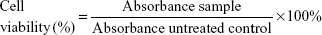Back to Journals » Drug Design, Development and Therapy » Volume 10
Flavokawain derivative FLS induced G2/M arrest and apoptosis on breast cancer MCF-7 cell line
Authors Mohd Ali N, Akhtar NM, Ky H, Lim KL, Abu N, Zareen S, Wan Yong H, Alan-Ong H, Tan SW, Alitheen NB , Ismail J, Yeap S, Kamarul T
Received 9 December 2015
Accepted for publication 7 January 2016
Published 10 June 2016 Volume 2016:10 Pages 1897—1907
DOI https://doi.org/10.2147/DDDT.S102164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Norlaily Mohd Ali,1 M Nadeem Akhtar,2 Huynh Ky,3 Kian Lam Lim,1 Nadiah Abu,4 Seema Zareen,2 Wan Yong Ho,5 Han Kiat Alan-Ong,1 Sheau Wei Tan,6 Noorjahan Banu Alitheen,4 Jamil bin Ismail,2 Swee Keong Yeap,6 Tunku Kamarul7
1Faculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, Selangor, 2Department of Industrial Biotechnology, Faculty of Industrial Sciences & Technology, Universiti Malaysia Pahang, Pahang, Malaysia; 3Department of Agriculture Genetics and Breeding, College of Agriculture and Applied Biology, Cantho University, CanTho City, Vietnam; 4Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 5School of Biomedical Sciences, The University of Nottingham Malaysia Campus, 6Institute of Bioscience, Universiti Putra Malaysia, Selangor, 7Tissue Engineering Group, National Orthopaedic Centre of Excellence for Research and Learning, Department of Orthopaedic Surgery, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia
Abstract: Known as naturally occurring biologically active compounds, flavokawain A and B are the leading chalcones that possess anticancer properties. Another flavokawain derivative, (E)-1-(2'-Hydroxy-4',6'-dimethoxyphenyl)-3-(4-methylthio)phenyl)prop-2-ene-1-one (FLS) was characterized with 1H-nuclear magnetic resonance, electron-impact mas spectrometry, infrared spectroscopy, and ultraviolet (1H NMR, EI-MS, IR, and UV) spectroscopic techniques. FLS cytotoxic efficacy against human cancer cells (MCF-7, MDA-MB-231, and MCF-10A) resulted in the reduction of IC50 values in a time- and dose-dependent mode with high specificity on MCF-7 (IC50 of 36 µM at 48 hours) against normal breast cell MCF-10A (no IC50 detected up to 180 µM at 72 hours). Light, scanning electron, and fluorescent microscopic analysis of MCF-7 cell treated with 36 µM of FLS displayed cell shrinkage, apoptotic body, and DNA fragmentation. Additionally, induction of G2/M cell arrest within 24 hours and apoptosis at subsequent time point was discovered via flow cytometry analysis. The roles of PLK-1, Wee-1, and phosphorylation of CDC-2 in G2/M arrest and proapoptotic factors (Bax, caspase 9, and p53) in promotion of apoptosis of FLS against MCF-7 cell were discovered using fluorometric, quantitative real-time polymerase chain reaction, and Western blot analysis. Interestingly, the presence of SCH3 (thiomethyl group) on ring B structure contributed to the selective cytotoxicity against MCF-7 cell compared to other chalcones, flavokawain A and B. Overall, our data suggest potential therapeutic value for flavokawain derivative FLS to be further developed as new anticancer drug.
