Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 14
Fracture Resistance and Fracture Behaviour of Monolithic Multi-Layered Translucent Zirconia Fixed Dental Prostheses with Different Placing Strategies of Connector: An in vitro Study
Authors Heidari N , Amawi R, Seweryniak P , Bakitian F , Vult von Steyern P
Received 18 October 2021
Accepted for publication 3 February 2022
Published 22 March 2022 Volume 2022:14 Pages 61—69
DOI https://doi.org/10.2147/CCIDE.S344941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Nada Heidari,1 Rasha Amawi,1 Przemek Seweryniak,2 Fahad Bakitian,3 Per Vult von Steyern1
1Department of Materials Science and Technology, Malmö University, Malmö, Sweden; 2Commercial Dental Laboratory, Malmö, Sweden; 3Department of Restorative Dentistry, Umm Al-Qura University, Makkah, Saudi Arabia
Correspondence: Fahad Bakitian, Department of Restorative Dentistry, Faculty of Dentistry, Umm Al-Qura University, Makkah, 21511, Saudi Arabia, Tel +966500507661, Email [email protected]
Purpose: To evaluate the effect of different placing strategies performed in the connector area on fracture resistance and fracture behaviour of monolithic multi-layered translucent zirconia fixed dental prostheses (FDPs).
Materials and Methods: Thirty 3-unit monolithic FDPs were produced and divided into three groups (n = 10) based on the different strategies for placing the connector area of FDPs in multi-layered zirconia blank with varying contents of yttria ranging from 4 to 5 mol%. The groups were as follows: FDPs with connectors placed in dentin layer with 4 mol% yttria content, FDPs with connectors placed in gradient layer, and FDPs with connectors placed in translucent layer with 5 mol% yttria content. A final group (n = 10) of conventional monolithic zirconia with a monolayer of yttria content (4 mol%) has been used as a control group. The specimens were artificially aged using thermocycling and pre-loading procedures and subsequently loaded to fracture using a universal testing machine. Fracture loads and fracture behaviour were analyzed using one-way ANOVA and Fisher’s exact tests and statistically evaluated (p ≤ 0.05).
Results: There were no significant differences in fracture loads among the groups based on the placing strategies of the connector area of the FDPs in the multi-layered translucent zirconia blank (p > 0.05). There was no significant difference in fracture loads between monolithic multi-layered translucent zirconia and conventional monolithic translucent zirconia materials (p > 0.05). Fracture behaviour of FDPs with connector area placed in translucent layer differed significantly compared to FDPs with connector area placed in dentin layer and FDPs in control group (p = 0.004).
Conclusion: The placing strategies of the connector used in the computer aided design and manufacturing procedures do not considerably affect fracture resistance of monolithic FDPs made of multi-layered translucent zirconia. Monolithic FDPs made of multi-layered translucent zirconia show comparable strength to FDPs made of conventional translucent zirconia, but with different fracture behaviour.
