Back to Journals » Journal of Pain Research » Volume 9
Fixed-site high-frequency transcutaneous electrical nerve stimulation for treatment of chronic low back and lower extremity pain
Authors Gozani S
Received 21 April 2016
Accepted for publication 2 June 2016
Published 28 June 2016 Volume 2016:9 Pages 469—479
DOI https://doi.org/10.2147/JPR.S111035
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael Schatman
Shai N Gozani
NeuroMetrix, Inc., Waltham, MA, USA
Objective: The objective of this study was to determine if fixed-site high-frequency transcutaneous electrical nerve stimulation (FS-TENS) is effective in treating chronic low back and lower extremity pain.
Background: Transcutaneous electrical nerve stimulation is widely used for treatment of chronic pain. General-purpose transcutaneous electrical nerve stimulation devices are designed for stimulation anywhere on the body and often cannot be used while the user is active or sleeping. FS-TENS devices are designed for placement at a pre-determined location, which enables development of a wearable device for use over extended time periods.
Methods: Study participants with chronic low back and/or lower extremity pain self-administered an FS-TENS device for 60 days. Baseline, 30-, and 60-day follow-up data were obtained through an online questionnaire. The primary outcome measure was the patient global impression of change. Pain intensity and interference were assessed using the Brief Pain Inventory. Changes in use of concomitant pain medications were evaluated with a single-item global self-rating.
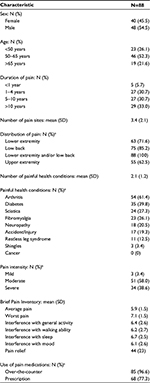
Results: One hundred and thirty participants were enrolled, with 88 completing the 60-day follow-up questionnaire. Most participants (73.9%) were 50 years of age or older. At baseline, low back pain was identified by 85.3%, lower extremity pain by 71.6%, and upper extremity pain by 62.5%. Participants reported widespread pain, at baseline, with a mean of 3.4 (standard deviation 1.1) pain sites. At the 60-day follow-up, 80.7% of participants reported that their chronic pain had improved and they were classified as responders. Baseline characteristics did not differentiate non-responders from responders. There were numerical trends toward reduced pain interference with walking ability and sleep, and greater pain relief in responders. There was a large difference in use of concomitant pain medications, with 80.3% of responders reporting a reduction compared to 11.8% of non-responders.
Conclusion: FS-TENS is a safe and effective option for treating chronic low back and lower extremity pain. These results motivate the use of FS-TENS in development of wearable analgesic devices.
Keywords: chronic pain, transcutaneous electrical nerve stimulation, wearable, patient global impression of change
Introduction
The Functioning and Disability Supplement of the 2012 National Health Interview Survey estimated that 40 million US adults have pain every day or most days, and another 87 million have pain on some days.1 Many people with chronic pain also have low quality sleep, anxiety, depression, and poor overall health.2 The annual economic cost of chronic pain is US $600 billion in the US alone.3 The past few decades have seen a dramatic increase in use of prescription opioids for chronic pain despite concerns about their adverse effects and potential for addiction.4 Therefore, there is a need for non-pharmacological options for treatment of chronic pain.
Transcutaneous electrical nerve stimulation (TENS) is the delivery of electricity across the intact surface of the skin to activate sensory nerve fibers. The technology was originally developed as a screening technique for predicting which chronic pain patients would respond to implantable stimulators. However, it became apparent that a significant percentage attained pain relief from TENS alone. Since that time, the efficacy of TENS for the treatment of chronic pain has been studied extensively. When evaluated with proper attention to methodological and technical factors,5 TENS has generally been shown to be safe and effective in various forms of chronic pain.6-11
A conceptual model for how sensory nerve stimulation leads to pain relief was proposed by Melzack and Wall in 1965.12 Their theory stipulates that activation of sensory nerves (Aβ fibers) closes a “pain gate” in the spinal cord that inhibits the transmission of pain signals carried by nociceptive afferents (C and Aδ fibers) to the brain. In the past 20 years, anatomic pathways and molecular mechanisms that may underlie the pain gate have been identified. Sensory nerve stimulation activates the descending pain inhibition system, primarily the periaqueductal gray and rostroventral medial medulla located in the midbrain and medulla sections of the brainstem respectively.13 The periaqueductal gray has neural projections to the rostroventral medial medulla, which in turn has diffuse bilateral projections into the spinal cord dorsal horn14,15 that inhibit ascending pain signal transmission.13-15
General purpose TENS (GP-TENS) devices are designed to enable stimulation essentially anywhere on the body under the assumption that analgesia is limited to the vicinity of the electrodes. However, activation of descending inhibition leads to analgesia beyond the stimulation site.16-20 This suggests an alternative approach, fixed-site high-frequency TENS (FS-TENS), in which the device is designed for a pre-determined location rather than according to the patient’s pain distribution. A priori knowledge of the anatomy and neurophysiology of a target site enables development of wearable devices that can be used for extended time without disrupting daytime activity or sleep, which is not feasible with GP-TENS. For example, the mechanical design of the device can be optimized for the specific anatomical location. Similarly, the electrode dimensions and electrical specifications can be matched to the peripheral nerves to be stimulated. An emerging benefit of FS-TENS is integration of wearable technology such as accelerometers, gyroscopes, and thermosensors.21 These measurements can provide objective feedback for therapy optimization, potentially in real-time. For example, accelerometer readings from the leg may be combined with a lower extremity biomechanical model to quantitatively track activity, falls, gait, and sleep, which are all influenced by chronic pain.22-25 Finally, FS-TENS can better co-exist with other devices, such as pacemakers,26 that may be disturbed by nearby electrical stimulation.
