Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 12
Forelimb Resistance Exercise Protects Against Neuromuscular Junction Denervation in the SOD1-G93A Rat Model of ALS
Authors Nishimune H, Stanford KG, Chen J, Odum JD, Rorie AD, Rogers RS, Wheatley JL, Geiger PC, Stanford JA
Received 9 September 2022
Accepted for publication 15 November 2022
Published 22 November 2022 Volume 2022:12 Pages 145—155
DOI https://doi.org/10.2147/DNND.S388455
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Thomas Müller
Hiroshi Nishimune,1,2 Kimberly G Stanford,3 Jie Chen,1 James D Odum,3 Alexander D Rorie,3 Robert S Rogers,4 Joshua L Wheatley,3 Paige C Geiger,3 John A Stanford3
1Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS, USA; 2Tokyo Metropolitan Institute of Gerontology, Neurobiology of Aging, Tokyo, Japan; 3Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA; 4Departments of Physiology and Basic Sciences, Kansas City University of Medicine and Biosciences, Joplin, MO, USA
Correspondence: John A Stanford, Department of Molecular & Integrative Physiology, University of Kansas Medical Center, 3901 Rainbow Blvd., MS 3051, Kansas City, KS, 66160, USA, Tel +913-588-7416, Fax +913-588-5677, Email [email protected]
Introduction: The symptoms of Amyotrophic Lateral Sclerosis (ALS) include muscle weakness and eventual paralysis. These symptoms result from denervation of the neuromuscular junction (NMJ) and motor neuron cell death in the brain and spinal cord. Due to the “dying back” pattern of motor neuron degeneration, protecting NMJs should be a therapeutic priority. Although exercise has the potential to protect against NMJ denervation, its use in ALS has been controversial. Most preclinical studies have focused on aerobic exercise, which report that exercise can be beneficial at moderate intensities. The effects of resistance exercise on NMJ preservation in limb muscles have not been explored.
Methods: We trained male SOD1-G93A rats, which model ALS, to perform a unilateral isometric forelimb resistance exercise task. This task allows within-animal comparisons of trained and untrained forelimbs. We then determined the effects of isometric resistance exercise on NMJ denervation and AMP kinase (AMPK) activation in forelimb muscles.
Results: Our results revealed that SOD1-G93A rats were able to learn and perform the task similarly to wildtype rats, even after loss of body weight. SOD1-G93A rats exhibited significantly greater NMJ innervation in their trained vs their untrained forelimb biceps muscles. Measures of activated (phosphorylated) AMPK (pAMPK) were also greater in the trained vs untrained forelimb triceps muscles.
Discussion: These results demonstrate that isometric resistance exercise may protect against NMJ denervation in ALS. Future studies are required to determine the extent to which our findings generalize to female SOD1-G93A rats and to other subtypes of ALS.
Keywords: strength training, resistance, isometric, neuromuscular
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a devastating and fatal disease that begins with neuromuscular denervation and muscle weakness in adults. These initial symptoms progress rapidly to paralysis and death within a few years. The only FDA-approved treatments for ALS are riluzole and edaravone, which have nominal effects on survival.1 Interventions that protect against denervation of the neuromuscular junction (NMJ) should preserve muscle function and improve survival. Although exercise is an obvious candidate, its use in ALS has been controversial.2 This points to the need for further study in preclinical animal models.
