Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9(10):867–878. doi:10.1007/s13238-017-0384-8
157. Szoor A, Toth G, Zsebik B, et al. Trastuzumab derived HER2-specific CARs for the treatment of trastuzumab-resistant breast cancer: CAR T cells penetrate and eradicate tumors that are not accessible to antibodies. Cancer Lett. 2020;484:1–8. doi:10.1016/j.canlet.2020.04.008
158. Teng R, Zhao J, Zhao Y, et al. Chimeric Antigen Receptor-modified T Cells Repressed Solid Tumors and Their Relapse in an Established Patient-derived Colon Carcinoma Xenograft Model. J Immunother. 2019;42(2):33–42. doi:10.1097/CJI.0000000000000251
159. Terlikowska KM, Dobrzycka B, Terlikowski SJ. Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment. Int J Mol Sci. 2021;22(7):658. doi:10.3390/ijms22073495
160. Boice M, Salloum D, Mourcin F, et al. Loss of the HVEM Tumor Suppressor in Lymphoma and Restoration by Modified CAR-T Cells. Cell. 2016;167(2):405–418 e413. doi:10.1016/j.cell.2016.08.032
161. Jung M, Yang Y, McCloskey JE, et al. Chimeric Antigen Receptor T Cell Therapy Targeting ICAM-1 in Gastric Cancer. Mol Ther Oncolytics. 2020;18:587–601. doi:10.1016/j.omto.2020.08.009
162. Wei H, Wang Z, Kuang Y, et al. Intercellular Adhesion Molecule-1 as Target for CAR-T-Cell Therapy of Triple-Negative Breast Cancer. Front Immunol. 2020;11:573823. doi:10.3389/fimmu.2020.573823
163. Pituch KC, Miska J, Krenciute G, et al. Adoptive Transfer of IL13Ralpha2-Specific Chimeric Antigen Receptor T Cells Creates a Pro-inflammatory Environment in Glioblastoma. Mol Ther. 2018;26(4):986–995. doi:10.1016/j.ymthe.2018.02.001
164. Hong H, Stastny M, Brown C, et al. Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J Immunother. 2014;37(2):93–104. doi:10.1097/CJI.0000000000000018
165. Kunkele A, Taraseviciute A, Finn LS, et al. Preclinical Assessment of CD171-Directed CAR T-cell Adoptive Therapy for Childhood Neuroblastoma: CE7 Epitope Target Safety and Product Manufacturing Feasibility. Clin Cancer Res. 2017;23(2):466–477. doi:10.1158/1078-0432.CCR-16-0354
166. Tang L, Yang J, Liu W, et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology. 2009;137(4):
167. Montemagno C, Cassim S, Pouyssegur J, Broisat A, Pages G. From Malignant Progression to Therapeutic Targeting: current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci. 2020;21(11):32. doi:10.3390/ijms21114067
168. Schoutrop E, El-Serafi I, Poiret T, et al. Mesothelin-Specific CAR T Cells Target Ovarian Cancer. Cancer Res. 2021;81(11):3022–3035. doi:10.1158/0008-5472.CAN-20-2701
169. Yeo D, Castelletti L, van Zandwijk N, Rasko JEJ. Hitting the Bull’s-Eye: mesothelin’s Role as a Biomarker and Therapeutic Target for Malignant Pleural Mesothelioma. Cancers. 2021;13(16):14. doi:10.3390/cancers13163932
170. Supimon K, Sangsuwannukul T, Sujjitjoon J, et al. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci Rep. 2021;11(1):6276. doi:10.1038/s41598-021-85747-9
171. You F, Jiang L, Zhang B, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci. 2016;59(4):386–397. doi:10.1007/s11427-016-5024-7
172. Han Y, Xie W, Song DG, Powell DJ
173. He C, Zhou Y, Li Z, et al. Co-Expression of IL-7 Improves NKG2D-Based CAR T Cell Therapy on Prostate Cancer by Enhancing the Expansion and Inhibiting the Apoptosis and Exhaustion. Cancers. 2020;12(7):54987. doi:10.3390/cancers12071969
174. Zhang Y, Li X, Zhang J, Mao L. Novel cellular immunotherapy using NKG2D CAR-T for the treatment of cervical cancer. Biomed Pharmacother. 2020;131:110562. doi:10.1016/j.biopha.2020.110562
175. Liu M, Wang X, Li W, et al. Targeting PD-L1 in non-small cell lung cancer using CAR T cells. Oncogenesis. 2020;9(8):72. doi:10.1038/s41389-020-00257-z
176. Nelson MA, Ngamcherdtrakul W, Luoh SW, Yantasee W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev. 2021;40(2):519–536. doi:10.1007/s10555-021-09968-0
