Back to Journals » Journal of Pain Research » Volume 14
Foraminoplasty at the Base of the Superior Articular Process with Bone Drilling for Far-Downward Discs in Percutaneous Endoscopic Lumbar Discectomy: A Retrospective Study
Authors Yang F , Li P, Zhao L, Chang C, Chen B
Received 17 September 2021
Accepted for publication 21 December 2021
Published 30 December 2021 Volume 2021:14 Pages 3919—3925
DOI https://doi.org/10.2147/JPR.S339883
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Fengkai Yang,1,2 Pengfei Li,1,2 Long Zhao,1 Chengbing Chang,1 Bin Chen1
1Department of Minimally Invasive Spine Surgery, Chengde Medical University Affiliated Hospital, Chengde, Hebei, People’s Republic of China; 2Chengde Medical University, Chengde, Hebei, People’s Republic of China
Correspondence: Bin Chen
Department of Minimally Invasive Spine Surgery, Chengde Medical University Affiliated Hospital, Chengde, 067000, Hebei, People’s Republic of China
Tel +8615633142760
Fax +863142279598
Email [email protected]; [email protected]
Purpose: Percutaneous endoscopic lumbar discectomy (PELD) is usually used to treat lumbar disc herniation (LDH). This study aims to describe PELD by foraminoplasty in the treatment of far-downward migrated LDH and to demonstrate the clinical efficacy by a retrospective evaluation.
Patients and Methods: Between January 2017 and July 2018, 41 patients with far-downward migrated LDH were treated with PELD by foraminoplasty at the base of the superior articular process (SAP). Clinical efficacy was evaluated with a visual analogue scale (VAS) score, the Oswestry disability index (ODI), and the modified Macnab criteria. Postoperative follow-up data (1 month, 6 months, 18 months) were recorded.
Results: The surgical levels included L2/3 (1 patient), L3/4 (1 patient), L4/5 (17 patients), and L5/S1 (22 patients). The VAS and ODI scores indicated a significant improvement 18 months after surgery (mean ± standard deviation, VAS, 6.9± 1.3 versus 0.5± 0.8; ODI, 66.3± 12.2 versus 14.0± 8.2, respectively). Based on the modified Macnab criteria, 92.7% of patients had a good-to-excellent rate. There were three patients with a dural tear, and one patient had recurrent disc herniation.
Conclusion: PELD by foraminoplasty at the base of the superior articular process is a good method for treating far-downward migrated LDH.
Keywords: lumbar disk herniation, migrated disc herniation, foraminoplasty, percutaneous endoscopic lumbar discectomy
Introduction
Percutaneous endoscopic lumbar discectomy (PELD) is a safe and effective minimally-invasive technique for the treatment of lumbar disc herniation (LDH). With the improvement of related surgical techniques, positioning techniques, and optical imaging systems, it has become more widely used in the treatment of lumbar degenerative diseases.1 Due to the anatomical obstacles of this region, such as the facet joint process, pedicle, exiting nerve root, and intervertebral foramina stenosis, the correct insertion of the working cannula and the range of motion under the microscope are limited.2 There are concerns about residual nucleus pulposus that may cause poor postoperative results and recurrence. PELD was once considered limited to a few selective cases and not suitable for the treatment of migrated LDH. After the surgeons accumulated experience and improved surgical skills, clinical studies have shown that there is no significant difference in clinical efficacy between PELD and traditional open surgery in the treatment of prolapsed LDH.3–5 PELD indications have expanded, and it has been applied to various types of intervertebral disc herniations and even spinal stenosis.6 Lee et al7 divided disk migration into four zones based on the direction and distance from the intervertebral space on preoperative sagittal magnetic resonance imaging (MRI). A migration ranging from the center to the inferior margin of the lower pedicle is considered a far-downward disc herniation. Since traditional PELD technique have some shortcomings such as insufficient exposure, poor visualization, and difficult to reach and grasp highly migrated discs. We chose to remove prolapsed discs by the transforaminal approach, relying on foraminoplasty at the base of the superior articular process (SAP) with the assistance of a common instrument-bone drilling. In order to provide a reference for the effectiveness of PELD in the treatment of LDH with far-downward migrated.