This study evaluated the efficacy of an FS-TENS device, placed on the upper calf, for chronic low back and lower extremity pain. The first study aim was to determine whether this FS-TENS device was effective in providing pain relief. The second aim was to identify baseline factors predictive of a positive response. The relationship between the anatomic distribution of pain and the response to FS-TENS was of particular interest. The third aim was to identify which chronic pain domains were most influenced by FS-TENS. The implications of this study are that if FS-TENS is safe and effective, then opportunities exist for development of wearable analgesic devices for treating chronic pain.
Methods
Participants
Study participants were recruited from a cohort of 300 chronic pain sufferers who participated in an online research project to evaluate attitudes about alternative approaches to pain management. Inclusion criteria for the original cohort were primary residence in the US, age 40 years or more, minimum household income of US $50,000, pain for most days during the past 3 months or longer, pain distribution that included one or more sites in the low back, legs or feet, and at least one painful health condition among diabetes, sciatica, fibromyalgia, neuropathy, shingles, and restless leg syndrome. The last condition was included because the sensory symptoms are often described as painful27,28 and because of its high association with neuropathies,29 fibromyalgia,30 and multi-site chronic pain.31 Exclusion criteria were any contraindication to use of the FS-TENS device, which included having a cardiac pacemaker, implanted defibrillator, or other implanted metallic or electronic device. Participants received US $50 compensation and the option to keep the FS-TENS device upon completing the study.
Procedures and outcome variables
Recruitment and data collection was managed by an independent research firm. Enrolled participants completed an online baseline questionnaire and were then sent a commercially available, over-the-counter (OTC), FS-TENS device. They had access to the same instructional resources as commercial users. The device instructions encourage daily use to manage pain. Participants did not receive special instructions or training. Participants self-administered the device for 60 days. At 30 and 60 days following delivery of the device, follow-up data were obtained through an online questionnaire that took about 10 minutes to complete. Participants had 1 week to complete the questionnaires, and up to two reminder emails were sent. If the questionnaire was not completed within the 1-week period then the participant was deemed lost to follow-up. De-referenced data files were provided to the study author. All participants signed digital informed consent. Institutional review board approval was not sought because the study was conducted using a commercially available OTC device consistent with its US Food and Drug Administration (FDA) cleared instructions and participant involvement was limited to voluntarily completing three online surveys. The principles outlined in the World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects, were followed.32
The baseline variables included demographics, pain characteristics, and use of pain medications. Pain was characterized by duration, anatomical distribution, painful health conditions, and a prospectively selected subset of the Brief Pain Inventory – Short Form (BPI-SF).33 The anatomical distribution was defined as one or more among nine sites that included feet, legs, lower back, chest, hands, arms, abdomen, neck, and head. Lower extremity sites included feet and legs; upper extremity sites included arms, hands, and neck. The location of the pain was not qualified as unilateral or bilateral because of the potential for contralateral secondary hyperalgesia and allodynia.34 Painful health conditions included arthritis, diabetes, sciatica, neuropathy, fibromyalgia, restless leg syndrome, shingles, cancer, and accident/injury. Cardiovascular risk factors were recorded because of their high prevalence in chronic pain,35,36 but were not treated as painful conditions. BPI-SF items included average and worst pain; interference with sleep, general activity, walking ability, and mood; and pain relief. This final item asks participants to rate the percentage relief they feel their current pain treatments provide (0% for no pain relief to 100% for complete pain relief). This item represents pain treatment satisfaction.33
Follow-up data include patient global impression of change (PGIC), the aforementioned BPI-SF items, use of concomitant pain medications, and self-reported device utilization. The primary outcome measure was the PGIC. Participants were asked to rate changes in their chronic pain and overall health since the beginning of the study using a 5-point scale comprised of: much worse, worse, no change, improved, or much improved. Responders were participants with a rating of improved or much improved. Participants reported changes in use of concomitant pain medications with a single-item global self-rating comprised of: decreased a lot, decreased a little, no change, increased a little, or increased a lot. Device utilization was self-reported as: several times a day, once or twice every day, several times a week, or several times a month.