177. Zhao W, Jia L, Zhang M, et al. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res. 2019;9(8):1846–1856.
178. Priceman SJ, Gerdts EA, Tilakawardane D, et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology. 2018;7(2):e1380764. doi:10.1080/2162402X.2017.1380764
179. Wu D, Lv J, Zhao R, et al. PSCA is a target of chimeric antigen receptor T cells in gastric cancer. Biomark Res. 2020;8:3. doi:10.1186/s40364-020-0183-x
180. Wang G, Zhou X, Fuca G, et al. Fully human antibody VH domains to generate mono and bispecific CAR to target solid tumors. J Immunother Cancer. 2021;9(4):548. doi:10.1136/jitc-2020-002173
181. Zuccolotto G, Penna A, Fracasso G, et al. PSMA-Specific CAR-Engineered T Cells for Prostate Cancer: CD28 Outperforms Combined CD28-4-1BB “Super-Stimulation”. Front Oncol. 2021;11:708073. doi:10.3389/fonc.2021.708073
182. Katz SC, Burga RA, McCormack E, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res. 2015;21(14):3149–3159. doi:10.1158/1078-0432.CCR-14-1421
183. Tchou J, Zhao Y, Levine BL, et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol Res. 2017;5(12):1152–1161. doi:10.1158/2326-6066.CIR-17-0189
184. Zhang Y, Zhang Z, Ding Y, et al. Phase I clinical trial of EGFR-specific CAR-T cells generated by the piggyBac transposon system in advanced relapsed/refractory non-small cell lung cancer patients. J Cancer Res Clin Oncol. 2021;147(12):3725–3734. doi:10.1007/s00432-021-03613-7
185. Liu Y, Guo Y, Wu Z, et al. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy. 2020;22(10):573–580. doi:10.1016/j.jcyt.2020.04.088
186. Hiltbrunner S, Britschgi C, Schuberth P, et al. Local delivery of CAR T cells targeting fibroblast activation protein is safe in patients with pleural mesothelioma: first report of FAPME, a phase I clinical trial. Ann Oncol. 2021;32(1):120–121. doi:10.1016/j.annonc.2020.10.474
187. Junghans RP, Ma Q, Rathore R, et al. Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate. 2016;76(14):1257–1270. doi:10.1002/pros.23214
188. Feng K, Liu Y, Guo Y, et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018;9(10):838–847. doi:10.1007/s13238-017-0440-4
189. Chen YJ, You ML, Chong QY, et al. Autocrine Human Growth Hormone Promotes Invasive and Cancer Stem Cell-Like Behavior of Hepatocellular Carcinoma Cells by STAT3 Dependent Inhibition of CLAUDIN-1 Expression. Int J Mol Sci. 2017;18(6):54. doi:10.3390/ijms18061274
190. Bebnowska D, Grywalska E, Niedzwiedzka-Rystwej P, et al. CAR-T Cell Therapy-An Overview of Targets in Gastric Cancer. J Clin Med. 2020;9(6):14. doi:10.3390/jcm9061894
191. Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Tureci O. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49(9):870–876. doi:10.1093/jjco/hyz068
192. Wen Y, Graybill WS, Previs RA, et al. Immunotherapy targeting folate receptor induces cell death associated with autophagy in ovarian cancer. Clin Cancer Res. 2015;21(2):448–459. doi:10.1158/1078-0432.CCR-14-1578
193. Ma DW, Finnell RH, Davidson LA, et al. Folate transport gene inactivation in mice increases sensitivity to colon carcinogenesis. Cancer Res. 2005;65(3):887–897.