Methods
Patient Population
From January 2017 to July 2018, 41 consecutive patients were treated with single-level PELD. The inclusion criteria were as follows: 1) far-downward migrated LDHs verified by preoperative MRI and computed tomography (CT); 2) unilateral leg symptoms (pain, sensory changes, motor weakness); 3) symptoms were not relieved despite conservative treatment for more than 6 weeks. The exclusion criteria were as follows: 1) spinal stenosis confirmed by MRI and CT; 2) segmental instability; and 3) coexistent pathologic conditions, such as fractures, acute inflammation, infection, or tumors. This retrospective study was approved by the Ethics Committee of Chengde Medical University Affiliated Hospital (CYFYLL2021101). Informed written consent was obtained from all patients.
Surgical Techniques
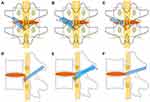
The patient was placed in a lateral decubitus position, and a soft roll was put under the lumbar area above the iliac crest to increase the area of the intervertebral foramen. The skin entry point was determined according to the position of the iliac crest and the target segment. The location was usually 8–12 cm from the posterior midline and 1–3 cm above the iliac crest. The whole procedure was performed under local anesthesia with 50 mL of 0.8% lidocaine. The specific anesthesia steps were carried out according to a previous study.8 Under the guidance of fluoroscopy, an 18-gauge needle was inserted percutaneously, pointing to the base of the SAP. The needle core was replaced by a guidewire, and a skin incision was done at the entry point. The TomShidi needle was used to penetrate the ventral part of the SAP in the direction of the guidewire [Figure 1]. Sequential bone drills (4 mm, 6 mm, 7 mm, 8 mm; MaxMore spine, Hoogland Spine Products GmbH, Germany) were used to create a working channel by resecting the base of the ventral part of the SAP. Whenever possible and when the front end of the drill was close to the midline, the lateral recesses were simultaneously enlarged [Figure 2A–D]. Foraminoplasty was then carried out by the “bottom-up” technique with a bone drill [Figure 2E and F]. The endoscope was introduced after the bevel-ended working cannula was placed [Figure 3]. The endoscope system (MaxMore spine, Hoogland Spine Products GmbH, Germany) was connected to a 0.9% saline solution for continuous rinsing.
 |
Figure 1 Sagittal (A) and anteroposterior (B) fluoroscopic images of the Tom Shidi. |
 |
Figure 3 Positioning of the working cannula in fluoroscopic view ((A), sagittal; (B), anteroposterior). |
Soft tissue, ligamentum flavum and bone debris were removed with nucleus fragment forceps, and by using tip-flexible electrode bipolar radiofrequency ablation (Elliquence LLC, New York, USA) to stop bleeding. The prolapsed intervertebral disc tissue was exposed in a clear field of vision and then removed with forceps. We used semi-flexible forceps for removal of far-downward disc debris. In order to achieve complete decompression, including that at the disc level, we adjusted the position of the working cannula from the bottom to the top [Figure 4]. Annuloplasty was performed by electrode ablation after all attainable loose disc tissue around the disc crevasse was removed. The nerve root was sufficiently decompressed and pulsed freely with the heart rate as assessed by endoscopic visualization. A drainage tube was placed, and the incision was sutured. Routine follow-up MRI was performed in all patients [Figure 5].
Patient Evaluation
The clinical outcomes were assessed using the visual analog scale (VAS) score and the Oswestry Disability Index (ODI) score. All patients in the study were followed up for at least 18 months after the surgery. Surgical efficiency was assessed by using the modified Macnab criteria9 at the final follow-up.