Keywords: (E)-1-(2'-Hydroxy-4',6'-dimethoxyphenyl)-3-(4-methylthio)phenyl)prop-2-ene-1-one (FLS), MCF-7, G2/M arrest, apoptosis, cell cycle, PLK-1, p53, caspase
Introduction
Breast cancer is the leading cause of cancer death in females worldwide, accounting for ~25% (1.68 million cases) of the total new cancer cases and ~14% (521,900 deaths) of the total cancer deaths in 2012.1,2 Among the various types of cancer, breast cancer is the most frequently diagnosed and the most common cancer that affects Malaysian women from all ethnicities.3 According to recent reports, an estimated 232,670 new cases of breast cancer have been expected and diagnosed among women and about 2,360 new cases in men in the US during 2014.4,5 Although numerous efforts have been made to decrease the mortality rate of breast cancer,6 to-date, no ultimate chemotherapeutic drug is available to overcome this disease. Thus, effort in searching alternative or better candidates of antibreast cancer compound from natural phytochemical or synthetic chemistry is still ongoing.7
A number of studies have reported the broad spectrum bioactivities of chalcones including antioxidant, cytotoxic, anti-inflammation, antihistaminic, and antimicrobial effects.8 Flavokawains (either A, B, or C) are among the well-studied chalcones that were extracted from Piper methysticum. Flavokawain A (FLA) constituted as the highest amount of flavokawain found in kava extract, while flavokawain B (FLB) is the most well studied flavokawain. Both flavokawain possess various level of cytotoxicity against several cancer cell line including colon, bladder, and breast.9–12 An ideal cytotoxic compound should possess good selectivity against the targeted cancer cell while inducing limited or no toxicity against normal cell.9 Comparing the cytotoxic effect of FLA and FLB, these two compounds were recorded with different degrees of in vitro cytotoxicity against estrogen-dependent MCF-7 (FLA: ~25 μM; FLB: ~33 μM) and estrogen-independent MDA-MB-231 (FLA: ~17 μM; FLB: ~12 μM) breast cancer cell lines. However, both flavokawains also exhibited cytotoxicity on normal MCF-10A (FLA: ~95 μM; FLB: ~45 μM) breast cell line.9,10 Synthesis of bioactive compounds allows the possibility of modifying the lead compound for better efficacy and desired cytotoxic action. For example, Badisa et al7 have shown that synthetic piperidinyl-diethylstilbestrol and pyrrolidinyl-diethylstilbestrol possessed better selectivity against breast cancer MCF-7 cell than normal breast MCF-10A cell. In this investigation, a flavokawain derivative, (E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(4-(methylthio)phenyl)prop-en-1-one (FLS) was synthesized via a Claisen–Schmidt condensation reaction. Thus, the aim of this study was to compare the selectivity of flavokawain derivative FLS on breast cancer and normal cell lines. In addition, the regulation of cell cycle and apoptotic inducing effect of FLS against MCF-7 were also observed.
Materials and methods
Synthesis of FLS
FLS was synthesized via a Claisen–Schmidt condensation reaction. Acetophenone, 4,6-dimethoxy-2′-hydroxyacetophenone 3.0 g (9.0 mmol) and 4-(methylthio)benzaldehyde (1.4 mL, 10 mmol) were dissolved in methanol. A 40% water solution of potassium hydroxide 5.0 g and 50 mL methanol was mixed in a 500 mL round bottle flask and left for 15 minutes at room temperature. The crude product was transferred into separating funnel (500 mL) and then added with 200 mL distilled water. The mixture was then acidified with the addition of 2–3 mL hydrochloride. The product was extracted with 100 mL ethyl acetate. The organic layer was then successively washed with brine and dried over anhydrous sodium sulfate. The crude product was finally purified by flash column chromatography with ethyl acetate and hexane (5:5) as eluants and recrystallized in ethyl acetate and drops of MeOH.
MTT cell viability assay
MCF-7, MDA-MB-231, and MCF-10A cells (American Type Culture Collection [ATCC], Manassas, VA, USA) were seeded overnight in 96-well plates at a concentration of 0.8×105 cells/mL at 37°C CO2 incubator in Roswell Park Memorial Institute medium-1640, Dulbecco’s Modified Eagle’s Medium (DMEM), and DMEM-F12 supplemented with hydrocortisone (0.5 μg/mL), insulin (10 μg/mL), human epidermal growth factor (20 ng/mL) (Sigma-Aldrich Co., St Louis, MO, USA) medium, respectively. Then, respective cells were treated with FLS (ranging between 180 and 3 μM) for 24, 48, and 72 hours. MTT solution (5 mg/mL) was added to all wells 3 hours before end of incubation time. Absorbance was read at wavelength of 570 nm (BioTek Instruments, Winooski, VT, USA).13 Percentage viability of the tested cell line was calculated using the following formula:
|
IC50 value indicating concentration that inhibited 50% of the tested cell lines was obtained from the graph plotted with percentage of cell viability against concentration of FLS.