Keywords: all-ceramic restorations, computer-aided design\manufacturing, fracture load, multi-layered zirconia, Y-TZP
Introduction
Yttria-stabilized tetragonal zirconia polycrystal (Y-TZP) is the most commonly used oxide ceramic material in Restorative Dentistry. This is related to its superior fracture strength and unique toughening properties.1,2 However, owing to its poor optical properties, Y-TZP based restorations must be veneered with translucent glass-ceramic materials in many clinical situations. Although the high success rate of veneered Y-TZP restorations has been reported to be over 90%, clinical complications such as veneer chipping and connector fracture still occur.3–5 Moreover, the use of veneered Y-TZP restorations requires removing more underlying tooth substance to provide enough space for the material. For those reasons, there is a general preference for shifting toward monolithic Y-TZP restorations, with challenges in achieving esthetical requirements without compromising the overall strength.6–8
The main drawback of using Y-TZP material as a monolithic restoration is the low translucency, resulting in poor esthetics.6,9–11 Scattering of light in Y-TZP and subsequent reduction of light transmittance mainly occurs at grain boundaries, pores, and secondary phases.6,9–11 However, enhanced optical properties of this material have been achieved by modifying the microstructure, for example, through altering the yttria (Y2O3) content and applying different sintering conditions.12,13 Shorter sintering times result in smaller grain size and thus an increase of the light transmittance of the final dental zirconia.12 Furthermore, it has been shown that the change of dopant contents, such as lanthanum oxide and aluminum oxide, improved the optical properties of zirconia.14 From a material point of view, the mechanical properties of Y-TZP are negatively affected by enhancing the translucent properties of the material.15,16 The more translucent the zirconia is, the lower the fracture strength.15,16
Recently, a new multi-layered translucent zirconia material, with a natural progression of shade and translucency, has emerged in the dental market to mimic natural teeth closely. This material is indicated to produce monolithic restorations in both the anterior and posterior regions. There are two types of multi-layered translucent zirconia on the market: 1) Multi-layered zirconia with different colour saturations in the different layers but the same yttria content throughout all layers, and 2) Multi-layered zirconia with different translucency in the different layers as a result of varying yttria contents in the different layers. Thus, the strength and toughness of the layers with different yttria contents are expected to be different. During computer-aided design and manufacturing (CAD/CAM) procedures, dental technicians can use different placing strategies to place the fixed dental prosthesis (FDP) in multi-layered translucent zirconia blank before milling. Previous studies showed that the main fracture origin leading to the failure of the prostheses is located at the gingival side of the connector area, which is linked to the development of stress concentrations in the connector when different loads are applied to the FDPs.17,18 Accordingly, in practice, the fracture resistance of the FDP, especially in the connector area, might be affected depending on how the placing strategy has been performed by the dental technician during CAD/CAM procedures. It is not known, however, if the different placing strategies of the connector, during computer manufacturing of zirconia blanks, might affect the fracture resistance of the final restoration made of the new multi-layered translucent zirconia material, since the strength varies between the different layers of zirconia.
Therefore, the present study aimed to evaluate the effect of the different placing strategies performed in the connector area on fracture resistance and fracture behaviour of monolithic FDPs made of multi-layered translucent zirconia. The null hypothesis is that there is no difference in fracture resistance and fracture behaviour of the FDPs made of multi-layered translucent zirconia based on the placing strategies performed in the connector area.
Materials and Methods
Study Design
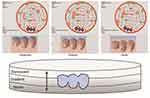
Thirty 3-unit monolithic FDPs were produced and divided into three groups (n=10) according to the different strategies for placing the connector area of the FDPs in the multi-layered translucent zirconia blank (IPS e.max ZirCAD MT Multi, Ivoclar Vivadent, Schaan, Liechtenstein) (Figure 1). The groups were as follows: FDPs produced with the connectors placed in the dentin layer with 4 mol% yttria, FDPs produced with the connectors placed in the gradient layer, and FDPs produced with the connectors placed in the translucent layer with 5 mol% yttria. A final group (n=10) of conventional monolithic zirconia with monolayer of 4 mol% yttria content has been used as a control group (IPS e.max ZirCAD, Ivoclar Vivadent, Schaan, Liechtenstein). The FDPs were cemented using compatible resin cement onto abutment models made of a polymer material (POM C glass infiltrated). The specimens were artificially aged using both thermocycling and cyclic fatigue procedures before they were loaded to fracture. Fracture loads and fracture behaviour were subsequently analyzed and evaluated statistically p ≤0.05.
