Back to Journals » Infection and Drug Resistance » Volume 16
First Emergence of NDM-5 and OqxAB Efflux Pumps Among Multidrug-Resistant Klebsiella pneumoniae Isolated from Pediatric Patients in Assiut, Egypt
Authors Abdelbary ER, Elsaghier AM , Abd El-Baky RM , Waly NG, Ramadan M, Abd- Elsamea FS, Ali ME, Alzahrani HA, Salah M
Received 1 June 2023
Accepted for publication 16 August 2023
Published 8 September 2023 Volume 2023:16 Pages 5965—5976
DOI https://doi.org/10.2147/IDR.S421978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Eman R Abdelbary,1 Ashraf M Elsaghier,2 Rehab M Abd El-Baky,3,4 Nancy GFM Waly,3 Mohammed Ramadan,1 Fatma S Abd- Elsamea,5 Mohamed E Ali,1 Hayat A Alzahrani,6 Mohammed Salah7
1Microbiology and Immunology Department, Faculty of Pharmacy, Al-Azhar University-Assiut Branch, Assiut, 11651, Egypt; 2Gastroenterology and Hepatology Unit, University Children Hospital, Faculty of Medicine, Assiut University, Assiut, 11651, Egypt; 3Microbiology and Immunology Department, Faculty of Pharmacy, Minia University, Minia, 61519, Egypt; 4Microbiology and Immunology Department, Faculty of Pharmacy, Deraya University, Minia, 11566, Egypt; 5Medical Microbiology and Immunology Department, Faculty of Medicine, Assiut University, Assiut, 11651, Egypt; 6Department of Medical Laboratory Technology, Faculty of Applied Medical Science, Northern Border University, Arar, 91431, Saudi Arabia; 7Microbiology and Immunology Department, Faculty of Pharmacy, Port Said University, Port Said City, 42526, Egypt
Correspondence: Rehab M Abd El-Baky, Email [email protected]
Introduction: New Delhi metallo-β-lactamase (NDM)-producing K. pneumoniae poses a high risk, especially among Egyptian pediatric patients who consume carbapenems antibiotics very widely and without adequate diagnostic sources. In addition, presence of efflux pump genes such as OqxAB increases resistance against many groups of antimicrobials which exacerbates the problem faced for human health. This study aimed to determine NDM variants among K. pneumoniae strains isolated from pediatric patients in Egypt, analyze the presence of OqxAB genes, and molecular characterization of blaNDM-5-positive K. pneumoniae.
Methods: Fifty-six K. pneumoniae isolates were recovered from pediatric patients, and tested for carbapenemase by modified carbapenem inactivation methods (mCIM) test. Minimum inhibitory concentrations of meropenem and colistin were determined by meropenem E-test strips and broth microdilution, respectively. PCR was used for the detection of the resistant genes (ESBL gene (blaCTX-M), carbapenemase genes (blaNDM, blaKPC) colistin resistant (mcr1, mcr2)) and genes for efflux pump (oqxA and oqxB). BlaNDM was sequenced. The effect of efflux pump in NDM-5-producing isolates was assessed by measuring MIC of ciprofloxacin and meropenem before and after exposure to the carbonyl cyanide 3-chlorophenylhydrazone (CCCP). The horizontal gene transfer ability of blaNDM-5 was determined using liquid mating assay and PCR-based replicon typing (PBRT) was done to determine the major plasmid incompatibility group.
Results: Twenty-nine isolates were positive for blaNDM-1, nine isolates were positive for blaNDM-5, and 15 isolates were positive for blaKPC. There is a significant increase of meropenem MIC of NDM-5-positive isolates compared with NDM-1-positive isolates. In addition, 38 isolates were positive for CTX-M, and 15 isolates were positive for mcr1. Both OqxA and OqxB were detected in 26 isolates and 13 isolates were positive for OqxA while 11 isolates were positive for OqxB only. All NDM-5-producing isolates except one isolate could transfer their plasmids by conjugation to their corresponding transconjugants (E. coli J53). Plasmid replicon typing showed that FII was predominant in NDM-5-producing K. pneumoniae. Similar strains were found between the three isolates and similarity was also detected between the two isolates.
Conclusion: The highly resistant K. pneumoniae producing blaNDM-5 type was firstly isolated from pediatric patients. The association of efflux pump genes such as OqxAB is involved in resistance to ciprofloxacin. This highlighted the severity risk of blaNDM-5-positive K. pneumonia as it could transfer blaNDM-5 to other bacteria and has more resistance against carbapenems. This underlines the importance of continuous monitoring of infection control guidelines, and the urgent need for a national antimicrobial stewardship plan in Egyptian hospitals.