MCF-7 cell treatment
Based on the MTT assay (Table 1), reduction in IC50 values of FLS was observed after treatment at 24–72 hours on MCF-7 verifying that FLS effect is time- and dose-dependent. To standardize the treatment, one concentration of FLS (36 μM) was used for the remaining assays. MCF-7 cell was seeded at 0.8×105 cells/mL overnight in 6-well plate. After reaching 80%–90% confluence, the cell was treated with 36 μM (IC50 at 48 hours) of FLS for 24, 48, and 72 hours. Untreated control was prepared simultaneously. After 24, 48, or 72 hours of incubation, cell was harvested using TrypLE (Thermo Fisher Scientific, Waltham, MA, USA), washed with phosphate-buffered saline and subjected to the subsequent assays.
  | Table 1 Comparison values of IC50 of FLS, FLA, and FLB in MCF-7, MDA-MB-231 and MCF-10A cells |
Light and scanning electron microscopic examination
Cell was subjected to microscopic examination using inverted light microscope (Nikon Corporation, Tokyo, Japan) and scanning electron microscope (SEM; JEOL, Tokyo, Japan) after 72 hours of treatment. Prior to SEM observation, cell was seeded on poly-L-lysine coated round coverslips in a 6-well plate. After 72 hours, untreated control and FLS treated MCF-7 cell were fixed in 4% glutaraldehyde, washed with 0.1 M sodium cocodylate buffer, fixed with 1% osmium tetroxide and underwent series of dehydration in acetone and dried using CO2. Then, the samples were mounted onto the stubbing, coated with gold in sputter and viewed under JEOL SEM under the 15 kV setting.
Acridine orange/propidium iodide dual staining
Harvested cell was stained with 10 μg/mL of acridine orange and propidium iodide (PI) each. The morphology of cell was observed under fluorescent microscope (Nikon) with combination of excitation filter (450–490 nm) and long pass filter (520 nm). Random scoring of 200 cells for both untreated and FLS-treated MCF-7 was taken to determine the percentage of viable (green intact cell), apoptotic (green cell with condensed or fragmented nucleus), and necrotic (red cell) cells.
Cell cycle analysis
Cell cycle progression was evaluated using BD Cycletest Plus kit and subjected to BD FACS Calibur flow cytometer (BD, Franklin Lakes, NJ, USA) analysis. In brief, harvested cell was incubated with 250 μL of trypsin buffer for 10 minutes followed by 200 μL of trypsin inhibitor with RNase buffer. The samples were finally stained with 200 μL of PI solution and analyzed.
Annexin V/PI apoptosis study
Apoptosis of cell was evaluated using BD Annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis detection kit. In brief, harvested cell was washed and added with 100 μL of 1× binding buffer. The cell was then stained with 5 μL of Annexin V-FITC and 5 μL of PI solution, incubated for 15 minutes and diluted with 400 μL of 1× binding buffer. All samples were analyzed with BD FACS Calibur flow cytometer (Becton Dickinson).
Quantitative reverse transcription real-time PCR assay
RNA was extracted from cell using RNeasy mini plus kit (Qiagen NV, Venlo, the Netherlands). The purity and concentration of the isolated RNA were quantified prior to DNA conversion using iScript cDNA synthesis kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Expression of polo-like kinase 1 (PLK1), WEE1 G2 checkpoint kinase (WEE-1), c-Jun proto-oncogene (c-Jun), Glucose transporter (GLUT) were quantified by quantitative real-time PCR (qRT-PCR) using SYBR select master mix (Thermo Fisher Scientific) on iQ-5 real-time polymerase chain reaction (PCR) machine (Bio-Rad) with normalization to three housekeeping genes, β-actin, 18srRNA, and GAPDH. All primers are listed in Table 2. PCR condition for this experiment was 1 cycle of 50°C/2 minutes for Uracil-DNA glycosylase (UDG) activation; 1 cycle of 95°C/2 minutes for DNA polymerase activation; 40 cycles of 95°C 2 seconds for denature, 60°C for 30 seconds for anneal and extend. Normalization of reference genes was carried out using geNorm algorithm.