The most widely used preclinical models of ALS are transgenic rodents that express mutant forms of superoxide dismutase.3,4 Mimicking ALS, these animals exhibit a relatively normal pre-symptomatic stage followed by muscle weakness, atrophy and eventual paralysis. Also like ALS, they exhibit neuromuscular denervation prior to loss of motor neurons.5 Most preclinical research into exercise for ALS has focused on aerobic or endurance exercise. As in the human literature, preclinical studies examining the effects of exercise in these models report mixed results. Moderate exercise delayed disease onset and increased lifespan in several studies, but high-intensity exercise hastened disease onset.6–10 While preclinical endurance training protocols have great translational validity, their inherent aerobic effects introduce disease-modifying confounds.11 Findings from the high-intensity exercise studies are consistent with accelerated disease progression in SOD1-G93A rats following chronic phrenic nerve hyperstimulation.12 In the physical therapeutic context, resistance or strength training can reverse muscle weakness and is often prescribed to individuals who lack the physical capacity for endurance or aerobic exercise. Like aerobic exercise, the use of strength training as an intervention in ALS remains controversial.13
The goal of the current study was to determine whether long-term resistance exercise affects neuromuscular denervation in the SOD1-G93A rat model of ALS. We also wanted to measure activated (phosphorylated) AMP-Kinase (AMPK). Phosphorylated AMPK (pAMPK) increases with increased metabolic demand in cells14 and has been reported to be detrimental in the SOD1-G93A C. elegans model.15 To answer these questions, we used a unilateral forelimb resistance training task that allows for within-animal comparisons between muscles in the trained and untrained forelimbs. After operant training, rats performed the task daily until they reached disease end stage.
Experimental Procedures
Animals and Forelimb Training
Male SOD1-G93A (n = 6) rats and wildtype (n = 5) littermates were used in our study. Our protocols were approved by the University of Kansas Institutional Animal Care and Use Committee and adhered to the Guide for the Care and Use of Laboratory Animals. Rats were bred in our colony and genotyped by Transnetyx. At approximately 2 months of age, rats were gradually water restricted and placed in modified Gerbrands operant chambers for training (chambers are described in detail in 16, 17). It should be noted that all rats received sufficient water to avoid dehydration (per our animal protocol) and maintain weight gain throughout the experiment until SOD1-G93A rats exhibited disease-related weight loss. Operant behavioral training consisted of shaping rats through successive approximations to reach out of a window in the front panel of the chamber with their right forelimb and press an operandum to raise a water dipper so that the rat could drink from the dipper cup (Figure 1). Due to the spatial arrangement of the operandum and dipper well, strength training was limited to the right forelimb. The operandum (an 18-mm diameter disc rigidly attached to Model 31 load cells; Sensotec) continuously measured the downward force exerted by the forelimb. A LabMaster interface (Scientific Solutions) received the analog signals from the load cells, converted the signals to digital form, and routed the signals to a computer. Computer software recorded the force output at 100 Hz with a force resolution of 0.33 g equivalent weights. The force requirement to raise and maintain the dipper in the raised position was 20 g (ie, the rats licked water while they pressed the operandum). When the 0.5 mL dipper cup was depleted, the rat released its force to refill the dipper and initiated another press-hold-release bout (Figure 2). After rats successfully learned the task (~13 weeks of age) they engaged in one 8 min training session per day, 5 days/week, for approximately 3 months (the number of sessions differed due to heterogeneity in disease progression). Training sessions continued until individual SOD1-G93A rats exhibited signs of limb paralysis or no longer performed the task. Attempts were made to harvest tissue from wildtype rats at a similar age as SOD1-G93A rats.