Keywords: blaNDM-5, OqxAB, carbapenem resistant K. pneumoniae, efflux pumps
Introduction
Currently, there is increased awareness of the impact of Gram-negative bacteria that are resistant to carbapenem as it is the last choice treatment for bacterial infections.1,2 Enterobacteriaceae exhibit resistance to carbapenems by three possible mechanisms: production of carbapenemase enzymes, efflux pump activity, and porin loss.3,4 Carbapenemase production constitutes the basic mechanism of carbapenem resistance and study of this mechanism is essential for the best choice of recently effective antibiotics.5,6
New Delhi metallo-β-lactamase (NDM) is considered the latest threat for public health and numerous variants of NDM-type carbapenemases have been identified among Gram-negative bacteria worldwide.7 Among them, NDM-5 was a variant with increased carbapenemase activity in comparison with NDM-1.8 The blaNDM-5 gene has been identified in E. coli and Klebsiella pneumoniae from many countries.9 In addition, blaNDM-5 was reported to be carried on different incompatibility typing plasmids that are responsible for transferring as IncF, IncN and IncX3.10 These plasmids can facilitate the dissemination of blaNDM-5 among the members of Enterobacteriaceae through horizontal gene transfer11 that cause the bacterial cells to become a severely hazardous strain.12
Efflux pumps are found in nearly all bacteria and are responsible for mediating resistance to antimicrobials by reducing intracellular concentration of antibiotics and promoting site mutation accumulation. Presence of OqxAB efflux pump confers resistance to many drugs such as quinolones, quinoxalines, tigecycline, chloramphenicol and nitrofurantoin.13,14 Efflux pumps are considered as one of the key mechanisms of antibiotic resistance in K. pneumoniae isolates. OqxAB, encoded by the oqxA and oqxB genes, is a plasmid-encoded multi-drug efflux pump first identified in E. coli (located on a conjugative plasmid). Their presence was found to develop multi-drug resistance against different groups of antimicrobials, detergents, and disinfectants and on the chromosome of K. pneumoniae.15 This study aimed to determine NDM variants among K. pneumoniae strains isolated from pediatric patients in Assiut governorate, Egypt, analyze the presence of OqxAB genes and carry out the molecular characterization of blaNDM-5-producing K. pneumoniae.
To the best of our knowledge, this study is the first to isolate K. pneumoniae carrying blaNDM-5 and OqxAB from pediatric patients in Egypt.
Materials and Methods
K. pneumoniae Isolates and Identification
Fifty-six K. pneumoniae isolates were isolated from pediatric patients admitted to different departments at Assiut University Children’s Hospital over a period of 6 months, from September 2022 to February 2023. This study was approved by scientific research ethics committee at the faculty of pharmacy, Al-Azhar University, Egypt (reference number of ZA-AS/PH/12/C/2022). K. pneumoniae were isolated from blood samples, sputum, endotracheal aspirate samples, urine samples, stool, wound or throat swabs. Isolates were identified by API20E kit (BioMerieux, Marcy L Etoile. France). Demographic and clinical data of patients were obtained from the hospital information system.
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility of the isolated K. pneumoniae was tested by the Kirby-Bauer disc diffusion method according to recommendations of the clinical laboratory standards institute.16 The following 14 commercial antimicrobial discs were used: ampicillin (10 μg), amoxicillin-clavulanic acid (20/10 μg), piperacillin (100 μg), ceftriaxone (30 μg), ceftazidime (30 μg), cefoperazone (75 μg), meropenem (10 μg), trimethoprim/sulfamethoxazole (1.25/23.75 μg), aztreonam (30 μg), amikacin (30 μg), nitrofurantoin (300 μg), levofloxacin (5 μg), tetracycline (30 μg), and cefepime (30 μg). The E. coli ATCC 25922 isolate was used for quality control. The test was repeated twice for each isolate.
Detection of Carbapenemase-Producing Isolates
Isolates were considered carbapenemase producing by modified carbapenem inactivation methods (mCIM) test.17 In addition, the minimum inhibitory concentrations (MIC) of meropenem against the tested K. pneumoniae were tested using Meropenem E-test strips (bioMérieux, Solna, Sweden).