  | Table 2 The accession number and sequence of the primers used in the quantitative real-time PCR assay |
Western blot analysis
Harvested cell was lysed with radioimmunoprecipitation assay (RIPA) buffer supplemented with phosphatase inhibitor cocktail (Hoffman-La Roche Ltd., Basel, Switzerland) prior to protein quantification using Bradford assay (Sigma-Aldrich Co.). Next, 100 μg of cell lysate was electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad), transferred to nitrocellulose membrane using Pierce Fast Semi-Dry Blotter (Pierce, Thermo Fisher Scientific, Waltham, MA, USA), blocked with 0.5% skimmed milk and incubated with primary antibodies (anti-CDC2, anti-p-CDC2, anti-p53, anti-Bax, anti-Bcl-2, and anti-β-actin at dilution 1:1,000) (Abcam, San Francisco, CA, USA) for 1 hour. Following that, membrane was washed, incubated with goat anti-rabbit immunoglobulin G (IgG) and haematoxylin and eosin stain (H&E) conjugated to alkaline phosphatase (Abcam) in 1:5,000 dilution and developed under chemiluminescence condition (SuperSignal West Pico, Pierce) using ChemiDoc XRS (Bio-Rad). The bands, intensity was analyzed using Quantity One 1D analysis software (Bio-Rad).
Caspase 9 and cytochrome c detection study
Extracted protein from previous Western blot analysis was also subjected to detection of caspase 9 and cytochrome c using CaspGLOW Red Active Caspase-9 staining kit (BioVision, Milpitas, CA, USA) and human cytochrome c platinum enzyme-linked immunosorbent assay (ELISA) (eBioscience Affymetrix, Santa Clara, CA, USA). The fluorescence intensity in active caspase 9 was measured at Ex/Em =540/570 nm using microplatefluorometer (Thermo Scientific). On the other hand, absorbance for human cytochrome c was measured using ELISA plate reader at wavelength of 450 nm (BioTek Instruments). Fold change of caspase 9 and cytochrome c were calculated by dividing fluorescence intensity or absorbance of FLS treated MCF-7 with untreated control MCF-7.
Statistical analysis
The data are presented as average of mean ± SE (standard error of the mean), one-way analysis of variance was used to assess differences between time point groups. Results were considered statistically significant when P<0.05.
The Ethical Committee for Research in Universiti Putra Malaysia, decided that ethical approval was unnecessary for using commercial human cell lines.
Results
Synthesis of FLS
FLS was the yellow flat crystals with 78.6% yield through the synthesis. IR (CHCl3): 1,642–1,644 (C=O), 1,550–1,568 (C=C–C=O), 1,458–14,728 (C=C, Ar), and 1,200–1,100 (C–O) cm−1. 1HNMR (CDCl3, 500 MHz): δ14.30 (chelated OH), 7.86 (IH, J=15.5 Hz, Hβ), 7.53 (d, 1H, J=15.5 Hz, Hα), 7.50 (br, d, 2H, H-2,6), 7.26 (m, 3H, H-3,4,5), 5.95 (d, J=2.5 Hz, 1H, H-3′), 5.90 (d, 1H, J=2.5 Hz, H-5′), 3.90 (s, 3H, OMe, C-6′), 3.82 (s, 3H, OMe, C-4′), 2.54 (s, 3H, S-CH3). Electron-Impact Mas Spectrometry has indicated that synthesised FLS has molecular weight of 330.32 with molecular formula C18H18O4S. Molecular structures of FLS, FLA, and FLB are shown in Figure 1.
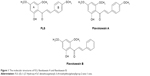  | Figure 1 The molecular structures of FLS, flavokawain A and flavokawain B. |
FLS selectively reduced the viability of MCF-7 cell
FLS provoked a time-dependent cytotoxicity against both MCF-7 and MDA-MB-231 cells with 50% reduction in a reducing concentration from 24 to 72 hours. The IC50 value of FLS on MDA-MB-231 was 1.5-fold higher than on MCF-7 indicating that FLS is more sensitive to MCF-7 (IC50 ~33 μM) than to MDA-MB-231 (IC50 ~48.5 μM) (Table 1). On contrary, no cytotoxicity against normal breast cell MCF-10A can be detected up to 180 μM. Selective index (SI) was calculated based on the IC50 value of FLS on MCF-10A comparing to MCF-7 or MDA-MB-231. FLS exhibited greater selectivity of MCF-7 (SI >5) cell than MDA-MB-231 (SI >3) against MCF-10A.