Specimen Preparation
For the preparation of the teeth, a plastic model of a mandibular jaw was used (KaVo YZ; KaVo Dental GmbH, Biberach, Germany). The preparations were made on the canine (43) and premolar (45) and were designed to provide space for Y-TZP material with a 120° chamfer and 15° convergence angle. The teeth preparations were conducted by prosthodontist. After the preparations were conducted, a full-arch impression using silicone material (President; Coltene AG, Altstätten, Switzerland) was made and poured with die stone material (Vel-Mix; Kerr Corp, Orange, CA). A master cast was produced from the die stone, and subsequently, a wax-up (1.5–3 mm) of the FDP was made by professional dental technician. The wax-up was scanned with a double-scan technique using a dental laboratory scanner (D900L; 3Shape, Copenhagen, Denmark). Data from the scanner were transferred to a computer loaded with computer-aided design (CAD) software. The design of the FDP connector was a round shape and the dimensions for all the FDP connectors were adjusted to 3 mm x 3 mm. The occlusal thickness of the retainer core was set to 1 mm, and the axial wall thickness was set to 0.8 mm with a 0.5 mm cervical margin. After the adjustments, the CAD file was sent to a milling center (Cosmodent AB, Malmö, Sweden) to produce the FDPs. The same sintering protocol for the two zirconia materials has been used following the manufacturer instructions. The CAD file was used to produce the abutment models made from a polymer material (POM-C GF25; Mekaniska AB, Simrishamn, Sweden) with a modulus of elasticity comparably close to dentin (9 GPa).
Artificial Aging, Cementation, and Load to Fracture Test
All FDPs were subjected to artificial aging, beginning with thermocycling. In a thermocycling device (THE-1100; SD Mechatronik GmbH, Feldkirchen-Westerham, Germany) containing two water baths, the FDPs underwent 10,000 thermocycles at two different temperatures, 5 and 55°C. Each cycle lasted for 60 seconds, 20 seconds in each bath and 20 seconds to complete the transfer between the baths.4,19–22 The cementation of the FDPs to the abutment models was completed using a dual-polymerized resin cement (Panavia V5; Kuraray Medical Inc., Okayama, Japan) according to the manufacturer’s recommendations. However, before cementation, the abutment models were air-abraded with 50 µm aluminum oxide using an air abrasion device (Basic Quattro IS; Renfert GmbH, Hilzingen, Germany) as well as treated with two primers (Tooth Primer, Clearfil and Ceramic Primer; Kuraray Medical Inc) following the manufacturers’ instructions. The FDPs were cemented to the abutment models with a standardized seating load of 15 N in the direction of insertion. A calibrated curing lamp (Heraeus Translux® Power Blue®, Heraeus Kulzer GmbH) was used according to the manufacturer’s recommendations to initiate the curing. Ultimately, excess cement was removed with a scalpel (AESCULAP® no. 12, Aesculap AG & Co, Tuttlingen, Germany). The specimens were stored in a humid environment at a temperature of 37°C before cyclic fatigue. The last step of artificial aging was cyclic fatigue using a pre-loading machine (MTI Engineering AB; Lund, Sweden/Pamaco AB, Malmö, Sweden). The cemented FDPs were submerged in distilled water at 10° of inclination towards the tooth axis and went through 10,000 cycles of 30–300 N at a frequency of 1 Hz. A 4 mm stainless ball was placed on the occlusal surface of the connector area between teeth 45 and 44 of the bridges to apply mechanical cyclic loads.4,19–22
After artificial aging, all FDPs were installed in a test jig at 10° inclination towards the axial direction using a universal testing machine (Instron 4465, Instron Co. Ltd, Norwood, MA, USA), (Figure 2) as was suggested in previous laboratory studies.4,19–22 The load was applied on the pontic using a specialized stainless-steel intender. Throughout loading, all the FDPs were submerged in water. The crosshead speed was set at 0.255 mm/min, and the fracture was defined as follows: visible crack, load drop or an acoustic event, whatever occurred first.4,19–22 The load at fracture was then registered.
 |
Figure 2 Illustration of the specimen in a test jig at 10° inclination in cyclic fatigue and load to fracture tests. All specimens were submerged in water during the tests. |
Fracture Behaviour Analysis
The fracture surfaces of the FDPs were analyzed by two examiners. A gross visual and microscopic assessments (Leica DFC 420, Leica Application Suite v. 3.3.1, Leica Microsystems CMS GmbH, Wetzlar, Germany) were performed to classify fracture behaviour according to the location of fracture into: fracture at the distal connector, fracture at the mesial connector, complete fracture of the FDP (involving fracture of the retainer).
