Back to Journals » Journal of Pain Research » Volume 16
Fremanezumab for Chronic Migraine Prevention in Japanese Patients: Subgroup Analysis from Two International Trials
Authors Saigoh K, Takeshima T, Nakai M , Shibasaki Y, Ishida M, Ning X, Barash S, Isogai Y, Koga N
Received 26 October 2022
Accepted for publication 4 April 2023
Published 20 April 2023 Volume 2023:16 Pages 1311—1319
DOI https://doi.org/10.2147/JPR.S393854
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Alexandre F DaSilva
Kazumasa Saigoh,1 Takao Takeshima,2 Masami Nakai,3 Yoshiyuki Shibasaki,4 Miki Ishida,5 Xiaoping Ning,6 Steve Barash,6 Yuki Isogai,4 Nobuyuki Koga7
1Department of Neurology, Kindai University School of Medicine, Osaka, Japan; 2Headache Center, Department of Neurology, Tominaga Hospital, Osaka, Japan; 3Medical Affairs, Otsuka Pharmaceutical Co., Ltd., Osaka, Japan; 4Medical Affairs, Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan; 5Headquarters of Clinical Development, Otsuka Pharmaceutical Co., Ltd., Osaka, Japan; 6Teva Branded Pharmaceutical Products R&D, Inc., West Chester, PA, USA; 7Medical Affairs, Otsuka Pharmaceutical Co., Ltd., Tokushima, Japan
Correspondence: Masami Nakai, Medical Affairs, Otsuka Pharmaceutical Co., Ltd., 3-2-27 Otedori, Chuo-ku, Osaka, 540-0021, Japan, Tel +81-80-9026-3806, Email [email protected]
Purpose: Fremanezumab monoclonal antibody therapy has demonstrated efficacy for chronic migraine (CM) with rapid onset and good tolerability. This subgroup analysis of two clinical trials (Japanese and Korean CM Phase 2b/3 [NCT03303079] and HALO CM Phase 3 [NCT02621931]) aimed to evaluate the efficacy and safety of fremanezumab in Japanese patients.
Patients and Methods: Both trials randomly assigned eligible patients at baseline (1:1:1 ratio) to subcutaneous monthly fremanezumab, quarterly fremanezumab, or placebo at 4-week intervals. The primary endpoint was the mean change from baseline in the monthly (28-day) average number of headache days of at least moderate severity during the 12-week period after the first dose of study medication (analyzed by ANCOVA over 12 weeks and MMRM over initial 4 weeks). Secondary endpoints examined other aspects of efficacy, including medication use and disability.
Results: A total of 479 and 109 patients were Japanese in the Japanese and Korean CM Phase 2b/3 and HALO CM trials, respectively. Baseline and treatment characteristics were generally similar between treatment groups for both trials. Results of subgroup analyses for the primary endpoint according to ANCOVA demonstrated the superiority of fremanezumab over placebo in Japanese patients (quarterly fremanezumab, p=0.0005; monthly fremanezumab, p=0.0002 in both trials). Results using the MMRM analysis confirmed the rapid onset of action in this population. Results of the secondary endpoints further supported the efficacy of fremanezumab in Japanese patients. Fremanezumab was well tolerated with nasopharyngitis and injection-site reactions representing the most common adverse events in all treatment groups.
Conclusion: Despite the limitations of subgroup analyses, these consistent results confirm the efficacy and tolerability of fremanezumab in Japanese patients with CM.