Statistical Analyses
For statistical analyses, the clinical results were analyzed using SPSS version 26 (IBM, Armonk, USA). The mean outcome scores (mean ± standard deviation) from pre- and postoperative variables were compared by paired Student’s t-tests or Wilcoxon rank sum test. P < 0.05 was considered statistically significant.
Results
There were no terminated operations due to intraoperative pain or nerve injury. The segmental level was L2/3 in one case, L3/4 in one case, L4/L5 in 17 cases, and L5/S1 in 22 cases. The mean age of patients was 44.8±14.5 years. The sex ratio (male/female) was 1.2 (22/19). The mean operation time was 66.8±13.1 minutes. The mean preoperative VAS-leg pain score and ODI score were 6.9±1.3 and 66.3±12.2, respectively. Compared with the preoperative state, the patient’s VAS score and ODI score improved to 0.5±0.8 and 14.0±8.2, 18 months after surgery. (P < 0.05) The VAS-leg pain score and ODI score were significantly improved at each postoperative time points [Table 1]. The good-to-excellent rate in patients was 92.7% (38/41), 27 reported excellent results, 11 reported good results, 3 evaluated there results as fair and none reported poor outcome.
 |
Table 1 Changes of Preoperative and Postoperative VAS, ODI Scores |
Complications
Three patients had an intraoperative dural tear, but without cerebrospinal fluid leakage postoperatively. No neurological sequelae were observed in the follow-up period. One patient suffered recurrent disc herniation 3 months after surgery and underwent revision surgery. No patient had residual fragments as assessed by postoperative MRI. There were no reports of infection, cauda equina syndrome, vascular injury, or other serious complications.
Discussion
The lumbar intervertebral disc can prolapse to the cranial, caudal or even dorsal side, but most commonly migrates to the caudal side.10 The traditional transforaminal surgical approach is inadequate because it is difficult to remove the prolapsed disc due to obstruction caused by the articular process, pedicle, and other inherent anatomical structures of the lumbar spine. There is a high failure rate in the treatment of prolapsed intervertebral disc herniation, especially since in patients with far-downward prolapse, the risk of residual disc tissue is significantly increased.7,11,12 The key step of PELD is to approximate the front end of the working cannula as close as possible to the herniation. For far-downward discs, the working cannula of the traditional transforaminal approach is located at the disc level, with a steep trajectory angle, being unable to access the disc in the distal portion of the epidural space. In order to remove the prolapsed disc, it is necessary to increase the angle of inclination toward the head, which is likely to cause irritation of the exiting nerve root.
A variety of different PELD approaches have emerged to improve clinical efficacy. Choi et al13 successfully removed a prolapsed disc of the L4-L5 segment by the L5-S1 interlaminar approach. This technique required a wide interlaminar space. Kim et al14 tried to access the target via a contralateral transforaminal route, overcoming the limitation of obstruction of the visual field by the pedicle. The cranial tilt angle was reduced, thereby reducing the risk of damage to the exiting nerve root. However, the scope of indications was limited and there was a risk of damage to the dural sac and contralateral nerve root. Krzok et al15 used a trephine or bone drill to directly cut through the pedicle from the posterolateral side to establish a working channel. The technique required high accuracy of the hole-forming position, and avoidance of pedicle fractures was difficult. Some surgeons performed PELD via the superior vertebral pedicle notch approach.16–18 They needed to remove part of the upper edge of the pedicle with a bone drill or trephine. The scope of inspection under the endoscopic was enlarged to reduce the difficulty of the operation. At present, there is no consensus on which approach to use for lumbar disc herniated tissues with different degrees of migration. Most surgeons use an individually-optimized surgical approach for treatment.