Forelimb Force Measures
Data from sessions were analyzed by computer programs that provided several measures of data for each training session. The first measure was one of overall task engagement. Time-on-task, which is analogous to operant response rate, was defined as the amount of time a rat spent applying at least 1 gram of force with its trained forelimb. Following the session, each rat’s raw data were parsed into individual 4.36 s force-time waveforms identified by a custom computer program. Individual waveforms were averaged for each animal across its session. Because the first second of each response typically involved an overshoot of the force requirement (see Figure 2), mean hold force was an average of the force emitted during the second 3.36 s of the stationary hold portion of the press-hold-release bouts. To obtain a measure of effort, we calculated time integral of force as the area under the force-time function. We then calculated force-related tremor (integrated power) by summing the area in the power spectrum between the 10–25 Hz frequency band, as determined by Fourier analysis of the 3.36 s stationary component of the force-time waveforms, as in our previous studies.16,17 The ratio of tremor to force output was also calculated to normalize these two related variables as we have found this measure to decrease with repeated resistance exercise.18
Tissue Harvest for Morphological Analysis of Neuromuscular Junctions and Muscle AMPK Protein Levels
Rats were anesthetized with isoflurane, and biceps muscles were dissected bilaterally for NMJ analysis. Transverse sections taken from the middle of the muscle using a cryostat were 20 µm thick, and the number of sections necessary to quantify at least 30 NMJs from each animal was typically two to four. We quantified all the NMJs found on a section to reduce bias; therefore, the number of NMJs quantified differed slightly between animals. This quantification was conducted under the epi-fluorescence microscope by eye and by focusing up and down the thickness of each identified NMJ (ie, 2D images were not used for quantification). Each muscle sample was analyzed to determine the percentage of innervated, partially denervated and fully denervated neuromuscular junctions in each muscle using immunohistochemical detection of nerves (antibodies against neurofilament (SMI-312R, Covance) and SV2 (SV2 DSHB)) and acetylcholine receptors (Alexa594-alpha-bungarotoxin, Molecular Probes) as described in detail previously.19 Forelimb triceps muscles were dissected bilaterally for AMPK protein levels (total and phosphorylated). Antibodies for pAMPK (T172) and total AMPK were purchased from Cell Signaling Technology (Beverly, MA, USA), and protein levels were quantified using Western blot detection. Protein levels of AMPK were quantified relative to tubulin, while pAMPK was quantified relative to total AMPK.
Statistics
All statistics were performed using SYSTAT software (systat.com). For behavioral variables, significance was assessed by two-way ANOVA with group (SOD1-G93A vs wildtype controls) as the between-subjects factor and time as the within-subjects, repeating factor. For NMJ innervation and AMPK protein analyses, two-way ANOVA with group (SOD1-G93A vs wildtype controls) and side (trained vs untrained limb) as the within-subjects, repeating factor. Statistical significance was determined by a p value ≤0.05.
Results
Task Engagement and Performance
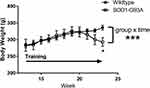
Rats in both groups learned to perform the task equally well. Body weight peaked in the SOD1-G93A rats at 19 weeks of age and declined in this group but not in the wildtype rats (Figure 3), leading to a significant group × time interaction (F = 6.145, p < 0.001). While this is not a novel finding in preclinical ALS studies, these data are included for reference to the other outcome measures reported here. There was a statistically significant decline in task engagement (Time on Task) across time (F = 3.948; p < 0.05; Figure 4A). Although the decline was driven primarily by the SOD1-G93A group, neither the group effect nor the interaction effect achieved statistical significance due to high variability in the measure. Likewise, the time integral of force also did not differ significantly between the two groups (Figure 4B). Although this measure declined in the SOD1-G93A rats across time, neither the group × time interaction nor the main effect for time reached statistical significance. Likewise, mean hold force trended greater for the SOD1-G93A group across the three time points (Figure 5A), but this measure did not differ significantly between-groups nor was there an effect of time or a group × time interaction. Although the values were greater in the wildtype rats at the first two time points, integrated power in the 10–25 Hz force band did not differ between the two groups (Figure 5B), as a function of time, or in the group × time interaction. Repeated performance resulted in a significant decrease in the ratio of integrated 10–25Hz power to force (F = 5.379, p < 0.05; Figure 5C). Although the decline in this ratio trended greater in the wildtype rats, the interaction effect did not reach statistical significance (p = 0.06). Overall, the SOD1-G93A rats performed similarly to their wildtype counterparts, even after loss of body weight, indicating functional preservation of their trained forelimbs during the training period. Survival (age in days in which a rat exhibited signs of limb paralysis or no longer performed the task) was 162 + 1.4 days in SOD1-G93A rats.