Morphology observation of MCF-7 treated with FLS
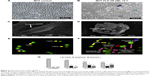
Untreated MCF-7 cells were confluent with normal morphology (Figure 2A). FLS (36 μM) reduced the cell number of MCF-7 and induced cell shrinkage after 72 hours of treatment. Also, detachment of cell was also observed (Figure 2B). Scanning electron microscopy has provided a clearer comparison between ultrastructure of untreated and FLS treated MCF-7 after 72 hours of incubation. Untreated control MCF-7 was observed with normal cell morphology with extended structure and microvilli observed indicating the control cell was healthy and stably attached on the glass slide (Figure 2C). In contrast, morphological changes related to apoptosis including cell shrinkage, irregular surface, large hole, and apoptotic body were observed in the FLS treated MCF-7 (Figure 2D). In addition, apoptosis, necrosis, and viability of MCF-7 cell treated with FLS were also investigated under fluorescent microscope after staining with acridine orange and PI. Viable cell was stained as green intact cell (Figure 2E). On the other hand, MCF-7 cell treated with FLS was observed with significant increase of apoptotic population (P<0.05), which was marked by chromatin condensation, cell shrinkage and membrane blebbing (Figure 2F). Based on random scoring of ~200 cells, untreated control MCF-7 cell was recorded with >95% of viable population. Unlike control, FLS treatment induced ~26% of apoptosis and 14.5% of necrosis or late apoptosis after 72 hours of incubation (Figure 2G).
FLS induced G2/M cell cycle arrest followed by apoptosis on MCF-7
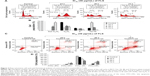
Cell cycle progression of FLS (36 μM) treated MCF-7 was examined by flow cytometry PI staining assay. Result of cell cycle analysis is summarized in Figure 3. Significant (P<0.05) G2/M arrest was noticed after 24 hours of FLS treatment. Subsequently, 48 and 72 hours of FLS treatment were found with drastic increase of sub G0/G1 population on MCF-7 indicating the possibility of DNA fragmentation to happen. To confirm the apoptotic inducing effect of FLS on MCF-7 at 48 and 72 hours, Annexin V/PI apoptosis flow cytometry was done to evaluate the late and early apoptosis indicated by externalization of phosphatidylserine with or without loss of membrane integrity (that allow PI to enter and stain the DNA), respectively. Based on the Annexin V/PI result, early apoptosis was noticed with constant increase from 9.18% to 36.72% from 24 to 72 hours. Besides, 11.86% of late apoptosis was observed after 48 hours and further increased to 17.29% at 72 hours on FLS treated MCF-7 cell (Figure 3). These results indicated that FLS treatment promoted G2/M cell cycle arrest in early time point and subsequently induced apoptosis at later time point.
FLS downregulated the mRNA expression of PLK1 and GLU1 but upregulated the mRNA expression of WEE-1 and c-Jun
Differential expression of PLK1, WEE-1, c-Jun, and GLUT on untreated control and FLS treated MCF-7 cell was quantified by qRT-PCR assay on the cDNA synthesized from the extracted RNA. The results indicated that FLS significantly (P<0.05) downregulated expression (>2 fold) of PLK1 and GLUT mRNA expression in MCF-7 cell. On the other hand, it upregulated WEE-1 expression for all evaluated time points but only significantly increased c-Jun expression after 72 hours of treatment (Figure 4).