Statistical Analysis
The differences in fracture resistance among the groups were analyzed using one-way ANOVA, followed by Tukey’s post hoc test (IBM SPSS Statistics 25). The differences in fracture behaviour among the groups were analyzed using Fisher’s exact test. The level of significance was set to p ≤0.05. The statistical analysis was performed by an experienced professional statistician. Power analysis was based on previous studies where differences regarding the level of significance and standard deviation were detected among the zirconia-based specimens.17,19–21
Results
Loads at fracture, levels of significance, fracture behaviour for all groups are summarized in Tables 1 and 2. There were no significant differences in fracture loads among the groups based on the different strategies for placing the connector area of the FDPs in the multi-layered zirconia blank (p >0.05). There was no significant difference (p >0.05) in fracture loads between the two different materials: monolithic multi-layered translucent zirconia and conventional monolithic translucent zirconia materials.
 |
Table 1 Load at Fracture in Newton (N) |
 |
Table 2 Distribution of Fracture Behaviour |
Three types of fracture behaviour were registered after load to fracture test: fracture at the mesial connector propagating through the pontic, fracture at the distal connector propagating through the pontic, and complete fracture involving the retainer (Figure 3). Fracture behaviour of the FDPs with connector area placed in the translucent layer (5Y-TZP) differed significantly compared to the FDPs with connector area placed in the dentin layer (4Y-TZP) and the FDPs in the control group (p ≤0.05).
 |
Figure 3 Different types of fracture behaviour. (A) Fracture at distal connector; (B) complete fracture; (C) fracture at mesial connector. |
Discussion
The null hypothesis of this study was rejected since fracture resistance of the FDPs showed no significant differences among the groups based on the different placing strategies performed in the connector area during computer manufacturing of the FDPs. However, the results showed that the different placing strategies performed in the connector area affect fracture behaviour of the three-unit FDPs.
One of the common methods to improve the translucency of dental zirconia is by changing the amount of yttria content, which results in a greater portion of the optically isotropic cubic phase without light scattering at the grain boundaries.14,15 The major phenomena related with the enhanced translucency of polycrystalline zirconia-based ceramics is the reduction of birefringence, the light scattering promoted by a material with anisotropic refractive index. Tetragonal zirconia phase is birefringent, however, by increasing yttria content the precipitation of cubic zirconia, which is isotropic and do not experience birefringence, is favoured and an enhancement of the transmitted light fraction is experienced.23–25 This, on the other hand, compromises the strength and toughness of the cubic zirconia because it does not undergo stress-induced transformation.14,23–25 In the present study, the FDPs made of multi-layered translucent zirconia were divided into three groups: dentin, gradient, and translucent, based on the content of yttria ranging from 4 to 5 mol%. The groups with the connectors placed in the gradient and the translucent layers presented higher standard deviation values than the dentin and control groups. This might be explained by the fact that the gradient layer combines different microstructures of both the translucent and the dentin layers, which results in varying mechanical properties. Thus, the FDPs with the connectors placed in the layer consisting of a microstructure primarily composed of dentin (4Y-TZP) withstand higher fracture loads. The opposite applies to the FDPs with the connectors placed in the layer consisting of a mainly translucent microstructure, namely 5Y-TZP. These findings are in line with previous studies, which concluded that translucency affects the mechanical properties of zirconia.15,23–25 Although the differences of the results were not statistically significant, the numerical differences among the groups in this study, together with the findings of previous studies, confirm the effect of enhanced translucency on the mechanical properties of Y-TZP. Moreover, it is noteworthy that the limitations of the methodology used in this study might have influenced the results. For geometric reasons, it is impossible to place the whole reconstruction in one layer in the multi-layered translucent zirconia blank without infringing the minimum dimensional demands of the FDP. This means that the critical part of the connector area, the gingival portion, where the highest stress concentrations occur during loading, will probably not be entirely located in solely one layer.17,18 This technical limitation means that study findings need to be interpreted cautiously.