NMJ Innervation
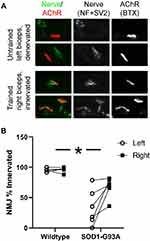
Immunohistochemistry of NMJs from an untrained vs trained biceps muscle in an SOD1-G93A rat is shown in Figure 6. Overlap of the nerve and receptor indicates full innervation, while lack of overlap indicates a denervated receptor due to the nerve dying back. Forelimb biceps muscle denervation was significantly greater in the SOD1-G93A group than in the wildtype group (F = 25.353, p = 0.001). Forelimb training had a significant effect on this measure, as the percentage of fully-intervated NMJs was significantly greater in the trained (right) than in the untrained (left) biceps muscles (F = 8.830, p < 0.05). There was a significant group × side interaction, as the training effect was greater in the SOD1-G93A group (who exhibited denervation in their untrained forelimbs) than in the wildtype group (F = 6.773, p < 0.05). The extent of this protection was present even in the faster-progressing rats that had greater denervation in their untrained forelimbs.
AMPK Activation
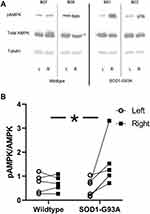
Western blot analyses revealed that pAMPK was greater in the trained forelimb triceps from SOD1-G93A rats but not in the wildtype rats, leading to a significant group × side (trained vs untrained limb) interaction (F = 7.514, p < 0.05; see Figure 7). This effect remained significant even when the outlier data point from the trained SOD1-G93A rat (see Figure 6) was removed (F = 7.400, p < 0.05).
Discussion
We report here that unilateral resistance exercise protects against forelimb muscle weakness and NMJ denervation in SOD1-G93A rats. SOD1-G93A rats were able to perform the forelimb exercise task even when their untrained forelimb reached the point of paralysis. This functional and neuroprotective effect coincided with greater AMPK activation in the trained forelimb muscles of SOD1-G93A rats. These results support resistance exercise as a treatment for muscle weakness in ALS, and a potentially positive role for muscle AMPK activation in this effect.
Previous studies testing aerobic exercise in animal models of ALS report dose-dependent effects. Moderate intensity exercise has been reported to delay disease onset and increased lifespan, while high-intensity exercise hastened disease onset.6–8,10 If the effects of aerobic exercise generalize to resistance exercise, our results suggest that the force requirements we implemented were of moderate intensity. This would be consistent with the ability of Sprague-Dawley rats to produce forces exceeding 60 g in this task.18 Alternatively, it is possible that the detrimental effects of high-intensity aerobic exercise do not occur with resistance exercise. Further studies comparing the cellular effects of exercise in this model as a function of exercise modality are necessary to test this hypothesis.
The positive effects of resistance exercise on forelimb strength and NMJ protection reported here contrast with our recent study examining tongue force training in SOD1-G93A rats.20 Compared to unexercised controls, ALS rats that were required to lick a force-sensing disc with higher forces exhibited similar (~50%) NMJ denervation in their genioglossus muscle and earlier tongue motility deficits. While speculative, it is possible that differences between limb and tongue muscles, as reported by others in the context of aging21,22 accounted for this effect. Unlike the tongue, limb muscles attach to bones on both ends. This relationship is not merely mechanical, however. Both muscle and bone act as endocrine organs that secrete proteins that influence each other through a complex bone-muscle crosstalk (reviewed in23). Further studies are needed to determine the extent to which mechanical vs secretory factors may account for differences between these muscle groups.
The etiology of ALS is unknown, but muscle use and exercise have long been implicated as disease-facilitating factors.24–30 These findings contribute to the controversy regarding exercise in ALS. Increased incidence in athletes and greater symptoms in the dominant limbs of affected individuals support the muscle use hypothesis. The muscle use hypothesis is nearly impossible to test in humans, precluding testing symptom onset as an outcome measure in a controlled experiment. In retrospective studies, confounding variables such as trauma and exposure to toxins complicate interpretations of causality.26,31 Selection bias in case–control studies or recall accuracy in retrospective studies also affects interpretation. Our results do not support the muscle use hypothesis in ALS disease progression, at least for the SOD1-G93A familial form of ALS.