Keywords: calcitonin gene-related peptide, chronic migraine, fremanezumab, Japanese
Introduction
Chronic migraine (CM) is defined as the occurrence of “characteristic headaches on at least 15 days per month for at least 3 months”.1 CM has a prevalence of approximately 1–2%,2 and negatively affects quality of life and functioning, especially when headaches are more frequent and severe.3 Further, CM can be complicated by headache from overuse of acute medications (eg, triptans).4 Compared with episodic migraine (EM), CM is associated with higher use of medical resources, including headache-specific medication and healthcare visits, in addition to total costs.5,6 Therefore, more effective treatments for CM continue to be needed to reduce the burden of these consequences. Indeed, a Japanese real-world treatment survey cited issues with efficacy more frequently compared with other issues (eg, compliance, sedation, and long-term safety concerns).7
Monoclonal antibodies, among the latest treatments in migraine, act either against the calcitonin gene-related peptide (CGRP) or the CGRP receptor,8 and have been developed for both prevention and management of acute episodes.9,10 Fremanezumab, a fully humanized monoclonal antibody, has been extensively investigated for CM and EM. Previously, a large-scale international phase 3 trial (HALO CM, NCT02621931) in patients with CM found that monthly or quarterly fremanezumab treatment significantly reduced the average number of headache days of at least moderate severity and improved the response rate compared with placebo.11 More recently, a phase 2b/3 trial in Japanese and Korean patients with CM confirmed the efficacy and safety of subcutaneous monthly or quarterly fremanezumab compared with placebo for migraine prevention in this subpopulation.12 In Japanese patients with CM or EM receiving fremanezumab, long-term safety has also been demonstrated in a 52-week, open-label, randomized, parallel-group trial.13
Confirming the safety and efficacy of drug treatments specifically in Japanese populations is important for local approval and expansion of population-specific data, which can be efficiently performed using the concept of ethnic bridging studies.14 Consequently, this subgroup analysis of the abovementioned international and Japanese/Korean clinical trials (Japanese and Korean CM Phase 2b/3 and HALO CM), included only Japanese patients.
Materials and Methods
Study Design
This subanalysis used two multicenter, randomized, double-blind, placebo-controlled, parallel-group trials in CM patients: the HALO CM trial (NCT02621931),11 and the phase 2b/3 trial in Japanese and Korean patients (NCT03303079).12 The HALO CM trial and the phase 2b/3 trial in Japanese patients start and completion dates were March 22, 2016 and April 11, 2017, and December 19, 2017 and November 30, 2019, respectively. Details of the design, patient populations, and inclusion/exclusion criteria for each trial have been published previously.11,12
Written informed consent was obtained from all patients in both trials. Protocols for both trials were approved by the institutional review board or independent ethics committee/ethics committee of each individual study center as outlined in the original trials (see Supplementary Tables 1 and 2 for list of independent ethics committees for each trial). Both trials complied with the principles of the Declaration of Helsinki.
Treatment
Informed consent and initial eligibility screening were conducted at Visit 1. Eligible patients were then randomly assigned at baseline (Visit 2) to receive monthly fremanezumab, quarterly fremanezumab, or placebo via subcutaneous injection in a 1:1:1 ratio (Supplementary Figure 1). All groups received a total of 3 doses of study treatment at 4-week (“monthly”) intervals. Patients in the monthly fremanezumab group received fremanezumab 675 mg as 3 active injections (225 mg/1.5 mL each) at baseline followed by fremanezumab 225 mg as a single active injection (225 mg/1.5 mL) at month 1 (Visit 3) and month 2 (Visit 4). Patients in the quarterly fremanezumab group received fremanezumab 675 mg at baseline and placebo as a single 1.5 mL injection at month 1 (Visit 3) and month 2 (Visit 4). Placebo recipients received three placebo injections (1.5 mL each) at Visit 2 and a single placebo injection (1.5 mL) at Visit 3 and Visit 4.
Outcomes
Post hoc analyses were performed only in Japanese patients using primary and secondary outcome data from both trials. As described previously in both trials, the primary endpoint was the “mean change from baseline in the monthly (28-day) average number of headache days of at least moderate severity during the 12-week period after the first dose of study medication”. Secondary endpoints used in both trials and assessed during the 12-week period after the first dose of study medication were the: (i) mean change in the monthly average number of migraine days from baseline, (ii) proportion of patients with a ≥50% reduction in the monthly average number of headache days of at least moderate severity, (iii) mean change in the monthly average number of days with use of any acute headache medications from baseline, (iv) mean change in the monthly average number of headache days of at least moderate severity in patients not receiving concomitant preventive migraine medications from baseline, and (v) mean change in disability score, as measured by the HIT-6 assessment item from baseline.15 Both trials also assessed safety, which was analyzed in relation to enrolled Japanese patients only.