Many studies have investigated the adverse effects of the other methods of enhancing the optical properties of zirconia on mechanical properties. For instance, although doping of metal oxides improves the optical properties of zirconia, this may affect adversely the mechanical and biological (cytotoxic) properties of zirconia.14 Other fabrication techniques such as colouring of pre-sintered zirconia for enhancing the optical properties might be necessary in many clinical cases. Previous studies have shown the effect of such colouring techniques on the mechanical and optical properties.26,27 Nevertheless, a very recent study investigating new multi-layered translucent zirconia material showed no differences in neither microstructure nor translucency between the different layers.28 Only colour pigment composition is different between the layers within each multi-layered translucent zirconia blank. The same study revealed that lanthanum oxide doping improved the translucency without diminishing the mechanical properties of the multi-layered translucent zirconia, which is the main goal when developing high esthetical monolithic dental zirconia.
Considering fracture behaviour, this study showed that most fractures started from the connector area (mesial or distal) and propagated through the pontic during loading. This is in agreement with previous studies, which concluded that critical tensile stresses mostly develop in the gingival embrasure of the connector, result in failure of prosthesis.17,18 However, there were significantly more complete fractures (involving the retainer) in the FDPs with connector area placed in the translucent layer (5Y-TZP) compared to the FDPs of the other groups. This finding could be expected theoretically since the translucent layer has a microstructure that is less resistant to fractures, as previous studies have shown.15,23–25 It is noteworthy that fracture behaviour analysis in this study aimed to show the fracture initiation and propagation pattern under a light microscope and evaluate the ability of the test to mimic the clinical failures of dental restorations. Sophisticated fractographic analysis using a scanning electron microscope, however, might provide more details on fracture behaviour.
When conducting an in vitro study to evaluate the mechanical properties of new material, a laboratory setup simulating the oral environment and the complex forces of mastication is of great importance. One of the limitations of in vitro studies is the difficulty to choose which aging procedures would produce comparable clinical results. Previous studies have investigated the effect of artificial aging procedures, that used to mimic the clinical situation, on the longevity of ceramics. Despite that some of those studies fail to show a direct relationship between aging procedures and fracture resistance of ceramics,29 most agree that they have a significant effect on the longevity of ceramic materials.30–32 Therefore, there is no consensus regarding the effectiveness of aging tests or a specific aging protocol, but it was reasonable, however, to perform such procedures in the present study to allow for comparison of the results of other studies carried out by the same research group with this specific protocol.4,19–21 The FDPs were mounted with a 10 of inclination relative to the load direction in the load to fracture test. This angle of inclination has been used in many previous studies and was initially suggested by Yoshinari and Derand.4,19–22 However, the mechanical load to fracture test performed in a laboratory study can never completely reproduce loads and environmental influences as in the clinical situation but can still give important information. Furthermore, to obtain realistic fracture load values and compare these values with previous studies, replicating the real clinical situation concerning mechanical support is crucial.33 Therefore, all FDPs were cemented onto abutment models made of a material with a modulus of elasticity close to dentin. The cementation procedure was performed according to the manufacturer’s recommendations, and the same cement was used for all groups. Since in vitro studies have shown that thermocycling affects the bond strength of cements, all FDPs were cemented after this stage to avoid partly loose prostheses at the subsequent cyclic fatigue and load to fracture tests.31,32
Since adequate communication between the dentist and the dental technician is essential for successful dental restorations, it is a prerequisite for dentists to gain knowledge of the dental material that is required. This study has shown that the different strategies for placing the FDP in the blank during the CAD/CAM process do not have a critical effect on the mechanical properties of the translucent multi-layered zirconia FDPs. Thus, this facilitates the process of ordering for the dentist who does not have to pay regard to the technical aspects. In vitro studies, in line with the present one, are of great importance to evaluate new dental materials before using them in a clinical situation, thus safeguarding patient safety.