194. Shivange G, Urbanek K, Przanowski P, et al. A Single-Agent Dual-Specificity Targeting of FOLR1 and DR5 as an Effective Strategy for Ovarian Cancer. Cancer Cell. 2018;34(2):331–345 e311. doi:10.1016/j.ccell.2018.07.005
195. Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108(3):619–626. doi:10.1016/j.ygyno.2007.11.020
196. Zuo S, Wen Y, Panha H, et al. Modification of cytokine-induced killer cells with folate receptor alpha (FRalpha)-specific chimeric antigen receptors enhances their antitumor immunity toward FRalpha-positive ovarian cancers. Mol Immunol. 2017;85:293–304. doi:10.1016/j.molimm.2017.03.017
197. van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med (Berl). 1996;74(1):13–33. doi:10.1007/BF00202069
198. Ziprin P, Ridgway PF, Pfistermuller KL, Peck DH, Darzi AW. ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-alpha: a potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun Adhes. 2003;10(3):141–154.
199. Jung WC, Jang YJ, Kim JH, et al. Expression of intercellular adhesion molecule-1 and e-selectin in gastric cancer and their clinical significance. J Gastric Cancer. 2012;12(3):140–148. doi:10.5230/jgc.2012.12.3.140
200. Min IM, Shevlin E, Vedvyas Y, et al. CAR T Therapy Targeting ICAM-1 Eliminates Advanced Human Thyroid Tumors. Clin Cancer Res. 2017;23(24):7569–7583. doi:10.1158/1078-0432.CCR-17-2008
201. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93(1):136–140. doi:10.1073/pnas.93.1.136
202. Lv J, Zhao R, Wu D, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J Hematol Oncol. 2019;12(1):18. doi:10.1186/s13045-019-0704-y
203. Sotoudeh M, Shirvani SI, Merat S, Ahmadbeigi N, Naderi M. MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. J Cell Biochem. 2019;120(4):5010–5017. doi:10.1002/jcb.27776
204. Beatty GL, O’Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: defining the challenges and next steps. Pharmacol Ther. 2016;166:30–39. doi:10.1016/j.pharmthera.2016.06.010
205. Morello A, Sadelain M, Adusumilli PS. Mesothelin-Targeted CARs: driving T Cells to Solid Tumors. Cancer Discov. 2016;6(2):133–146. doi:10.1158/2159-8290.CD-15-0583
206. Watanabe K, Luo Y, Da T, et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3(7):75. doi:10.1172/jci.insight.99573
207. Zhang Q, Liu G, Liu J, et al. The antitumor capacity of mesothelin-CAR-T cells in targeting solid tumors in mice. Mol Ther Oncolytics. 2021;20:556–568. doi:10.1016/j.omto.2021.02.013
208. Zhao R, Cui Y, Zheng Y, et al. Human Hyaluronidase PH20 Potentiates the Antitumor Activities of Mesothelin-Specific CAR-T Cells Against Gastric Cancer. Front Immunol. 2021;12:660488. doi:10.3389/fimmu.2021.660488
209. Stojanovic A, Correia MP, Cerwenka A. The NKG2D/NKG2DL Axis in the Crosstalk Between Lymphoid and Myeloid Cells in Health and Disease. Front Immunol. 2018;9:827. doi:10.3389/fimmu.2018.00827
210. Basu S, Eriksson M, Pioli PA, et al. Human uterine NK cells interact with uterine macrophages via NKG2D upon stimulation with PAMPs. Am J Reprod Immunol. 2009;61(1):52–61. doi:10.1111/j.1600-0897.2008.00661.x
211. Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immun. 2013;13:8.