FS-TENS device
All participants used the same FDA cleared OTC FS-TENS device (Quell®; NeuroMetrix Inc., Waltham, MA, USA). This device is comprised of a one-channel stimulator, a stretchable band to secure the stimulator to the upper calf, and an electrode (Figure 1). The electrode is an array of four hydrogel pads, connected in two pairs that provide a 60 cm2 stimulation surface area. When located on the upper calf, the electrode array is circumferential and will stimulate sensory dermatomes S2-L4 independent of rotational placement. The stimulator generates bipolar, symmetrical, current-regulated pulses with alternating leading phase polarity. The stimulation pulse has zero net current flow to prevent development of polar concentrations with extended use that may cause adverse skin reactions.37,38 The peak output voltage and current are 100 V and 100 mA, respectively. The phase duration scales from 100 to 200 μsec with the stimulation intensity. The inter-pulse intervals vary randomly such that the simulation frequency has a mean of 80 Hz with a uniform distribution between 60 and 100 Hz. High-frequency stimulation induces an elevation in enkephalins that act through the δ-opioid receptor39,40 whereas prescription opioids primarily act through the μ-opioid receptor at concentrations associated with clinical use.41 Both receptors are involved in descending pain inhibition, including in the dorsal horn of the spinal cord where they inhibit pain signal transmission.13,39 Individuals taking prescription opioids or who have developed tolerance remain responsive to high-frequency induced analgesia.42
Currently published evidence suggests that stimulation intensity directly influences the degree of analgesia in a dose-dependent fashion.43,44 Stimulation below the level of sensory perception does not produce analgesia, and the degree of analgesia is correlated to the stimulation intensity. These and other studies suggest that stimulation should be delivered at a “strong but comfortable” level. Prior to first use, the device is calibrated to the user’s sensation threshold by an algorithm using both ascending and descending method of limits. Subsequent stimulation is automatically controlled. The initial therapeutic level is set so that the pulse charge is 6 decibels above sensation. This level is typically perceived as “strong but comfortable”. The stimulation intensity is then periodically increased by an adaptive algorithm to compensate for nerve de-sensitization and to activate deep tissue sensory afferents.45 The user may also manually decrease or increase intensity. Each therapy session is 60 minutes, with sessions automatically starting every other hour. The 1-hour duration matches the time course of endogenous opioid levels in the cerebrospinal fluid in response to high-frequency peripheral nerve stimulation.46,47 These studies showed that a statistically significant increase in cerebrospinal fluid opioid concentration can be measured after 20–45 minutes of stimulation and remains elevated for 60 minutes with continued stimulation. Further stimulation beyond 60 minutes decreases opioid levels.
Chronic pain is often worse in the evening and overnight.48 Accordingly, the majority of chronic pain patients complain of disturbed sleep and daytime lethargy.49 Polysomnography studies show that compared to normal subjects, chronic pain patients have shorter sleep duration, lower sleep efficiency, and greater numbers of periodic leg movements.25 Despite these sleep abnormalities; GP-TENS is typically not used during sleep to help control pain. In fact, in the US, TENS carry an FDA mandated warning against use during sleep. The safety concern is the potential for unnoticed electrode peeling leading to small skin-electrode contact area and an inversely high current density that may cause discomfort, or in the extreme, tissue damage. The FS-TENS used in this study is cleared by the FDA for use during sleep because of its mechanical design and an algorithm that detects electrode peeling. The device also has a tri-axial accelerometer to monitor body orientation and movement, from which it determines when a user is sleeping using actigraphy methods.50 If a user is determined to be sleeping, then stimulation intensity is reduced to lessen the likelihood of disrupting sleep.
The FS-TENS device may be used with an optional smartphone program (“app”) that tracks utilization and objective sleep metrics. These data have the potential to influence study compliance, which has been identified as an issue in clinical studies of self-administered TENS.51 Although the app was available to participants, its use was not required nor assessed. Therefore its impact and potential benefits could not be assessed.
Data analysis
The study cohort was partitioned into two groups; responders and non-responders as defined in “Procedures and outcome variables” section. The primary analysis was to compare demographic characteristics, clinical variables, and pain measures between the two groups at baseline and change from baseline to study end. Baseline variables were quantified by their mean and standard deviation (SD) if continuous, and by frequency counts if categorical. Confidence intervals (CIs) for frequency counts were determined using the modified Wald method. Although BPI-SF items are ordinal variables, they were analyzed as continuous values as has typically been the practice in pain studies. Group differences among continuous and ordinal variables were evaluated by the non-parametric Mann–Whitney U test. Dichotomous categorical variables (eg, sex) were compared using the two-sample z-test. The Pearson’s chi-squared test was applied to contingency tables (eg, age categories, pain duration categories) to evaluate how likely observed differences arose by chance.