One mechanism by which muscle activity might influence neuromuscular disease processes in ALS is through enhanced cellular bioenergetics. Despite impaired muscle mitochondrial function,32 increased metabolism has been reported both in SOD1 transgenic models of ALS33,34 and in sporadic ALS.35,36 The fact that muscle contraction increases muscle metabolic activity suggests that strength training could exacerbate hypermetabolism and accelerate neuromuscular dysfunction in ALS. This hypothesis is supported by two recent studies involving AMPK, a sensor for cellular energy balance that is activated under conditions of metabolic/ATP demand.37 The first study found that decreasing AMPK activity improves motor function in SOD1-G93A C. elegans (unfortunately, muscle denervation was not measured in this study).15 Another study reported that chronic AMPK stimulation impairs adaptive signaling in dystrophic skeletal muscle.38 Our results, however, reveal a positive relationship between AMPK activation and NMJ preservation, at least in muscle tissue. At the group level, greater pAMPK levels accompanied greater NMJ innervation in the trained forelimb muscles of SOD1-G93A rats compared to lower pAMPK and NMJ innervation in their untrained forelimbs. Our findings are consistent with a recent study reporting that AMPK activation via carbamazepine resulted in the protection of NMJs and motor neurons and extended survival in ALS SOD1-G93A mice.39 Further studies examining cell-specific (muscle vs neuronal) changes in AMPK activation are needed to address this controversy.
Our findings of a resistance training effect on the ratio of force-related tremor to force output are consistent with our previous study in healthy adult rats.18 Although the interaction between group and time did not reach statistical significance, the decline in this ratio trended greater in healthy controls. Isometric force-related tremor is a neurophysiological process related to motor neuron recruitment.40–42 Although this process can be modified by exercise and may be disrupted in ALS, the lack of a statistical significant interaction suggests that both groups exhibited this training effect. This phenomenon was not the primary measure in this study, and additional studies with adequately powered groups are required to investigate the effects of ALS on training-related neuromuscular function.
Conclusions
Our results support the use of resistance exercise to protect against muscle weakness and disease progression in ALS. The percentage of fully-intervated NMJs in the triceps of SOD1-G93A rats following repeated resistance exercise was comparable to that of interventions that include drugs, stem cells, gene silencing, and neurotrophic factors, in SOD1-G93A models.39,43–49 The fact that there remains ~25% room for further protection suggests that combining resistance exercise with other interventions may enhance neuromuscular protection and survival. We acknowledge several weaknesses of our study and caveats associated with our findings. The lack of a sedentary control group is a weakness for two reasons: we cannot rule out systematic laterality regarding NMJ denervation nor can we determine effects of our intervention on survival. Although paralysis onset is often observed in one limb in animal models of ALS, there are no data to our knowledge on laterality differences (much less systematic differences) in NMJ denervation in ALS. In fact, the muscle use hypothesis would predict greater denervation in trained forelimbs. Regarding survival, it is unlikely that protecting NMJ innervation in one forelimb would produce an effect, as evidenced by the fact that our rats survived an average of 162 days. Future tests implementing whole-body resistance exercise protocols are necessary to determine an effect on survival. By limiting our focus on NMJ denervation, we may have missed effects of resistance exercise on motor neurons in the spinal cord. Although the effects on motor neuron number may not be as apparent in the spinal cord given the “dying back” nature of degeneration in ALS,5 this is an empirical question that would be worthwhile to address. Another weakness is that we did not include female rats. This is also a future goal as the intervention may be more beneficial if female rats exhibit a different disease time course and severity. Finally, as in most preclinical ALS studies, we used SOD1 mutant rodents to model the disease. As we learn more about different subtypes of ALS, e.g.,50 interventions will likely be tailored to patients based on their unique genetic background. Overall, our unique unilateral forelimb resistance exercise task will allow for controlled preclinical testing in rodent models of ALS.
Acknowledgments
This work was supported by NIH grants GM103418, HD57850, NS078214, AG026491, AG031575, HD02528, and a KUMC Biomedical Research Training grant. The authors gratefully acknowledge the contributions of the late Stephen C. Fowler, PhD, in whose laboratory the isometric forelimb resistance exercise task originated.