Phenotypic Evaluation of Colistin Resistant K. pneumoniae
Minimum inhibitory concentrations (MICs) were determined by broth microdilution method according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for colistin against Enterobacteriaceae.18
PCR for Detection of Resistant Genes
DNA was extracted from overnight grown culture using Wizard Genomic DNA Purification Kit (Promega, WI, USA). The extracted DNA was stored at −20°C. K. pneumoniae isolates were tested by PCR for the detection of resistant genes. K. pneumoniae isolates were analyzed for the presence of the ESBL gene (blaCTX-M), carbapenemase genes (blaNDM, blaKPC)19 and for colistin resistant genes (mcr1, mcr2).20,21 Amplified DNA fragments of blaNDM were purified using QIAquick PCR Purification Kit (QIAGEN, Crawley, UK) and sequenced in both directions. Nucleotide and deduced amino acid sequences were analyzed and compared by BLAST, as implemented by the National Center for Biotechnology Information website (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Detection of oqxA and oqxB Genes Efflux Pump
The oqxA and oqxB genes were screened using a PCR-based technique. The presence of oqxA and oqxB was detected using primers oqxAF (5′-CTCGGCGCGATGATGCT-3′) and oqxAR (5′-CCACTCTTCACGGGAGACGA-3′), with products of 392 bp, and oqxBs (5′-TTCTCCCCCGGCGGGAAGTAC-3′) and oqxBa2 (5′-CTCGGCCATTTTGGCGCGTA-3′), with products of 512 bp, as described previously.15
Statistical Analysis
R-packages (version 4.2.3) were used in data analysis and visualization. Wilcoxon rank sum test and Mann–Whitney U-test were used to compare meropenem MIC between NDM-5 and NDM-1-positive isolates and meropenem MIC before and after addition of CCCP, respectively. P-value of less than 0.05 was regarded as significant. Clustering of samples was elucidated based on the Euclidean distance matrix.
Phenotypic Evaluation of the Efflux Pump in NDM-5-Producing K. pneumoniae
Phenotypic evaluation of the efflux pump in NDM-5-producing K. pneumoniae was assessed by measuring the minimum inhibitory concentrations (MICs) for ciprofloxacin and meropenem before and after exposure to the efflux pump inhibitor (EPI), carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Sigma-Aldrich, Dorset, UK) at a concentration of 20 mg/L. If the MIC values decreased 4-fold or greater in the presence of EPI, this was defined as a significant inhibition effect.22
Conjugation Experiment and Plasmid Replicon Typing
The horizontal gene transfer ability of blaNDM-5 was determined using liquid mating assay for nine K. pneumoniae isolates that harbored blaNDM-5. E. coli J53 was used as recipient isolate, and transconjugants selection was performed on MacConkey agar plates containing meropenem (2 µg/mL) and sodium azide (100 µg/mL). Transconjugants were tested for blaNDM-5 by PCR.23 PCR-based replicon typing (PBRT) was done to determine the major plasmid incompatibility group by using 14 pairs of primers in which multiplex PCR for FII, FIA and FIB was performed while 11 simplex PCRs were performed for IncL, IncM, IncT, FIC, FIIK, IncN, IncX3, IncH12, IncW, IncY and IncA/C.24
Genotyping by ERIC
PCR amplification was performed using the primers ERIC1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) for K. pneumoniae isolates.25 The gel bands were presented by PyElph version 1.4.26 The dendrogram was computed by the unweighted pair group method with arithmetic averages (UPGMA).
Results
Bacterial Isolates and Patient Details
Fifty-six K. pneumoniae isolates recovered from 56 (male 31, female 25) pediatric patients were analyzed; 21 patients were from chest unit and eight from gastroenterology, six from the intensive care unit, five from neurology, and five from hematology wards. Most of the isolates were recovered from endotracheal aspirate (25%, 14/56), followed by sputum (23.21%, 13/56) and blood (21.42%, 12/56). The clinical details of all patients are given in Table 1.
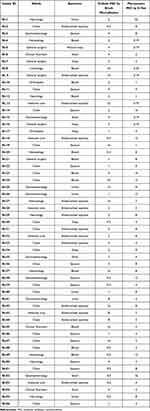 |
Table 1 Clinical Details and MIC of Colistin and Meropenem for All Isolates |
Antimicrobial Susceptibility Testing
The antibacterial susceptibility profiles to all isolates showed that all tested isolates (n = 56) were resistant to ampicillin, amoxicillin-clavulanate, piperacillin, ceftriaxone, ceftazidime, cefoperazone and aztreonam (Figure 1).
 |
Figure 1 Antibacterial resistance pattern of K. pneumoniae isolates. |
Isolates Producing Carbapenemase
mCIM test showed that 46 isolates (82.1%) of the tested K. pneumoniae were carbapenemase enzyme producers.
The MIC Values for Colistin and Meropenem
It was found that 67.85% (38/56) of K. pneumoniae isolates were meropenem resistant [MIC ≥4 μg/mL], 17.85% (10/56) isolates were meropenem sensitive [MIC ≤1 μg/mL] and 14.28% (8/56) isolates were meropenem intermediate [MIC >1<4 μg/mL] as illustrated in Table 1.