Results
A total of 130 participants were enrolled. Follow-up questionnaires were obtained for 94 (72.3%) after 30 days and 88 (67.7%) after 60 days. The only difference in baseline characteristics between participants who completed the 60-day questionnaire and for those in whom follow-up was not obtained, was that the former were less likely to be female (45.5% vs 69.4%, P=0.015). A per-protocol analysis was conducted using the 88 participants with 60-day follow-up data.
The FS-TENS device instructions encourage daily use to manage pain. At the 60-day follow-up, 55 (62.5%) reported using the device daily; with 31 (35.2%) using the device 3 or more hours per day. All participants reported using the device at least several times per week. The utilization level was unchanged from the 30-day follow-up where 58 (65.9%) reported daily use. There were no adverse events requiring termination from the study. One subject described a sore calf and another complained of skin irritation. These issues are known to occur infrequently with TENS and were resolved by temporarily stopping device usage.
Table 1 lists the baseline characteristics of the study cohort. Male sex was slightly more prevalent than female and most participants were 50 years of age or older. The study participants reported widespread pain with a mean of 3.4 pain sites. All 88 participants reported low back and/or lower extremity pain; with low back pain identified by 75 (85.3%) and lower extremity pain by 63 (71.6%). In addition, 55 (62.5%) noted upper extremity pain. Participants reported a mean of 2.1 painful health conditions. The most common were arthritis (61.4%), diabetes (39.8%), sciatica (27.3%), and fibromyalgia (26.1%).
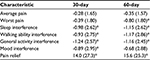
Figure 2 shows the PGIC ratings of the study participants. At the 60-day follow-up, 71 (80.7%, 95% CI 71.1%–87.7%) reported that their chronic pain and overall health was “improved” or “much improved” and were classified as responders. The remaining 17 (19.3%, 95% CI 12.3%–28.9%) reported no change or worsening and were classified as non-responders. Most of the improvement occurred within the first 30 days as the 60-day results were essentially the same as the first follow-up assessment. Table 2 shows 30-day and 60-day changes in BPI-SF items from baseline. At 60 days, there were statistically significant reductions in worst pain, pain interference with sleep, walking ability, and general activity, and increased pain relief. Most of the changes occurred within the first 30 days.
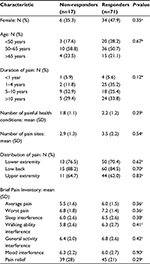
Table 3 compares baseline characteristics in non-responders and responders. There were no statistically significant differences. The total number of pain sites and proportion of participants with lower extremity, low back, and upper extremity pain were similar in the two groups. The number of painful health conditions was similar in the two groups. Inter-group differences for specific conditions were not examined because of the small sample sizes.
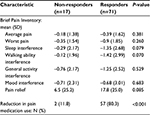
There was no difference in utilization between the non-responder and responder groups. Among the former, 53% used the device daily as compared to 65% in the latter (P=0.36). As shown in Table 4, there were numerical trends toward a greater reduction in pain interference with walking ability (P=0.071) and sleep (P=0.079), and greater pain relief (P=0.085) in responders as compared to non-responders. There was a large difference in pain medication use, with 80.3% of responders reporting a reduction (“decreased a lot” or “decreased a little”) compared to 11.8% of non-responders (P<0.001).
Discussion
This study investigated the efficacy of FS-TENS in chronic low back and lower extremity pain. An open label design was utilized because it was impractical to blind sham devices given the extensive online availability of information about TENS in general and this OTC device in particular. A total of 130 participants were enrolled, with 94 (72.3%) completing the 30-day questionnaire and 88 (67.7%) completing the 60-day questionnaire. The study used limited email contact and offered minimal financial benefits. As a result, it may not have been optimized for protocol adherence. With the exception of sex, baseline characteristics did not differentiate participants who completed the study from those who did not. About 70% of those lost to follow-up were female compared to approximately 45% who completed the study. There are sex differences in pain and analgesia,52 however it is not apparent how those differences would influence attrition in this study. The reasons for failed follow-up in the present study may have included absence of efficacy, side effects, and the inability or lack of interest in completing the online questionnaire. The relative contributions of these factors are unknown and therefore the potential for selection bias cannot be determined. In future studies, the utilization tracking capabilities of the device should be used to help differentiate true study dropouts from those who failed to complete follow-up questionnaires. Another potential source of selection bias is the inclusion criteria. The current study findings may not be generalizable to subjects under the age of 40 and those with household incomes below the inclusion threshold.