212. Baumeister SH, Murad J, Werner L, et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol Res. 2019;7(1):100–112. doi:10.1158/2326-6066.CIR-18-0307
213. Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma. Cancer Res. 2018;78(4):1031–1043. doi:10.1158/0008-5472.CAN-17-1788
214. Sun B, Yang D, Dai H, et al. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol Res. 2019;7(11):1813–1823. doi:10.1158/2326-6066.CIR-19-0026
215. Tao K, He M, Tao F, et al. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol. 2018;82(5):815–827. doi:10.1007/s00280-018-3670-0
216. de Vries NL, Swets M, Vahrmeijer AL, Hokland M, Kuppen PJ. The Immunogenicity of Colorectal Cancer in Relation to Tumor Development and Treatment. Int J Mol Sci. 2016;17:7. doi:10.3390/ijms17071030
217. Jiang W, Pan S, Chen X, Wang ZW, Zhu X. The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol Cancer. 2021;20(1):116. doi:10.1186/s12943-021-01406-7
218. Gong B, Kiyotani K, Sakata S, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J Exp Med. 2019;216(4):982–1000. doi:10.1084/jem.20180870
219. Yu H, Chen P, Xia L, et al. PD-1/PD-L1 inhibitor plus chemotherapy versus bevacizumab plus chemotherapy in first-line treatment for non-squamous non-small-cell lung cancer. J Immunother Cancer. 2021;9:11. doi:10.1136/jitc-2021-003431
220. Yang Y, Zhang X, Lin F, et al. Bispecific CD3-HAC carried by E1A-engineered mesenchymal stromal cells against metastatic breast cancer by blocking PD-L1 and activating T cells. J Hematol Oncol. 2019;12(1):46. doi:10.1186/s13045-019-0723-8
221. Li Q, Zhou ZW, Lu J, et al. PD-L1(P146R) is prognostic and a negative predictor of response to immunotherapy in gastric cancer. Mol Ther. 2021. doi:10.1016/j.ymthe.2021.09.013
222. Kobold S, Grassmann S, Chaloupka M, et al. Impact of a New Fusion Receptor on PD-1-Mediated Immunosuppression in Adoptive T Cell Therapy. J Natl Cancer Inst. 2015;107:8. doi:10.1093/jnci/djv146
223. Schlenker R, Olguin-Contreras LF, Leisegang M, et al. Chimeric PD-1:28 Receptor Upgrades Low-Avidity T cells and Restores Effector Function of Tumor-Infiltrating Lymphocytes for Adoptive Cell Therapy. Cancer Res. 2017;77(13):3577–3590. doi:10.1158/0008-5472.CAN-16-1922
224. Qin L, Zhao R, Chen D, et al. Chimeric antigen receptor T cells targeting PD-L1 suppress tumor growth. Biomark Res. 2020;8:19. doi:10.1186/s40364-020-00198-0
225. Saeki N, Gu J, Yoshida T, Wu X. Prostate stem cell antigen: a Jekyll and Hyde molecule? Clin Cancer Res. 2010;16(14):3533–3538. doi:10.1158/1078-0432.CCR-09-3169
226. Wei X, Lai Y, Li J, et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology. 2017;6(3):e1284722. doi:10.1080/2162402X.2017.1284722
227. Weimin S, Abula A, Qianghong D, Wenguang W. Chimeric cytokine receptor enhancing PSMA-CAR-T cell-mediated prostate cancer regression. Cancer Biol Ther. 2020;21(6):570–580. doi:10.1080/15384047.2020.1739952
228. Kloss CC, Lee J, Zhang A, et al. Dominant-Negative TGF-beta Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol Ther. 2018;26(7):1855–1866. doi:10.1016/j.ymthe.2018.05.003
229. Bai C, Gao S, Hu S, et al. Self-Assembled Multivalent Aptamer Nanoparticles with Potential CAR-like Characteristics Could Activate T Cells and Inhibit Melanoma Growth. Mol Ther Oncolytics. 2020;17:9–20. doi:10.1016/j.omto.2020.03.002
230. Maher J, Wilkie S, Davies DM, et al. Targeting of Tumor-Associated Glycoforms of MUC1 with CAR T Cells. Immunity. 2016;45(5):945–946. doi:10.1016/j.immuni.2016.10.014
231. Wang HF, Wang SS, Huang MC, Liang XH, Tang YJ, Tang YL. Targeting Immune-Mediated Dormancy: a Promising Treatment of Cancer. Front Oncol. 2019;9:498. doi:10.3389/fonc.2019.00498
232. Cheadle EJ, Gornall H, Baldan V, Hanson V, Hawkins RE, Gilham DE. CAR T cells: driving the road from the laboratory to the clinic. Immunol Rev. 2014;257(1):91–106. doi:10.1111/imr.12126