FLS promoted the level of CDC2 phosphorylation, Bax/Bcl-2 ratio, p53, active caspases, and cytochrome c
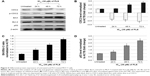
Based on the Western blot analysis, level of tumor suppressor p53 and proapoptotic Bax were upregulated in time-dependent manner. Furthermore, the level of antiapoptotic protein Bcl-2 was downregulated in the similar trend as well (Figure 5). The phosphorylation of CDC2 significantly increased with drastic reduction in CDC2 total protein levels at 24, 48, and 72 hours. Similarly, level of activated caspase 9 and cytochrome c were also detected to be significantly increased in FLS treated MCF-7 in time-dependent manner (Figure 6).
Discussion
Fighting cancer by combining knowledge of phytochemistry and synthetic chemistry has been utilized to discover potential chemotherapy agents with better selectivity against cancer than normal cell.7 Phytochemicals isolated from the natural sources have been proven with multiple bioactivities including anticancer activity but at the same time facing disadvantages such as lack of accessibility, and not being cost or time effective to obtain in large volume. These shortages can be overcome by synthetic compounds and even modified to increase the availability, bioactivities, and selectivity against cancer cell.14
Several studies have shown that chalcone is an important class for the anticancer properties against various cancer cell lines.14 Previously, we have synthesized FLA and FLB and investigated their cytotoxic effects antimetastatic effects on MCF-7 and MDA-MB-231 breast cancer cell lines in vivo and in vitro.9,10 In terms of FLA9 and FLB (Abu et al),10 they possessed greater selectivity against MDA-MB-231 (SI: 5.72 for FLA and 3.66 for FLB) than MCF-7 (SI: 3.98 for FLA and 1.33 for FLB) with IC50 on normal MCF-10A around 100 and 45 μM for FLA and FLB, respectively. Although FLA and FLB showed good cytotoxicity against MDA-MB-231 cell, their effect on MCF-7 was just marginal with poor SI especially FLB, which has lower IC50 value on normal breast cell line. In this study, flavokawain derivative (FLS) was synthesized and subjected to cytotoxicity evaluation. MTT assay indicated that synthetic FLS was more sensitive to MCF-7 than MDA-MB-231 compared to FLA and FLB (Table 1). More interestingly, FLS did not show IC50 value (>180) on normal breast MCF-10A cell thus giving the SI of 5.4 to MCF-10A/MCF-7. However, the SI of FLS against MCF-10A/MDA-MB-231 was lower than FLS against MCF-10A/MFC-7 since its cytotoxicity against MDA-MB-231 was low (Table 1). Chalcones consist of two aromatic rings (A and B) linked by a three-carbon unit αβ-unsaturated carbonyl moiety, which acts as a Michael acceptor. Apart from various substitutions on two rings, basic skeleton of chalcone (1,3-diphenyl-2-propenone) is potential in the treatment of human breast cancer.1,15–17 The difference in the structure of the compounds is the presence of methoxy (OCH3) in FLA and SCH3 (thiomethyl group) in FLS, meanwhile no substituent is present on ring B of FLB. The presence of SCH3 and OCH3 on ring B showed the contribution against maintaining cytotoxicity on MCF-7 cell but less toxic toward normal MCF-10A cell, which possibly due to the involvement of electron donating group, makes enrich scaffold αβ-unsaturated carbonyl moiety to further promote apoptosis in breast cancer MCF-7 cells but without further affecting of normal breast MCF-10A cells. Previous report by Ethiraj et al18 has shown that electron donating groups in chalcones have reduced the cytotoxic activity against cancer cell. FLB, free of electron donating methoxy or thiomethyl group, was found to be more sensitive to both breast cancer MDA-MB-231 and normal MCF-10A cell lines comparing to both FLA and FLS (Table 1). On the other hand, FLS and FLA with electron donating group possessed the advantage of less toxic in breast normal MCF-10A cell thus improved the selectivity against breast cancer.