Recent epidemiological studies indicate that most chronic pain sufferers have multiple sites of pain and painful health conditions.1 The participants in this study followed a similar pattern with a mean of 2.1 (SD 1.2) painful health conditions and 3.4 (SD 1.2) pain sites. Consistent with the study design, all participants had low back and/or lower extremity pain. However, 62.5% also reported upper extremity pain despite its absence from the inclusion criteria. This distributed nature of chronic pain presents a challenge to treatment with GP-TENS, and may help explain inconsistent results reported in the clinical literature.5,11,53 In fact, there is evidence that a barrier to effective use of TENS is the amount of effort needed to regularly apply the available devices.51,54,55
Efficacy of FS-TENS
In this study, 80.7% of participants reported an improvement in their chronic pain and overall health, on a 5-point PGIC scale, after 60 days of using the FS-TENS device. PGIC is recommended as a core outcome measure in chronic pain trials56 and has been used to define clinically important differences in pain intensity57 and quality of life.58 As a global assessment, PGIC aggregates the subject’s overall experience including changes in pain intensity, changes in sleep and function, side effects, and convenience into one measure. The advantage of PGIC is that it captures the outcomes of interest to each participant. The disadvantage is that it may be unclear how the rating relates to specific aspects of pain. Moreover, it is likely that this relationship varies across participants. When investigating the efficacy of a therapy that is applied with variable dosing and scheduling, a global rating may be necessary to capture the different ways that pain may be impacted.59 As an example, if a patient’s use of TENS is primarily to facilitate sleep, then the impact on pain intensity may be limited whereas sleep quality may be improved with a positive effect on quality of life as reflected in the PGIC.
There is no direct benchmark against which to compare the responder rate in this study. Despite the frequent use of PGIC in chronic pain clinical trials,56 it has only been used in one TENS study to our knowledge.60 In that study, TENS combined with local injections of cobalamin and/or lidocaine were evaluated in post-herpetic neuralgia. Therefore, the current study appears to be the first to use PGIC to directly assess the benefit of TENS in a diverse chronic pain cohort. Nevertheless, it is instructive to review pharmacological studies with similar designs. In an 8-week non-interventional, flexible dosing study comparing pregabalin and routine clinical care for chronic low back pain with accompanying neuropathic pain, 80.8% (pregabalin) and 45.4% (routine care) of subjects reported improvement on the PGIC.61 A 4-week open-label, flexible dosing study assessing the efficacy of pregabalin in patients with diabetic peripheral neuropathy or post-herpetic neuralgia under typical clinical conditions reported improvement on PGIC of 81.0%.62 In a study of pregabalin and milnacipran in fibromyalgia, 53.0% of subjects reported improvement in PGIC during a 4–12-week open label run-in period with pregabalin.63 These studies suggest that FS-TENS mediated improvement in chronic pain, as assessed using PGIC, may be similar to pharmacological interventions.
Relationship between baseline characteristics and FS-TENS efficacy
A key objective of this study was to determine if baseline characteristics predict response to FS-TENS. This information may enhance our scientific understanding of TENS induced analgesia and has clinical implications. None of the baseline characteristics showed statistically significant differences between the responder and non-responder groups. This finding may be interpreted within the traditional view that TENS generates localized analgesia. In particular, if the FS-TENS device is limited to providing lower extremity pain relief, consistent with its upper calf location, then we must assume that this local analgesia lowered the participants’ overall chronic pain sufficiently to result in a positive PGIC rating (ie, improved or much improved). While possible, this interpretation appears unlikely. First, lower extremity sites accounted for only 31.4% (SD 29.1%) of the pain sites among responders. Second, 29.6% of responders did not report any lower extremity pain sites. A more likely explanation is that a portion of the FS-TENS benefit was from analgesia outside of the stimulation area, either proximally in the low back region or extra-segmentally in the upper extremity. These analgesic effects are likely secondary to activations of descending pain inhibition.13,20 In support of this hypothesis, prior studies of experimental and chronic pain have demonstrated contralateral, proximal, and extrasegmental analgesia with TENS.64-71 Another possibility is a TENS induced reduction in sympathetic tone,72,73 because chronic pain is often associated with sympathetic overactivity.74-76 Additional mechanisms by which focal stimulation may evoke widespread analgesia are through reversal of maladaptive changes in the central nervous system (ie, central sensitization)77 and in the functional interactions between the cardiovascular and pain regulation systems.78
The fact that baseline characteristics did not predict response to FS-TENS is consistent with prior studies which have reported few reliable predictors of TENS response.79,80 Johnson et al used questionnaires to follow 179 long-term TENS users and were unable to find any correlation between patient characteristics and analgesic efficacy.80 Predicting the effect of TENS appears to depend on the choice of outcome measure.81 In one of the few studies that did identify predictors of TENS analgesia, Koke et al82 reported higher patient expectations, non-severe pain, and neuropathic pain as predictors of long-term (6 months) TENS use. They also reported a limited pain distribution (defined as ≤2 sites) and intermittent pain as predictors of a clinically important (≥33%) reduction in pain. These results contrast with those in the current study in which the number of pain sites and pain severity were not predictive of an analgesic response. However, the two studies cannot be directly compared due to differences in outcome measures, stimulation parameters, therapy period, and electrode size and placement.