Conclusion
Within the limitations of this laboratory study, the following conclusions can be drawn: the placing strategies of the connector used in the computer aided design and manufacturing procedures do not considerably affect fracture resistance of monolithic FDPs made of multi-layered translucent zirconia. Monolithic FDPs made of multi-layered translucent zirconia show comparable strength to FDPs made of conventional translucent zirconia, but with different fracture behaviour.
Acknowledgments
The authors thank Pelle von Wowern at Ivoclar Vivadent AB for providing materials for the study and Per-Erik Isberg for his assistance with statistics.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Guess PC, Schultheis S, Bonfante EA, et al. All-ceramic systems: laboratory and clinical performance. Dent Clin North Am. 2011;55:333–352. doi:10.1016/j.cden.2011.01.005
2. Zhang Y, Lawn BR. Evaluating dental zirconia. Dent Mater. 2019;35:15–23. doi:10.1016/j.dental.2018.08.291
3. Larsson C, Wennerberg A. The clinical success of zirconia-based crowns: a systematic review. Int J Prosthodont. 2014;27:33–43. doi:10.11607/ijp.3647
4. Bakitian F, Seweryniak P, Papia E, et al. Fracture strength of veneered translucent zirconium dioxide crowns with different porcelain thicknesses. Acta Biomater Odontol Scand. 2017;3:74–83. doi:10.1080/23337931.2017.1403288
5. Sulaiman TA. Materials in digital dentistry-A review. J Esthet Restor Dent. 2020;32:171–181. doi:10.1111/jerd.12566
6. Stawarczyk B, Keul C, Eichberger M, et al. Three generations of zirconia: from veneered to monolithic. Part I. Quintessence Int. 2017;48:369–380. doi:10.3290/j.qi.a38057
7. Ramos Nde C, Campos TM, Paz IS, et al. Microstructure characterization and SCG of newly engineered dental ceramics. Dent Mater. 2016;32:870–878. doi:10.1016/j.dental.2016.03.018
8. Sun T, Zhou S, Lai R, et al. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J Mech Behav Biomed Mater. 2014;35:93–101. doi:10.1016/j.jmbbm.2014.03.014
9. Shahmiri R, Standard OC, Hart JN, et al. Optical properties of zirconia ceramics for esthetic dental restorations: a systematic review. J Prosthet Dent. 2018;119:36–46. doi:10.1016/j.prosdent.2017.07.009
10. Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dent Mater. 2014;30:1195–1203. doi:10.1016/j.dental.2014.08.375
11. Belli R, Wendler M, de Ligny D, et al. Chairside CAD/CAM materials. Part 1: measurement of elastic constants and microstructural characterization. Dent Mater. 2017;33:84–98. doi:10.1016/j.dental.2016.10.009
12. Kim MJ, Ahn JS, Kim JH, et al. Effects of the sintering conditions of dental zirconia ceramics on the grain size and translucency. J Adv Prosthodont. 2013;5:161–166. doi:10.4047/jap.2013.5.2.161
13. Denry I, Kelly JR. Emerging ceramic-based materials for dentistry. J Dent Res. 2014;93:1235–1242. doi:10.1177/0022034514553627
14. Zhang F, Vanmeensel K, Batuk M, et al. Highly-translucent, strong and aging-resistant 3Y-TZP ceramics for dental restoration by grain boundary segregation. Acta Biomater. 2015;16:215–222. doi:10.1016/j.actbio.2015.01.037
15. Zhang F, Inokoshi M, Batuk M, et al. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent Mater. 2016;32:327–337. doi:10.1016/j.dental.2016.09.025
16. Muñoz EM, Longhini D, Antonio SG, et al. The effects of mechanical and hydrothermal aging on microstructure and biaxial flexural strength of an anterior and a posterior monolithic zirconia. J Dent. 2017;63:94–102.