Since FLS showed better cytotoxic potential against MCF-7 cell, the mode of cell death and cell cycle arrest induced and cell cycle arrest effects were evaluated on MCF-7 using IC50 value of FLS at 48 hours (36 μM). As indicated by the light microscope observation, reduction of cell number was recorded in FLS treated MCF-7 cell (Figure 2B). This result was further supported by the cell cycle analysis where G2/M arrest was observed as early as 24 hours post-FLS treatment. This effect might be contributed by the significant upregulation and downregulation of WEE-1 and PLK-1 in the treated cell, respectively (Figure 4). PLK1, a mitotic polo-like kinase, is a key regulator of G2/M phase progression while WEE-1 is the nuclear kinase that inhibits the entry of cell into mitosis.19 Overexpression of PLK1 is always reported in different types of cancer including breast cancer, while downregulation of PLK1 was found to link with G2/M arrest and subsequently apoptosis posttreatment with natural compound such as FLA.9 Cell cycle kinase subunit (CDC2) that binds to cyclin B1 to regulate progression from G2 to M transition was also found to be downregulated by FLS. FLS suggests that the downregulation of CDC2 protein level contributes to the increase of CDC2 phosphorylation. Suppression of PLK1 and upregulation of WEE-1 were reported to contribute to the downregulation of CDC2 via CDC2 Tyrosine-15 phosphorylation.20 Concomitant with this, similar trend was observed in FLS treated MCF-7 cell. Cyclin-dependent kinase inhibitor p21, which can be upregulated by p53, was promoted in FLA and FLB treated MCF-7 cell.9,10 In terms of FLS treated MCF-7 cell, although p53 was found to be upregulated (Figure 5), its role in the regulation of G2/M may be marginal because p21 was not significantly upregulated when evaluated using qRT-PCR (results not shown). Previous study has shown that G2/M arrest can occur with or without the involvement of tumor suppressor p53. Although the regulation of p21 in MCF-7 treated with FLS was difference comparing to FLA and FLB, all of these compounds were found to promote G2/M arrest via deregulation of PLK1.9,10,19
Apoptosis was significantly (P<0.05) enhanced in MCF-7 after 48 and 72 hours of FLS treatment in Annexin V/PI apoptosis study. The result was further supported by the morphology observations where FLS treated MCF-7 was found with typical characteristic of apoptosis including cell detach, cell shrinkage, membrane blebbing, hole, apoptotic body, and chromatin condensation (Figure 2). FLS modulated apoptosis via upregulation of p53, Bax, caspase 9, and cytochrome c. FLS induced DNA damage associated with tumor suppressor p53 upregulation. p53 was reported as a key regulator of BCL family where its upregulation was found to promote proapoptotic BAX and suppress antiapoptotic Bcl-2, which led to secretion of cytochrome c that stimulates the caspase cascade activation of cell death.21 Study has shown that mitochondria played a factor in the initiation of apoptotic process by releasing cytochrome c, apoptogenic factor from the outer mitochondria membrane space into cytosol of the apoptotic cells.22 Concomitant with this, upon cytochrome c leakage, caspase 9 was being activated. This suggests that FLS induced changes in the mitochondrial membrane potential in the early stages of apoptotic cell death via mitochondrial-dependent intrinsic pathway. In this study, raised BAX/Bcl-2 ratio, cytochrome c, and caspase 9 in the FLS treated MCF-7 indicated the involvement of FLS in promoting the intrinsic apoptosis as detected in flow cytometry and microscopic examinations. Our previous reports have shown higher sensitivity of FLA and FLB in mutant type p53 MDA-MB-231 cell than wild-type p53 MCF-7 cell. In this study, FLS was more potent in wild-type p53 MCF-7. The higher selectivity in wild-type p53 MCF-7 cell may be contributed by the exchange of SCH3 thiomethyl group in FLS. Previous study has shown that the presence of thiomethyl group improved the selectivity of the compound toward cancer cell and maintained its cytotoxicity via drastic upregulation of p53 and Bax status that subsequently promote apoptosis in leukemia cells. Similarly, regulation of FLS and this thiomethyl compound on Bcl-2 was marginal after 24 and 48 hours treatment.23
This study reveals that FLS showed good selectivity against breast cancer MCF-7 than normal MCF-10A cell line. The cytotoxicity of FLS on MCF-7 was mainly contributed by G2/M cell cycle arrest and induction of apoptosis. Downregulation of PLK1 and upregulation of WEE-1 associated with phosphorylation of CDC2 were the major regulators of the G2/M arrest. On the other hand, mitochondrial p53 pathway was found as the contributor of the FLS induced apoptosis in MCF-7 cell. The presence of SCH3 (thiomethyl group) on ring B structure might play a role in cytotoxicity and apoptosis of MCF-7 cell compared to other chalcones, FLA and FLB. Thus, FLS is more potent candidate in selectively combating MCF-7 breast cancer cell. Further in vivo study shall be carried out to evaluate the antibreast cancer efficacy of FLS comparing to FLA and FLB.