Disclosure
Dr Hiroshi Nishimune reports grants from NIH during the conduct of the study. The authors report no conflicts of interest in this work.
References
1. Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 2019;39(2):733–748. Cited in: PMID: 30101496. doi:10.1002/med.21528
2. Tsitkanou S, Della Gatta P, Foletta V, Russell A. The role of exercise as a non-pharmacological therapeutic approach for amyotrophic lateral sclerosis: beneficial or detrimental? Front Neurol. 2019;10:783. Cited in: PMID: 31379732; PMCID: PMC6652799. doi:10.3389/fneur.2019.00783
3. Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. Erratum in: Science 1995 Jul 14;269(5221):149. PMID: 8209258. doi:10.1126/science.8209258
4. Nagai M, Aoki M, Miyoshi I, et al. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21(23):9246–9254. PMID: 11717358; PMCID: PMC6763929. doi:10.1523/JNEUROSCI.21-23-09246.2001
5. Fischer LR, Culver DG, Tennant P, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185(2):232–240. PMID: 14736504. doi:10.1016/j.expneurol.2003.10.004
6. Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Regular exercise is beneficial to a mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2003;53(6):804–807. PMID: 12783429. doi:10.1002/ana.10597
7. Veldink JH, Bär PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13(9):737–743. PMID: 14561497. doi:10.1016/s0960-8966(03)00104-4
8. Mahoney DJ, Rodriguez C, Devries M, Yasuda N, Tarnopolsky MA. Effects of high-intensity endurance exercise training in the G93A mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2004;29(5):656–662. PMID: 15116368. doi:10.1002/mus.20004
9. Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57(5):649–655. PMID: 15852403. doi:10.1002/ana.20451
10. Carreras I, Yuruker S, Aytan N, et al. Moderate exercise delays the motor performance decline in a transgenic model of ALS. Brain Res. 2010;1313:192–201. PMID: 19968977; PMCID: PMC2892864. doi:10.1016/j.brainres.2009.11.051
11. Um HS, Kang EB, Leem YH, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22(4):529–539. PMID: 18813861.
12. Lepore AC, Tolmie C, O’Donnell J, et al. Peripheral hyperstimulation alters site of disease onset and course in SOD1 rats. Neurobiol Dis. 2010;39(3):252–264. PMID: 20381620; PMCID: PMC2910141. doi:10.1016/j.nbd.2010.03.021
13. Patel BP, Hamadeh MJ. Nutritional and exercise-based interventions in the treatment of amyotrophic lateral sclerosis. Clin Nutr. 2009;28(6):604–617. PMID: 19782443. doi:10.1016/j.clnu.2009.06.002
14. Kjøbsted R, Hingst JR, Fentz J, et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018;32(4):1741–1777. PMID: 29242278; PMCID: PMC5945561. doi:10.1096/fj.201700442R
15. Lim MA, Selak MA, Xiang Z, et al. Reduced activity of AMP-activated protein kinase protects against genetic models of motor neuron disease. J Neurosci. 2012;32(3):1123–1141. PMID: 22262909; PMCID: PMC3742882. doi:10.1523/JNEUROSCI.6554-10.2012
16. Stanford JA, Fowler SC. Dantrolene diminishes forelimb force-related tremor at doses that do not decrease operant behavior in the rat. Exp Clin Psychopharmacol. 2002;10(4):385–391. PMID: 12498335. doi:10.1037//1064-1297.10.4.385
17. Bethel-Brown CS, Morris JK, Stanford JA. Young and middle-aged rats exhibit isometric forelimb force control deficits in a model of early-stage Parkinson’s disease. Behav Brain Res. 2011;225(1):97–103. PMID: 21767573; PMCID: PMC3178104. doi:10.1016/j.bbr.2011.07.002