Pain domains impacted by FS-TENS
The primary outcome measure in this study was PGIC, which represents the participant’s overall assessment of changes in their chronic pain over the course of the study. An important ancillary objective is to identify those pain domains that improve with therapy and ostensibly integrate to yield a positive PGIC rating. Gladwell et al59 showed that subjects with chronic pain utilize TENS in disparate and occasionally surprising ways. This apportioning of the beneficial effects of TENS across multiple pain domains may complicate efforts to demonstrate its efficacy. Despite these challenges, the results from our study support several conclusions about how pain is impacted by FS-TENS. First, the influence on pain interference is greater than on pain intensity. In particular, interference with sleep and walking ability showed numerical trends toward a greater reduction in the responders. Similarly, pain relief showed a numerical trend toward greater improvement in the responder group. These findings are particularly relevant to the design of future studies. Most TENS studies have used changes in pain intensity as the primary outcome measure. However, the current and other studies59 suggest that pain intensity may not efficiently represent the impact of TENS. In fact, the focus on pain intensity may have contributed to low fidelity studies that have contributed to confusion regarding the clinical effectiveness of TENS.5
Changes in the use of concomitant pain medications are an important outcome measure in chronic pain clinical trials.56 There is no accepted standard method for measuring medication use. Patient self-reports are commonly used in both clinical and research settings. Although straightforward, studies show that these approaches can measure medication use effectively.83 In this study, changes in pain medications were recorded using a single-item global self-rating that ranged from “decreased a lot” to “increased a lot”. A large difference between responders and non-responders was found, with 80.3% of the former group reporting a reduction (“decreased a lot” or “decreased a little”) in pain medication use compared to 11.8% of non-responders (P<0.001). The design of this study as a series of online surveys made it challenging to monitor pain medication use. We were therefore unable to determine if specific classes of analgesics were most likely to be affected or to quantify changes in medication use. Nevertheless, these results mirror TENS associated reductions in post-surgical analgesic use.84
Summary
The results from this study suggest that FS-TENS is a safe and effective option for treating chronic low back and lower extremity pain. The most significant impact was a decrease in the use of concomitant pain medications. In addition, trends toward reduced interference with walking ability and sleep, and greater pain relief were observed. These results further motivate the use of FS-TENS in development of wearable analgesic devices. The study results also support the hypothesis that the effects of TENS can be widespread, most likely arising from activation of descending pain inhibition.
Disclosure
Dr Shai N Gozani is an employee and shareholder of NeuroMetrix, Inc., which funded this study. The author has no other conflicts of interest to disclose.
References
Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769-780. | ||
Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain. 2011;152(6):1249-1255. | ||
Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724. | ||
Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011;377(9784):2226-2235. | ||
Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain. 2011;152(6):1226-1232. | ||
Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain. 2007;130(1-2):157-165. | ||
Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4(3):197-209. | ||
Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother. 2011;11(5):735-753. | ||
Jin DM, Xu Y, Geng DF, Yan TB. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2010;89(1):10-15. | ||
Cruccu G, Aziz TZ, Garcia-Larrea L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14(9):952-970. | ||
Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013;93(10):1397-1402. | ||
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-979. | ||
DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10(6):492-499. | ||
Zemlan FP, Behbehani MM, Beckstead RM. Ascending and descending projections from nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat. Brain Res. 1984;292(2):207-220. | ||
Antal M, Petko M, Polgar E, Heizmann CW, Storm-Mathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73(2):509-518. | ||
Ainsworth L, Budelier K, Clinesmith M, et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120(1-2):182-187. | ||
Somers DL, Clemente FR. Contralateral high or a combination of high- and low-frequency transcutaneous electrical nerve stimulation reduces mechanical allodynia and alters dorsal horn neurotransmitter content in neuropathic rats. J Pain. 2009;10(2):221-229. | ||
Chan CW, Tsang H. Inhibition of the human flexion reflex by low intensity, high frequency transcutaneous electrical nerve stimulation (TENS) has a gradual onset and offset. Pain. 1987;28(2):239-253. | ||