17. Plengsombut K, Brewer JD, Monaco EA, et al. Effect of two connector designs on the fracture resistance of all-ceramic core materials for fixed dental prostheses. J Prosthet Dent. 2009;101:166–173. doi:10.1016/S0022-3913(09)60022-6
18. Modi R, Kohli S, Rajeshwari K, et al. A three-dimension finite element analysis to evaluate the stress distribution in tooth supported 5-unit intermediate abutment prosthesis with rigid and nonrigid connector. Eur J Dent. 2015;9:255–261. doi:10.4103/1305-7456.156847
19. Johansson C, Kmet G, Rivera J, et al. Fracture strength of monolithic all-ceramic crowns made of high translucent yttrium oxide-stabilized zirconium dioxide compared to porcelain-veneered crowns and lithium disilicate crowns. Acta Odontol Scand. 2014;72:145–153. doi:10.3109/00016357.2013.822098
20. Bakitian F, Seweryniak P, Papia E, et al. Load-bearing capacity of monolithic zirconia fixed dental prostheses fabricated with different connector designs and embrasure shaping methods. J Prosthodont. 2019;28:64–70. doi:10.1111/jopr.13002
21. Mahmood DJH, Linderoth EH, Von Steyern PV, et al. Fracture strength of all-ceramic (Y-TZP) three- and four-unit fixed dental prostheses with different connector design and production history. Swed Dent J. 2013;37:179–187.
22. Yoshinari M, Derand T. Fracture strength of all-ceramic crowns. Int J Prosthodont. 1994;7:329–338.
23. Jerman E, Lümkemann N, Eichberger M, et al. Evaluation of translucency, Marten’s hardness, biaxial flexural strength and fracture toughness of 3Y-TZP, 4Y-TZP and 5Y-TZP materials. Dent Mater. 2021;37:212–222. doi:10.1016/j.dental.2020.11.007
24. Li Q-L, Jiang -Y-Y, Wei Y-R, et al. The influence of yttria content on the microstructure, phase stability and mechanical properties of dental zirconia. Ceram Int. 2022;48:5361–5368. doi:10.1016/j.ceramint.2021.11.079
25. Alves MFRP, Abreu LG, Klippel GGP, et al. Mechanical properties and translucency of a multi-layered zirconia with color gradient for dental applications. Ceram Int. 2021;47:301–309. doi:10.1016/j.ceramint.2020.08.134
26. Sen N, Sermet IB, Cinar S. Effect of coloring and sintering on the translucency and biaxial strength of monolithic zirconia. J Prosthet Dent. 2018;119:
27. Shah K, Holloway JA, Denry IL. Effect of coloring with various metal oxides on the microstructure, color, and flexural strength of 3Y-TZP. J Biomed Mater Res B Appl Biomater. 2008;87:329–337. doi:10.1002/jbm.b.31107
28. Kolakarnprasert N, Kaizer MR, Kim DK, et al. New multi-layered zirconias: composition, microstructure and translucency. Dent Mater. 2019;35:797–806.
29. Sundh A, Molin M, Sjogren G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dent Mater J. 2005;21:476–482. doi:10.1016/j.dental.2004.07.013
30. Kohorst P, Dittmer MP, Borchers L, et al. Influence of cyclic fatigue in water on the load-bearing capacity of dental bridges made of zirconia. Acta Biomater. 2008;4:1440–1447. doi:10.1016/j.actbio.2008.04.012
31. Anusavice KJ, Kakar K, Ferree N. Which mechanical and physical testing methods are relevant for predicting the clinical performance of ceramic-based dental prostheses? Clin Oral Implants Res. 2007;18:218–231. doi:10.1111/j.1600-0501.2007.01460.x
32. Jung YG, Peterson IM, Kim DK, et al. Lifetime-limiting strength degradation from contact fatigue in dental ceramics. J Dent Res. 2000;79:722–731. doi:10.1177/00220345000790020501
33. Mahmood DJ, Linderoth EH, Vult von Steyern P. The influence of support properties and complexity on fracture strength and fracture mode of all-ceramic dental prostheses. Acta Odontol Scand. 2011;69:229–237. doi:10.3109/00016357.2010.549508
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.