18. Stanford JA, Vorontsova E, Fowler SC. The relationship between isometric force requirement and forelimb tremor in the rat. Physiol Behav. 2000;69(3):285–293. PMID: 10869594. doi:10.1016/s0031-9384(99)00248-6
19. Smittkamp SE, Spalding HN, Brown JW, et al. Measures of bulbar and spinal motor function, muscle innervation, and mitochondrial function in ALS rats. Behav Brain Res. 2010;211(1):48–57. PMID: 20211206; PMCID: PMC2872192. doi:10.1016/j.bbr.2010.03.007
20. Ma D, Shuler JM, Kumar A, et al. Effects of tongue force training on bulbar motor function in the female SOD1-G93A rat model of amyotrophic lateral sclerosis. Neurorehabil Neural Repair. 2017;31(2):147–156. PMID: 27573800; PMCID: PMC5243852. doi:10.1177/1545968316666956
21. Connor NP, Ota F, Nagai H, Russell JA, Leverson G. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. J Speech Lang Hear Res. 2008;51(4):818–827. PMID: 18658053; PMCID: PMC2892886. doi:10.1044/1092-4388(2008/059)
22. Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol. 2013;114(4):472–481. PMID: 23264540; PMCID: PMC3568981. doi:10.1152/japplphysiol.01370.2012
23. Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone. 2019;120:212–218. PMID: 30408611; PMCID: PMC6360108. doi:10.1016/j.bone.2018.11.002
24. McMenemey WH. Pathological aspects. Proc R Soc Med. 1962;55(12):1032–1033. PMID: 13932193; PMCID: PMC1897528.
25. Felmus MT, Patten BM, Swanke L. Antecedent events in amyotrophic lateral sclerosis. Neurology. 1976;26(2):167–172. PMID: 946326. doi:10.1212/wnl.26.2.167
26. Harwood CA, McDermott CJ, Shaw PJ. Physical activity as an exogenous risk factor in motor neuron disease (MND): a review of the evidence. Amyotroph Lateral Scler. 2009;10(4):191–204. PMID: 19263258. doi:10.1080/17482960802549739
27. Beghi E, Logroscino G, Chiò A, et al. Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph Lateral Scler. 2010;11(3):289–292. PMID: 20433412; PMCID: PMC3513269. doi:10.3109/17482960903384283
28. Turner MR, Wicks P, Brownstein CA, et al. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(8):853–854. PMID: 20562391. doi:10.1136/jnnp.2010.208413
29. Raymond J, Mehta P, Larson T, Factor-Litvak P, Davis B, Horton K. History of vigorous leisure-time physical activity and early onset amyotrophic lateral sclerosis (ALS), data from the national ALS registry: 2010–2018. Amyotroph Lateral Scler Frontotemporal Degener. 2021;24:1–10. PMID: 33896281. doi:10.1080/21678421.2021.1910308
30. Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(Pt 3):472–476. PMID: 15634730. doi:10.1093/brain/awh373
31. Armon C. Sports and trauma in amyotrophic lateral sclerosis revisited. J Neurol Sci. 2007;262(1–2):45–53. PMID: 17681549. doi:10.1016/j.jns.2007.06.021
32. Duffy LM, Chapman AL, Shaw PJ, Grierson AJ. Review: the role of mitochondria in the pathogenesis of amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2011;37(4):336–352. PMID: 21299590. doi:10.1111/j.1365-2990.2011.01166.x
33. Dupuis L, Oudart H, René F, de Aguilar JL G, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A. 2004;101(30):11159–11164. PMID: 15263088; PMCID: PMC503756. doi:10.1073/pnas.0402026101
34. Dupuis L, Loeffler JP. Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models. Curr Opin Pharmacol. 2009;9(3):341–346. PMID: 19386549. doi:10.1016/j.coph.2009.03.007
35. Desport JC, Preux PM, Magy L, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2001;74(3):328–334. PMID: 11522556. doi:10.1093/ajcn/74.3.328