Statistics
An analysis of covariance (ANCOVA) model was used to analyze the primary endpoint with fixed effects being treatment, sex, country, and baseline preventive medication use and covariates being baseline number of headache days of at least moderate severity and years since onset of migraines. A mixed-effects model for repeated measures (MMRM) analysis was also applied to the weekly change in average number of headache days of at least moderate severity over 4 weeks. This allowed assessment of how rapidly fremanezumab acted in this subgroup with treatment, sex, country, baseline preventive migraine medication use, month and treatment-by-month interaction included as fixed effects, and baseline value and years since onset of migraine as covariates.
The ANCOVA model was also applied to most secondary endpoints similar to that of the primary endpoint while the Cochran-Mantel-Haenszel test was applied to proportion of patients with a ≥50% reduction in the monthly average number of headache days of at least moderate severity. Differences between each fremanezumab group and the placebo group and two-sided 95% confidence interval (a Mantel-Haenszel estimator of the difference and its two-sided 95% confidence interval) were calculated. Data from patients who had monthly variables with <10 days of data and weekly variables with <3 days of data were considered missing. A headache day of at least moderate severity and a migraine day was normalized to 28 days for the monthly analysis and 7 days for the weekly analysis. Adverse events (AEs) were tabulated by frequency with the incidence rate determined for each event within groups. All statistical calculations were carried out using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Disposition and Baseline Characteristics
A total of 479 and 109 Japanese patients were included in the Japanese and Korean phase 2b/3 trial (fremanezumab monthly group, n=159; fremanezumab quarterly group, n=159; placebo group, n=161) and HALO CM trial (fremanezumab monthly group, n=38; fremanezumab quarterly group, n=35; placebo group, n=36), respectively. Table 1 summarizes baseline characteristics of Japanese patients enrolled in both trials and demonstrates the similarity between treatment groups for both trials with the exception of lower baseline concomitant preventive medication use in the Japanese and Korean phase 2b/3 trial compared with the HALO CM trial.
 |
Table 1 Demographic and Baseline Clinical Characteristics of Japanese Patients Enrolled in the Phase 2b/3 Trial of Japanese and Korean Patients and the HALO Trial |
Efficacy
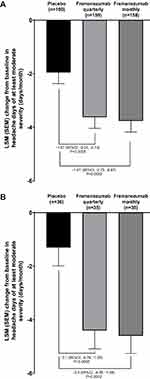
The primary endpoint (mean change from baseline in the monthly average number of headache days of at least moderate severity during the 12-week period after the first dose of study medication) by ANCOVA for both trials is shown in Figure 1. Differences between both fremanezumab treatment groups and placebo were statistically significant. In the Japanese and Korean phase 2b/3 trial, the mean (SE, 95% CI) difference in the primary endpoint compared with placebo was −1.67 (0.48, 95% CI −2.61, −0.74, p=0.0005) for the fremanezumab quarterly group and −1.81 (0.48, 95% CI −2.75, −0.87, p=0.0002) for the fremanezumab monthly group. In the HALO trial, corresponding differences in the primary endpoint compared with placebo were −3.1 (0.86, 95% CI −4.79, −1.39, p=0.0005) in the fremanezumab quarterly group and −3.3 (0.85, 95% CI −4.95, −1.58, p=0.0002) in the fremanezumab monthly group. In support of this finding, the LSM ± SE change in the average number of headache days of at least moderate severity from baseline by MMRM analysis over 3 months and the initial 4 weeks were also greater in the fremanezumab quarterly and monthly groups compared with placebo (Figure 2).
Table 2 and Supplementary Table 3 summarize the results of the secondary efficacy endpoints in Japanese patients for the Japanese and Korean phase 2b/3 trial and HALO trial, respectively. In the subgroup analysis of the Japanese and Korean phase 2b/3 trial, differences favoring fremanezumab (monthly and quarterly) compared with placebo were all statistically significant at the level of p<0.05 or greater. Among Japanese patients enrolled in the HALO trial, all differences between fremanezumab groups and placebo were also statistically significant with the exception of HIT-6 scores at 4 weeks after the last dose.