Dean J, Bowsher D, Johnson MI. The effects of unilateral transcutaneous electrical nerve stimulation of the median nerve on bilateral somatosensory thresholds. Clin Physiol Funct Imaging. 2006;26(5):314-318. | ||
Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554-2562. | ||
Piwek L, Ellis DA, Andrews S, Joinson A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016;13(2):e1001953. | ||
Patel KV, Dansie EJ, Turk DC. Impact of chronic musculoskeletal pain on objectively measured daily physical activity: a review of current findings. Pain Manag. 2013;3(6):467-474. | ||
Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302(20):2214-2221. | ||
Lalli P, Chan A, Garven A, et al. Increased gait variability in diabetes mellitus patients with neuropathic pain. J Diabetes Complications. 2013;27(3):248-254. | ||
Okura K, Lavigne GJ, Huynh N, Manzini C, Fillipini D, Montplaisir JY. Comparison of sleep variables between chronic widespread musculoskeletal pain, insomnia, periodic leg movements syndrome and control subjects in a clinical sleep medicine practice. Sleep Med. 2008;9(4):352-361. | ||
Carlson T, Andrell P, Ekre O, et al. Interference of transcutaneous electrical nerve stimulation with permanent ventricular stimulation: a new clinical problem? Europace. 2009;11(3):364-369. | ||
Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165(11):1286-1292. | ||
Cho YW, Song ML, Earley CJ, Allen RP. Prevalence and clinical characteristics of patients with restless legs syndrome with painful symptoms. Sleep Med. 2015;16(6):775-778. | ||
Lopes LA, Lins Cde M, Adeodato VG, et al. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28(11):2633-2636. | ||
Viola-Saltzman M, Watson NF, Bogart A, Goldberg J, Buchwald D. High prevalence of restless legs syndrome among patients with fibromyalgia: a controlled cross-sectional study. J Clin Sleep Med. 2010;6(5):423-427. | ||
Stehlik R, Ulfberg J, Hedner J, Grote L. High prevalence of restless legs syndrome among women with multi-site pain: a population-based study in Dalarna, Sweden. Eur J Pain. 2014;18(10):1402-1409. | ||
Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695-713. | ||
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138. | ||
Shenker NG, Haigh RC, Mapp PI, Harris N, Blake DR. Contralateral hyperalgesia and allodynia following intradermal capsaicin injection in man. Rheumatology (Oxford). 2008;47(9):1417-1421. | ||
Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113(3):331-339. | ||
Burns JW, Quartana PJ, Bruehl S, et al. Chronic pain, body mass index and cardiovascular disease risk factors: tests of moderation, unique and shared relationships in the Study of Women’s Health Across the Nation (SWAN). J Behav Med. 2015;38(2):372-383. | ||
Kantor G, Alon G, Ho HS. The effects of selected stimulus waveforms on pulse and phase characteristics at sensory and motor thresholds. Phys Ther. 1994;74(10):951-962. | ||
Fary RE, Briffa NK. Monophasic electrical stimulation produces high rates of adverse skin reactions in healthy subjects. Physiother Theory Pract. 2011;27(3):246-251. | ||
Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289(2):840-846. | ||
Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain. 2010;151(1):215-219. | ||
Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363-1381. | ||
Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain. 2011;12(2):213-221. | ||
Claydon LS, Chesterton LS, Barlas P, Sim J. Dose-specific effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: a systematic review. Clin J Pain. 2011;27(7):635-647. | ||
Moran F, Leonard T, Hawthorne S, et al. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12(8):929-935. | ||
Pantaleao MA, Laurino MF, Gallego NL, et al. Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia. J Pain. 2011;12(5):581-590. | ||
Salar G, Job I, Mingrino S, Bosio A, Trabucchi M. Effect of transcutaneous electrotherapy on CSF beta-endorphin content in patients without pain problems. Pain. 1981;10(2):169-172. | ||
Almay BG, Johansson F, von Knorring L, Sakurada T, Terenius L. Long-term high frequency transcutaneous electrical nerve stimulation (hi-TNS) in chronic pain. Clinical response and effects on CSF-endorphins, monoamine metabolites, substance P-like immunoreactivity (SPLI) and pain measures. J Psychosom Res. 1985;29(3):247-257. | ||
Gilron I, Bailey JM, Vandenkerkhof EG. Chronobiological characteristics of neuropathic pain: clinical predictors of diurnal pain rhythmicity. Clin J Pain. 2013;29(9):755-759. | ||
McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2002;7(2):75-79. | ||
Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259-267. | ||
Pallett EJ, Rentowl P, Johnson MI, Watson PJ. Implementation fidelity of self-administered transcutaneous electrical nerve stimulation (TENS) in patients with chronic back pain: an observational study. Clin J Pain. 2014;30(3):224-231. | ||
Greenspan JD, Craft RM, LeResche L, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 Suppl 1:S26-S45. | ||
Johnson MI, Walsh DM. Pain: continued uncertainty of TENS’ effectiveness for pain relief. Nat Rev Rheumatol. 2010;6(6):314-316. | ||