36. Desport JC, Torny F, Lacoste M, Preux PM, Couratier P. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters. Neurodegener Dis. 2005;2(3–4):202–207. PMID: 16909026. doi:10.1159/000089626
37. Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49(4):527–531. PMID: 10871188. doi:10.2337/diabetes.49.4.527
38. Ljubicic V, Khogali S, Renaud JM, Jasmin BJ. Chronic AMPK stimulation attenuates adaptive signaling in dystrophic skeletal muscle. Am J Physiol Cell Physiol. 2012;302(1):C110–21. PMID: 21940670. doi:10.1152/ajpcell.00183.2011
39. Zhang JJ, Zhou QM, Chen S, Le WD. Repurposing carbamazepine for the treatment of amyotrophic lateral sclerosis in SOD1-G93A mouse model. CNS Neurosci Ther. 2018;24(12):1163–1174. PMID: 29656576; PMCID: PMC6489874. doi:10.1111/cns.12855
40. Allum JH, Dietz V, Freund HJ. Neuronal mechanisms underlying physiological tremor. J Neurophysiol. 1978;41(3):557–571. PMID: 660226. doi:10.1152/jn.1978.41.3.557
41. Hömberg V, Reiners K, Hefter H, Freund HJ. The muscle activity spectrum: spectral analysis of muscle force as an estimator of overall motor unit activity. Electroencephalogr Clin Neurophysiol. 1986;63(3):209–222. PMID: 2419077. doi:10.1016/0013-4694(86)90087-8
42. McAuley JH, Rothwell JC, Marsden CD. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Exp Brain Res. 1997;114(3):525–541. PMID: 9187289. doi:10.1007/pl00005662
43. Kalmar B, Edet-Amana E, Greensmith L. Treatment with a coinducer of the heat shock response delays muscle denervation in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(4):378–392. PMID: 22591194. doi:10.3109/17482968.2012.660953
44. Pérez-García MJ, Burden SJ. Increasing MuSK activity delays denervation and improves motor function in ALS mice. Cell Rep. 2012;2(3):497–502. PMID: 22939980; PMCID: PMC3462266. doi:10.1016/j.celrep.2012.08.004
45. Yoo YE, Ko CP. Dihydrotestosterone ameliorates degeneration in muscle, axons and motoneurons and improves motor function in amyotrophic lateral sclerosis model mice. PLoS One. 2012;7(5):e37258. PMID: 22606355; PMCID: PMC3351454. doi:10.1371/journal.pone.0037258
46. Krakora D, Mulcrone P, Meyer M, et al. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol Ther. 2013;21(8):1602–1610. PMID: 23712039; PMCID: PMC3734670. doi:10.1038/mt.2013.108
47. Thomsen GM, Gowing G, Latter J, et al. Delayed disease onset and extended survival in the SOD1G93A rat model of amyotrophic lateral sclerosis after suppression of mutant SOD1 in the motor cortex. J Neurosci. 2014;34(47):15587–15600. PMID: 25411487; PMCID: PMC4298650. doi:10.1523/JNEUROSCI.2037-14.2014
48. Dadon-Nachum M, Ben-Yaacov K, Ben-Zur T, et al. Transplanted modified muscle progenitor cells expressing a mixture of neurotrophic factors delay disease onset and enhance survival in the SOD1 mouse model of ALS. J Mol Neurosci. 2015;55(3):788–797. PMID: 25330859. doi:10.1007/s12031-014-0426-0
49. Nizzardo M, Bucchia M, Ramirez A, et al. iPSC-derived LewisX+CXCR4+β1-integrin+ neural stem cells improve the amyotrophic lateral sclerosis phenotype by preserving motor neurons and muscle innervation in human and rodent models. Hum Mol Genet. 2016;25(15):3152–3163. PMID: 27270413. doi:10.1093/hmg/ddw163
50. Pampalakis G, Angelis G, Zingkou E, Vekrellis K, Sotiropoulou G. A chemogenomic approach is required for effective treatment of amyotrophic lateral sclerosis. Clin Transl Med. 2022;12(1):e657. PMID: 35064780; PMCID: PMC8783349. doi:10.1002/ctm2.657
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.