 |
Table 2 Summary of Primary and Secondary Endpoints of Japanese Patients Enrolled in the Japanese and Korean Phase 2b/3 Trial |
Safety
Table 3 and Supplementary Table 4 summarize the AEs observed in Japanese patients from the Japanese and Korean phase 2b/3 trial and HALO trial, respectively. In the Japanese and Korean phase 2b/3 trial subgroup analysis, a similar proportion of patients in the placebo (30.4%) and fremanezumab (quarterly, 34.0%; monthly, 30.2%) groups had at least one treatment-emergent AE potentially related to study drug. In the HALO trial subgroup analysis, the corresponding proportion was lower in the placebo group (11.1%) than in the fremanezumab quarterly (22.9%) or monthly (31.6%) groups. The most common treatment-emergent AEs were nasopharyngitis and injection-site reactions. In the Japanese and Korean phase 2b/3 trial, no major differences in the incidence of these AEs were seen between placebo and fremanezumab groups. However, in the HALO trial, the placebo group had a lower incidence of some injection-site reactions than the fremanezumab groups.
 |
Table 3 Adverse Events Overall and in Patients with Incidence of Adverse Events >2% Reported in Any Treatment Group (for Japanese Patients Enrolled in the Japanese and Korean Phase 2b/3 Trial) |
Discussion
This subanalysis of Japanese patients with CM confirmed the significant differences between fremanezumab and placebo for the primary endpoint previously noted in the two overall trial populations. Primary endpoint results using the MMRM analysis over 4 weeks highlighted the rapid onset of action of fremanezumab, which have been noted in separate trials, including among Japanese and Korean patients.16,17 The numerical differences between fremanezumab and placebo treatment groups in the primary endpoint were greater in Japanese patients enrolled in the HALO trial than those seen in the Japanese and Korean phase 2b/3 trial despite both being statistically significant to a similar degree. Placebo response in terms of primary endpoint efficacy in the overall population of the Japanese and Korean phase 2b/3 trial and the HALO trial were numerically similar. However, the responses to placebo among Japanese patients enrolled in both trials were numerically lower than those observed in the overall population. The numerically lower response to placebo in the HALO trial may have affected the size of the effect produced by fremanezumab, but the cause of this is unknown.
For secondary endpoints, the HALO trial population was small and the number of Japanese patients from the Japanese and Korean phase 2b/3 trial alone was adequate to draw conclusions. Hence, we mainly evaluated the results from the Japanese and Korean phase 2b/3 trial. As with the primary endpoint, all secondary efficacy endpoints were very similar to those from the main trial, including the proportion of patients with ≥50% reduction in the monthly average number of headache days of at least moderate severity, the average monthly number of migraine days, mean change from baseline in the monthly average number of days with use of any acute headache medication and HIT-6 score.
In terms of safety, the incidences of AEs were similar for Japanese patients in both fremanezumab groups compared with placebo in the Japanese and Korean phase 2b/3 trial but lower for placebo in the smaller Japanese population enrolled in the HALO trial. The most common adverse events were nasopharyngitis and injection-site reactions. The safety profile noted in this subgroup analysis is consistent with earlier phase 2 trials of fremanezumab in patients with CM,18,19 and a long-term analysis of Japanese patients with CM or EM.13
Limitations of this subgroup analysis study mainly relate to those of the previous trials, including the lack of inclusion of patients with more refractory disease or coexisting diseases and the short-term evaluation period. Other limitations relate to the post hoc, rather than prespecified, nature of the subgroup analyses such as the lack of statistical power to generate firm conclusions. These limitations may be addressed by further trials, including in patients with concomitant diseases and using longer follow-up periods. However, the consistency of these results with previous trials in both Japanese and non-Japanese patients represents a key strength of this subgroup analysis.
Conclusion
Based on the results of this subanalysis, fremanezumab appears to be an effective preventive medication with a favorable tolerability profile in Japanese patients with CM. In addition, fremanezumab appears to have a relatively rapid onset of action. Regarding safety, no additional concerns were raised, and the safety profile is consistent with earlier phase 2 trials of fremanezumab and a long-term analysis of Japanese patients with CM or EM.
Data Sharing Statement
De-indentified individual participant data underlying the results of this analysis as well as relevant study protocols may be shared with researchers to achieve aims prespecified in a methodologically sound research proposal upon request.