Acknowledgments
This work was jointly supported by University of Malaya HIR-MoE grant (reference number - UM.C/625/1/HIR/ MOHE/CHAN/03, account number - A000003-50001) and University Malaysia Pahang internal grants no (RDU 120373 and RDU 120389).
Disclosure
The authors report no conflict of interest in this work.
References
American Cancer Society. Global Cancer Facts and Figures. 3rd ed. Atlanta: American Cancer Society; 2015. Available from: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-044738.pdf. Accessed January 6, 2016. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. | ||
Loganathan R, Radhakrishnan AK, Selvaduray KR, Nesaretnam K. Selective anti-cancer effects of palm phytonutrients on human breast cancer cells. RSC Advances. 2015;5:1745–1753. | ||
US Mortality Data, National Center for Health Statistics, Centers for Disease Control and Prevention. American Cancer Society, Surveillance Research. Atlanta, GA: American Cancer Society; 2014. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. | ||
Badisa RB, Darling-Reed SF, Joseph P, Cooperwood JS, Latinwo LM, Goodman CB. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009;29:2993–2996. | ||
Batovska DI, Todorova IT. Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol. 2010;5:1–29. | ||
Abu N, Akhtar MN, Yeap SK, et al. Flavokawain A induces apoptosis in MCF-7 and MDA-MB231 and inhibits the metastatic process in vitro. PLoS One. 2014;9:e105244. | ||
Abu N, Akhtar M, Yeap S, et al. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases in vitro. BMC Complem Altern M. 2016;16:86. | ||
Kuo YF, Su YZ, Tseng YH, Wang SY, Wang HM, Chueh PJ. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic Biol Med. 2010;49:214–226. | ||
Tang Y, Simoneau AR, Xie J, Shahandeh B, Zi X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res (Phila). 2008;1:439–451. | ||
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. | ||
Abu N, Ali NM, Ho WY, Yeap SK, Aziz MY, Alitheen N. Damnacanthal: a promising compound as a medicinal anthraquinone. Anticancer Agents Med Chem. 2014;14:750–755. | ||
Go ML, Wu X, Liu X. Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem. 2005;12:481–499. | ||
Hsu YL, Kuo PL, Tzeng WS, Lin CC. Chalcone inhibits the proliferation of human breast cancer cell by blocking cell cycle progression and inducing apoptosis. Food Chem Toxicol. 2006;44:704–713. | ||
Sawle P, Moulton BE, Jarzykowska M, et al. Structure-activity relationships of methoxychalcones as inducers of heme oxygenase-1. Chem Res Toxicol. 2008;21:1484–1494. | ||
Ethiraj KR, Aranjani J, Khan FN. Potential cytotoxic and apoptosis inducing agents: Synthesis and evaluation of methoxy-substituted chalcones against human lung and cervical cancers. Med Chem Res. 2013;22:5408–5417. | ||
Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–924. | ||
Fletcher L, Cheng Y, Muschel RJ. Abolishment of the Tyr-15 inhibitory phosphorylation site on cdc2 reduces the radiation-induced G(2) delay, revealing a potential checkpoint in early mitosis. Cancer Res. 2002;62:241–250. | ||
Jiang X, Wang X. Cytochrome c-mediated apoptosis. Ann Rev Biochem. 2004;73:87–106. | ||
Looi CY, Arya A, Cheah FK, et al. Induction of apoptosis in human breast cancer cells via caspase pathway by vernodalin isolated from Centratherum anthelminticum (L.) seeds. PLoS One. 2013;8:e56643. | ||
Fimognari C, Nüsse M, Iori R, Cantelli-Forti G, Hrelia P. The new isothiocyanate 4-(Methylthio)Butylisothiocyanate selectively affects cell-cycle progression and apoptosis induction of human leukemia cells. Invest New Drugs. 2004;22:119–129. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.