The technique used in this study is a modified lumbar foraminoplasty. It is different from the traditional Tessys technique which advocates foraminoplasty at the tip of the SAP.19 Sequential bone drills were used to the upper edge of the pedicle to remove part of the base of the SAP and to carry out progressive enlargement of the intervertebral foramen. Commonly used instruments in foraminoplasty include bone drills, trephines, chisels, Kerrison rongeurs, high-speed drills, among others. Although the high-speed endoscopic drill system is also an option to consider, such equipment is not available in most hospitals, especially in developing countries. Additionally, it increases medical expenses. A bone drill was selected for this study according to the technical characteristics of our surgical approach. The tip of the drill had a blunt head, which avoids cutting nerve roots while entering the spinal canal. At the same time, the drill pushed the ligamentum flavum to wrap around the ventrolateral side of the dural sac for protection. With the tip of the drill anchored in the posterior wall of the vertebral body and used as a fulcrum, the bone drill could further enlarge the intervertebral foramen from the bottom of the SAP to its top. Adjusting the position of the working cannula was easy. After abrading the SAP, the bone debris were washed out with a saline flow under the endoscope, and the remaining small fragments were removed with nucleus forceps. There was little blood loss from the bone wound because the injured cancellous bone was ground and compressed. This was conducive to maintaining a clear operating vision. Patients were conscious and could provide timely feedback during the operation. We adjusted the depth of the bone drill into the spinal canal according to each case. It is not recommended to reach the midline when some patients may then suffer dural sac compression accompanied by irritation or injury, especially in cases with a congenitally small spinal canal.
Li et al12,20 specially designed an instrument for foraminoplasty at the base of the ventral SAP with graded trephines. These authors treated lateral recess stenosis and 90.6% (77/85) of patients experienced a favorable outcome. According to the Macnab’s score obtained, 92.5% (124/134) of complex uncontained LDH cases were classified as “excellent” or “good.” The effectiveness of this modified lumbar foraminoplasty method was therefore proven. In the present study, we report a case series of 41 patients of far-downward migrated LDH, where 92.7% of cases were classified as “excellent” or “good” by the modified Macnab score. Satisfactory therapeutic results were achieved by our surgical treatment. However, our study has several limitations. The sample size was limited and needed to be further expanded. More cases can help measure the relationship between the depth of bone drilling into the spinal canal and the size of the spinal canal space. The study also needs more detailed radiology data for further evaluation. Once we specify a suitable reference indicator, the safety and efficacy of this approach can be further improved. Considering this is a retrospective medical record analysis, a larger sample size and controlled trial are necessary to evaluate this method accurately.
Conclusion
PELD with foraminoplasty at the base of the SAP can be an effective treatment for far-downward migrated LDH. It may provide an alternative minimally invasive surgery method for patients with far-downward migrated LDH. We suggest that the procedure should be performed by a skillful surgeon who has several years of experience in PELD technique.
Abbreviations
CT, computed tomography; LDH, lumbar disc herniation; MRI, magnetic resonance imaging; ODI, Oswestry disability index; PELD, percutaneous endoscopic lumbar discectomy; SAP, superior articular process; VAS, visual analogue scale score.
Ethics Approval and Informed Consent
This retrospective study was approved by the Ethics Committee of Chengde Medical University Affiliated Hospital (CYFYLL2021101). Informed written consent was obtained from all patients. This study was conducted in accordance with the Declaration of Helsinki.
Funding
This research was supported by the Department of Health of Hebei Province (20150019).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pan M, Li Q, Li S, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. 2020;23(1):49–56.
2. Hu QF, Pan H, Fang YY, Jia GY. Percutaneous endoscopic lumbar discectomy for high-grade down-migrated disc using a trans-facet process and pedicle-complex approach: a technical case series. Eur Spine J. 2018;27(Suppl 3):393–402. doi:10.1007/s00586-017-5365-3
3. Choi G, Lee SH, Lokhande P, et al. Percutaneous endoscopic approach for highly migrated intracanal disc herniations by foraminoplastic technique using rigid working channel endoscope. Spine (Phila Pa 1976). 2008;33(15):E508–515. doi:10.1097/BRS.0b013e31817bfa1a
4. Wu C, Lee CY, Chen SC, Hsu SK, Wu MH. Functional outcomes of full-endoscopic spine surgery for high-grade migrated lumbar disc herniation: a prospective registry-based cohort study with more than 5 years of follow-up. BMC Musculoskelet Disord. 2021;22(1):58. doi:10.1186/s12891-020-03891-1
5. Ahn Y, Jang IT, Kim WK. Transforaminal percutaneous endoscopic lumbar discectomy for very high-grade migrated disc herniation. Clin Neurol Neurosurg. 2016;147:11–17.
6. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices. 2014;11(6):605–616. doi:10.1586/17434440.2014.940314
7. Lee S, Kim SK, Lee SH, et al. Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J. 2007;16(3):431–437.
8. Cheng XK, Chen B. Percutaneous transforaminal endoscopic decompression for geriatric patients with central spinal stenosis and degenerative lumbar spondylolisthesis: a novel surgical technique and clinical outcomes. Clin Interv Aging. 2020;15:1213–1219. doi:10.2147/CIA.S258702
9. Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53(5):891–903. doi:10.2106/00004623-197153050-00004
10. Son ES, Kim DH, Jung JW, Lee D. Analysis of migration patterns of disk fragments and contributing factors in extruded lumbar disk herniation. Pm r. 2017;9(1):15–20. doi:10.1016/j.pmrj.2016.06.007
11. Choi KC, Lee JH, Kim JS, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery. 2015;76(4):
12. Li ZZ, Hou SX, Shang WL, Song KR, Zhao HL. Modified percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar discectomy: instrument design, technique notes, and 5 years follow-up. Pain Physician. 2017;20(1):E85–e98. doi:10.36076/ppj.2017.1.E85
13. Choi G, Prada N, Modi HN, Vasavada NB, Kim JS, Lee SH. Percutaneous endoscopic lumbar herniectomy for high-grade down-migrated L4-L5 disc through an L5-S1 interlaminar approach: a technical note. Minim Invasive Neurosurg. 2010;53(3):147–152. doi:10.1055/s-0030-1254145
14. Kim JS, Choi G, Lee SH. Percutaneous endoscopic lumbar discectomy via contralateral approach: a technical case report. Spine (Phila Pa 1976). 2011;36(17):E1173–1178. doi:10.1097/BRS.0b013e3182264458
15. Krzok G, Telfeian AE, Wagner R, Iprenburg M. Transpedicular lumbar endoscopic surgery for highly migrated disk extrusions: preliminary series and surgical technique. World Neurosurg. 2016;95:299–303. doi:10.1016/j.wneu.2016.08.018
16. Ying J, Huang K, Zhu M, et al. The effect and feasibility study of transforaminal percutaneous endoscopic lumbar discectomy via superior border of inferior pedicle approach for down-migrated intracanal disc herniations. Medicine (Baltimore). 2016;95(8):e2899. doi:10.1097/MD.0000000000002899
17. Lee CW, Yoon KJ, Ha SS, Kang JK. Foraminoplastic superior vertebral notch approach with reamers in percutaneous endoscopic lumbar discectomy: technical note and clinical outcome in limited indications of percutaneous endoscopic lumbar discectomy. J Korean Neurosurg Soc. 2016;59(2):172–181. doi:10.3340/jkns.2016.59.2.172
18. Giordan E, Del Verme J, Coluzzi F, Canova G, Billeci D. Full-endoscopic transpedicular discectomy (FETD) for lumbar herniations: case report and review of the literature. Int J Surg Case Rep. 2020;72:137–141. doi:10.1016/j.ijscr.2020.05.085
19. Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol. 2005;17(6):641–661. doi:10.1007/s00064-005-1156-9
20. Li ZZ, Hou SX, Shang WL, Cao Z, Zhao HL. Percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar decompression for lateral recess stenosis through transforaminal approach: technique notes and 2 years follow-up. Clin Neurol Neurosurg. 2016;143:90–94. doi:10.1016/j.clineuro.2016.02.008
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.