233. Yu S, Li A, Liu Q, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10(1):78. doi:10.1186/s13045-017-0444-9
234. Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–581. doi:10.1038/nrc.2016.97
235. Caruana I, Diaconu I, Dotti G. From monoclonal antibodies to chimeric antigen receptors for the treatment of human malignancies. Semin Oncol. 2014;41(5):661–666. doi:10.1053/j.seminoncol.2014.08.005
236. Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. doi:10.1172/JCI83416
237. Quintarelli C, Guercio M, Manni S, et al. Strategy to prevent epitope masking in CAR.CD19+ B-cell leukemia blasts. J Immunother Cancer. 2021;9(6). doi:10.1136/jitc-2020-001514
238. Salguero-Aranda C, Amaral AT, Olmedo-Pelayo J, Diaz-Martin J, Alava E. Breakthrough Technologies Reshape the Ewing Sarcoma Molecular Landscape. Cells. 2020;9:4. doi:10.3390/cells9040804
239. Li C, Shen Q, Zhang P, et al. Targeting MUS81 promotes the anticancer effect of WEE1 inhibitor and immune checkpoint blocking combination therapy via activating cGAS/STING signaling in gastric cancer cells. J Exp Clin Cancer Res. 2021;40(1):315. doi:10.1186/s13046-021-02120-4
240. Shi J, Li F, Yao X, et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 2018;37(22):3022–3038. doi:10.1038/s41388-018-0204-5
241. Shi W, Zhang G, Ma Z, et al. Hyperactivation of HER2-SHCBP1-PLK1 axis promotes tumor cell mitosis and impairs trastuzumab sensitivity to gastric cancer. Nat Commun. 2021;12(1):2812. doi:10.1038/s41467-021-23053-8
242. Yu S, Hu C, Cai L, et al. Seven-Gene Signature Based on Glycolysis Is Closely Related to the Prognosis and Tumor Immune Infiltration of Patients With Gastric Cancer. Front Oncol. 2020;10:1778. doi:10.3389/fonc.2020.01778
243. Sawaisorn P, Atjanasuppat K, Anurathapan U, Chutipongtanate S, Hongeng S. Strategies to Improve Chimeric Antigen Receptor Therapies for Neuroblastoma. Vaccines. 2020;8:4. doi:10.3390/vaccines8040753
244. Thadi A, Khalili M, Morano WF, Richard SD, Katz SC, Bowne WB. Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis. Vaccines. 2018;6:3. doi:10.3390/vaccines6030054
245. Zhao D, Klempner SJ, Chao J. Progress and challenges in HER2-positive gastroesophageal adenocarcinoma. J Hematol Oncol. 2019;12(1):50. doi:10.1186/s13045-019-0737-2
246. Ahmed N, Salsman VS, Kew Y, et al. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–485. doi:10.1158/1078-0432.CCR-09-1322
247. Ahmed N, Salsman VS, Yvon E, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17(10):1779–1787. doi:10.1038/mt.2009.133
248. Sun M, Shi H, Liu C, Liu J, Liu X, Sun Y. Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res. 2014;16(3):R61. doi:10.1186/bcr3674
249. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:399. doi:10.1126/scitranslmed.aaa0984
250. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–167. doi:10.1038/s41571-019-0297-y
251. Schaft N. The Landscape of CAR-T Cell Clinical Trials against Solid Tumors-A Comprehensive Overview. Cancers. 2020;12:9. doi:10.3390/cancers12092567
252. Liu C, Qi T, Milner JJ, Lu Y, Cao Y. Speed and Location Both Matter: antigen Stimulus Dynamics Controls CAR-T Cell Response. Front Immunol. 2021;12:748768. doi:10.3389/fimmu.2021.748768
253. Mulazzani M, Frassle SP, von Mucke-heim I, et al. Long-term in vivo microscopy of CAR T cell dynamics during eradication of CNS lymphoma in mice. Proc Natl Acad Sci U S A. 2019;116(48):24275–24284. doi:10.1073/pnas.1903854116
254. Leko V, Rosenberg SA. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell. 2020;38(4):454–472. doi:10.1016/j.ccell.2020.07.013
255. Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35:4. doi:10.1042/BSR20150089
256. Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi:10.1038/mt.2010.24
257. Li D, Li X, Zhou WL, et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct Target Ther. 2019;4:35. doi:10.1038/s41392-019-0070-9