Acknowledgments
We would like to thank all patients for their participation in the related trials, and all trial sites, investigators, and all clinical research staff for their contributions. We also thank Yoshiko Okamoto, PhD, and Mark Snape, MBBS, of inScience Communications, Springer Healthcare, for helping write the outline and first draft of the manuscript. This medical writing assistance was funded by Otsuka Pharmaceutical Co., Ltd.
Disclosure
KS has received grants from Sumitomo Pharma Co., Ltd.; consulting fees from Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd.; payment for lectures from AMGEN Inc., Daiichi Sankyo Company, Eisai Co., Ltd., Eli Lilly Japan, Otsuka Pharmaceutical Co., Ltd., Sanofi Japan, Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd.; committee member for Japanese Society of Headache, Japanese Society of Human Genetics. TT has received honoraria for lectures from Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Amgen K.K., Eli Lilly and Company, Daiichi Sankyo; research funds under contract from Eisai Co., Ltd., Eli Lilly and Company, Amgen K.K., Allergan Japan K.K., Shionogi & Co., Ltd., Lundbeck Japan K.K. MN, YS, MI, YI, and NK are full-time employees of Otsuka Pharmaceutical Co., Ltd. XN and SB are full-time employees of Teva Branded Pharmaceutical Products. The authors report no other conflicts of interest in this work.
References
1. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi:10.1177/0333102413485658
2. Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30(5):599–609. doi:10.1111/j.1468-2982.2009.01941.x
3. Pradeep R, Nemichandra SC, Harsha S, Radhika K. Migraine disability, quality of life, and its predictors. Ann Neurosci. 2020;27(1):18–23. doi:10.1177/0972753120929563
4. Negro A, Martelletti P. Chronic migraine plus medication overuse headache: two entities or not? J Headache Pain. 2011;12(6):593–601. doi:10.1007/s10194-011-0388-3
5. Bloudek LM, Stokes M, Buse DC, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain. 2012;13(5):361–378. doi:10.1007/s10194-012-0460-7
6. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51(7):1058–1077. doi:10.1111/j.1526-4610.2011.01945.x
7. Ueda K, Ye W, Lombard L, et al. Real-world treatment patterns and patient-reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20(1):68. doi:10.1186/s10194-019-1012-1
8. Vikelis M, Spingos KC, Rapoport AM. A new era in headache treatment. Neurol Sci. 2018;39(Suppl 1):47–58. doi:10.1007/s10072-018-3337-y
9. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338–350. doi:10.1038/s41582-018-0003-1
10. Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A. Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol. 2022;21(3):284–294. doi:10.1016/S1474-4422(21)00409-9
11. Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–2122. doi:10.1056/NEJMoa1709038
12. Sakai F, Suzuki N, Kim BK, et al. Efficacy and safety of fremanezumab for chronic migraine prevention: multicenter, randomized, double-blind, placebo-controlled, parallel-group trial in Japanese and Korean patients. Headache. 2021;61(7):1092–1101. doi:10.1111/head.14169
13. Sakai F, Suzuki N, Ning X, et al. Long-term safety and tolerability of fremanezumab for migraine preventive treatment in Japanese outpatients: a multicenter, randomized, open-label study. Drug Saf. 2021;44(12):1355–1364. doi:10.1007/s40264-021-01119-2
14. Nagata R, Rafizadeh-Kabe JD. Japanese pharmaceutical and regulatory environment. Dialogues Clin Neurosci. 2002;4(4):470–474. doi:10.31887/DCNS.2002.4.4/rnagata
15. Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963–974. doi:10.1023/A:1026119331193
16. Takeshima T, Nakai M, Shibasaki Y, et al. Early onset of efficacy with fremanezumab in patients with episodic and chronic migraine: subanalysis of two phase 2b/3 trials in Japanese and Korean patients. J Headache Pain. 2022;23(1):24. doi:10.1186/s10194-022-01393-0
17. Bhugra D, Ventriglio A. Do cultures influence placebo response? Acta Psychiatr Scand. 2015;132(4):227–230. doi:10.1111/acps.12422
18. Winner PK, Spierings ELH, Yeung PP, et al. Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache. 2019;59(10):1743–1752. doi:10.1111/head.13654
19. Bigal ME, Dodick DW, Krymchantowski AV, et al. TEV-48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology. 2016;87(1):41–48. doi:10.1212/WNL.0000000000002801
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.