Davies HT, Crombie IK, Brown JH, Martin C. Diminishing returns or appropriate treatment strategy?--an analysis of short-term outcomes after pain clinic treatment. Pain. 1997;70(2-3):203-208. | ||
Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102(1-2):195-201. | ||
Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. | ||
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158. | ||
Hagg O, Fritzell P, Nordwall A; Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12(1):12-20. | ||
Gladwell PW, Badlan K, Cramp F, Palmer S. Direct and Indirect Benefits Reported by Users of Transcutaneous Electrical Nerve Stimulation for Chronic Musculoskeletal Pain: Qualitative Exploration Using Patient Interviews. Phys Ther. 2015;95(11):1518-1528. | ||
Xu G, Feng Y, Tang WZ, Lv ZW. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: a single-center randomized controlled trial. Am J Phys Med Rehabil. 2014;93(4):287-298. | ||
Taguchi T, Igarashi A, Watt S, et al. Effectiveness of pregabalin for the treatment of chronic low back pain with accompanying lower limb pain (neuropathic component): a non-interventional study in Japan. J Pain Res. 2015;8:487-497. | ||
Baron R, Brunnmuller U, Brasser M, May M, Binder A. Efficacy and safety of pregabalin in patients with diabetic peripheral neuropathy or postherpetic neuralgia: Open-label, non-comparative, flexible-dose study. Eur J Pain. 2008;12(7):850-858. | ||
Mease PJ, Farmer MV, Palmer RH, Gendreau RM, Trugman JM, Wang Y. Milnacipran combined with pregabalin in fibromyalgia: a randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther Adv Musculoskelet Dis. 2013;5(3):113-126. | ||
Brown L, Tabasam G, Bjordal JM, Johnson MI. An investigation into the effect of electrode placement of transcutaneous electrical nerve stimulation (TENS) on experimentally induced ischemic pain in healthy human participants. Clin J Pain. 2007;23(9):735-743. | ||
Tanaka K, Ikeuchi M, Izumi M, et al. Effects of two different intensities of transcutaneous electrical nerve stimulation on pain thresholds of contralateral muscles in healthy subjects. J Phys Ther Sci. 2015;27(9):2771-2774. | ||
Tsang HH. Diffuse Inhibition of Flexion Reflex by Transcutaneous Electrical Nerve Stimulation (Tens) in Man. [theses]. Montreal: McGill University; 1986. | ||
Rao VR, Wolf SL, Gersh MR. Examination of electrode placements and stimulating parameters in treating chronic pain with conventional transcutaneous electrical nerve stimulation (TENS). Pain. 1981;11(1):37-47. | ||
Zoppi M, Francini F, Maresca M, Procacci P. Changes of cutaneous sensory thresholds induced by non-painful transcutaneous electrical nerve stimulation in normal subjects and in subjects with chronic pain. J Neurol Neurosurg Psychiatry. 1981;44(8):708-717. | ||
Katz J, France C, Melzack R. An association between phantom limb sensations and stump skin conductance during transcutaneous electrical nerve stimulation (TENS) applied to the contralateral leg: a case study. Pain. 1989;36(3):367-377. | ||
Kawamura H, Ito K, Yamamoto M, et al. The Transcutaneous Electrical Nerve Stimulation Applied to Contralateral Limbs for the Phantom Limb Pain. J Phys Ther Sci. 1997;9(2):71-76. | ||
Giuffrida O, Simpson L, Halligan PW. Contralateral stimulation, using TENS, of phantom limb pain: two confirmatory cases. Pain Med. 2010;11(1):133-141. | ||
Abram SE, Asiddao CB, Reynolds AC. Increased skin temperature during transcutaneous electrical stimulation. Anesth Analg. 1980;59(1):22-25. | ||
Kaada B. Vasodilation induced by transcutaneous nerve stimulation in peripheral ischemia (Raynaud’s phenomenon and diabetic polyneuropathy). Eur Heart J. 1982;3(4):303-314. | ||
Wasner G. Vasomotor disturbances in complex regional pain syndrome--a review. Pain Med. 2010;11(8):1267-1273. | ||
Martinez-Lavin M. Is fibromyalgia a generalized reflex sympathetic dystrophy? Clin Exp Rheumatol. 2001;19(1):1-3. | ||
Zamuner AR, Barbic F, Dipaola F, et al. Relationship between sympathetic activity and pain intensity in fibromyalgia. Clin Exp Rheumatol. 2015;33(1 Suppl 88):S53-S57. | ||
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2-S15. | ||
Sacco M, Meschi M, Regolisti G, et al. The relationship between blood pressure and pain. J Clin Hypertens (Greenwich). 2013;15(8):600-605. | ||
Bates JA, Nathan PW. Transcutaneous electrical nerve stimulation for chronic pain. Anaesthesia. 1980;35(8):817-822. | ||
Johnson MI, Ashton CH, Thompson JW. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain. 1991;44(3):221-229. | ||
Oosterhof J, Samwel HJ, de Boo TM, Wilder-Smith OH, Oostendorp RA, Crul BJ. Predicting outcome of TENS in chronic pain: a prospective, randomized, placebo controlled trial. Pain. 2008;136(1-2):11-20. | ||
Koke AJ, Smeets RJ, Perez RS, et al. Can we “predict” long-term outcome for ambulatory transcutaneous electrical nerve stimulation in patients with chronic pain? Pain Pract. 2015;15(3):256-264. | ||
Gonzalez JS, Schneider HE, Wexler DJ, et al. Validity of medication adherence self-reports in adults with type 2 diabetes. Diabetes Care. 2013;36(4):831-837. | ||
Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7(2):181-188. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.