258. Luo F, Qian J, Yang J, et al. Bifunctional alphaHER2/CD3 RNA-engineered CART-like human T cells specifically eliminate HER2(+) gastric cancer. Cell Res. 2016;26(7):850–853. doi:10.1038/cr.2016.81
259. Katz SC, Point GR, Cunetta M, et al. Regional CAR-T cell infusions for peritoneal carcinomatosis are superior to systemic delivery. Cancer Gene Ther. 2016;23(5):142–148. doi:10.1038/cgt.2016.14
260. Ding N, Zou Z, Sha H, et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat Commun. 2019;10(1):1336. doi:10.1038/s41467-019-09296-6
261. Brown MP, Ebert LM, Gargett T. Clinical chimeric antigen receptor-T cell therapy: a new and promising treatment modality for glioblastoma. Clin Transl Immunol. 2019;8(5):e1050. doi:10.1002/cti2.1050
262. Proshkina G, Deyev S, Ryabova A, et al. DARPin_9-29-Targeted Mini Gold Nanorods Specifically Eliminate HER2-Overexpressing Cancer Cells. ACS Appl Mater Interfaces. 2019;11(38):34645–34651. doi:10.1021/acsami.9b10441
263. Kasaraneni N, Chamoun-Emanuelli AM, Wright G, Chen Z. Retargeting Lentiviruses via SpyCatcher-SpyTag Chemistry for Gene Delivery into Specific Cell Types. mBio. 2017;8:6. doi:10.1128/mBio.01860-17
264. Shramova EI, Shilova MV, Ryabova AV, et al. Barnase*Barstar-guided two-step targeting approach for drug delivery to tumor cells in vivo. J Control Release. 2021;340:200–208. doi:10.1016/j.jconrel.2021.11.001
265. Anandappa AJ, Wu CJ, Ott PA. Directing Traffic: how to Effectively Drive T Cells into Tumors. Cancer Discov. 2020;10(2):185–197. doi:10.1158/2159-8290.CD-19-0790
266. Zhang C, Burger MC, Jennewein L, et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J Natl Cancer Inst. 2016;108(5):658. doi:10.1093/jnci/djv375
267. Merker M, Wagner J, Kreyenberg H, et al. ERBB2-CAR-Engineered Cytokine-Induced Killer Cells Exhibit Both CAR-Mediated and Innate Immunity Against High-Risk Rhabdomyosarcoma. Front Immunol. 2020;11:581468. doi:10.3389/fimmu.2020.581468
268. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
269. Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med. 2020;382(7):610–621. doi:10.1056/NEJMoa1914510
270. Nadal-Serrano M, Morancho B, Escriva-de-romani S, et al. The Second Generation Antibody-Drug Conjugate SYD985 Overcomes Resistances to T-DM1. Cancers. 2020;12:3. doi:10.3390/cancers12030670
271. Gradishar WJ. Emerging approaches for treating HER2-positive metastatic breast cancer beyond trastuzumab. Ann Oncol. 2013;24(10):2492–2500. doi:10.1093/annonc/mdt217
272. Pegram MD, Miles D, Tsui CK, Zong Y. HER2-Overexpressing/Amplified Breast Cancer as a Testing Ground for Antibody-Drug Conjugate Drug Development in Solid Tumors. Clin Cancer Res. 2020;26(4):775–786. doi:10.1158/1078-0432.CCR-18-1976
273. Roukos DH. Targeting gastric cancer with trastuzumab: new clinical practice and innovative developments to overcome resistance. Ann Surg Oncol. 2010;17(1):14–17. doi:10.1245/s10434-009-0766-0
274. Mohammadi M, Jeddi-Tehrani M, Golsaz-Shirazi F, et al. A Novel Anti-HER2 Bispecific Antibody With Potent Tumor Inhibitory Effects In Vitro and In Vivo. Front Immunol. 2020;11:600883. doi:10.3389/fimmu.2020.600883
275. Lu YJ, Chu H, Wheeler LW, et al. Preclinical Evaluation of Bispecific Adaptor Molecule Controlled Folate Receptor CAR-T Cell Therapy With Special Focus on Pediatric Malignancies. Front Oncol. 2019;9:151. doi:10.3389/fonc.2019.00151
276. Luo X, Cui H, Cai L, et al. Selection of a Clinical Lead TCR Targeting Alpha-Fetoprotein-Positive Liver Cancer Based on a Balance of Risk and Benefit. Front Immunol. 2020;11:623. doi:10.3389/fimmu.2020.00623
277. Zhang Y, Li Y, Chen K, Qian L, Wang P. Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy. Cancer Cell Int. 2021;21(1):262. doi:10.1186/s12935-021-01972-2
278. NIH; US National Library of Medicine. ClinicialTrials/.gov [homepage]; 2022. Available at: https://www.clinicaltrials.gov/. Accessed July 11, 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.