MIC values for all 56 isolates for colistin by broth microdilution ranged from 0.5–32 µg/mL and meropenem MIC by E-test ranged from 0.19–32 µg/mL (Table 1). There is a significant increase of meropenem MIC of NDM-5-positive isolates (P-value = 1.704e-14) than MIC of NDM-1-positive isolates.
Detection of Resistant Genes and OqxAB by PCR
Out of 56 isolates, 29 isolates were positive for blaNDM-1, 9 isolates were positive for blaNDM-5, and 15 isolates were positive for blaKPC carbapenemase genes. In addition, 38 isolates were positive for blaCTX-M ESBL gene, and 15 isolates were positive for mcr1. Both OqxA and OqxB were detected in 26 isolates. Thirteen isolates were positive for OqxA only and 11 isolates were positive for OqxB only (Figure 2).
Effect of the Efflux Pump in NDM-5-Producing K. pneumoniae
MIC of ciprofloxacin in NDM-5-producing K. pneumoniae ranged from 4–10 µg/mL due to the inhibition of efflux pump by CCCP. Eight of the nine (8/9) NDM-5-producing K. pneumoniae had 4-fold or more decrease in MIC after CCCP exposure. Only isolates St-26 and St-42 had an MIC fold change of 2 after CCCP addition (P-value = 0.00042). Conversely, no change in MIC of meropenem after CCCP addition was observed (Table 2).
 |
Table 2 Characterization of NDM-5-Producing K. pneumoniae |
Plasmid Replicon Typing and Conjugation Experiment
All NDM-5-producing isolates except one isolate (St-29) could transfer their plasmids by conjugation to their corresponding transconjugants (E. coli J53). The blaNDM-5 gene was located on six different types of plasmids (FII, FIIK, FIB, FIC, L and Inc M) and the FII type was predominant in NDM-5-producing K. pneumoniae (Table 2).
Genotyping by ERIC
Dendrogram of NDM-5-producing K. pneumoniae presented by PyElph showed that similar strains were found between these isolates (St-37, St-29, St-40) from hematology, neurology and chest and similarity was also detected between two isolates (St-42, St26) from the chest and gastroenterology departments (Figure 3).
 |
Figure 3 Dendrogram of NDM-5-producing K. pneumoniae presented by PyElph. |
Discussion
Carbapenem antibiotics are used in the treatment of infections caused by multi-drug resistant K. pneumoniae. However, the emergence and spread of NDM-producing K. pneumoniae has been a serious challenge to manage in the clinic because of the rapid worldwide spread of multi-drug resistance.27 As one main type of carbapenemases, New Delhi metallo-β-lactamase (NDM) can resist almost all β-lactams, including carbapenems. NDM-5, compared with NDM-1, has two amino-acid substitutions (Val88→Leu) and (Met154→Leu), which confer enhanced hydrolytic activity against carbapenems.11 Most studies focus on the dissemination of carbapenemase-producing Gram negative strains among adult patients more than from children. There was a spread of carbapenem-resistant K. pneumoniae and endemicity of NDM was reported in many hospitals of our region.28,29
In this study 56 K. pneumoniae isolates were recovered from hospitalized pediatric patients at Assiut University Children’s Hospital. Mostly patients were from the chest unit and most of the specimens were recovered from endotracheal aspirate. We observed previously that many MDR K. pneumoniae isolates can maintain and spread easily in the ward of PICU of this hospital.30 So, it is essential to estimate molecular characterization of this pathogen from other wards in the same hospital and to show the responsibility of this pathogen to cause a great challenge for infection monitoring among pediatric patients. The antibacterial susceptibility profiles of all isolates showed that all tested isolates were multi-drug resistant as each isolate were resistant to at least three different classes of antimicrobial agents.31
Among the 56 K. pneumoniae isolates, 46 isolates were confirmed phenotypically to produce carbapenemase by mCIM. However, only 40 isolates were found to produce carbapenemase genes by PCR and six other isolates exhibited other unknown mechanisms of carbapenem-resistance. The high level of production of carbapenemase genes among the isolates is that mostly of pediatric patients treated with carbapenem empirically. Sequencing of NDM-producing isolates revealed that the majority of blaNDM genes were blaNDM-1 type as with many studies32–34 and we found nine blaNDM-5 type. It was the first to isolate NDM-5 type from Klebsiella pneumoniae isolates in Egypt but recovered previously from Escherichia coli isolates with associated OXA-181.35 There is a significantly higher meropenem MIC of NDM-5-positive isolates than of NDM-1-positive isolates. NDM-5-producing strains (containing the V88L substitution) have been reported repeatedly to exhibit higher MIC against carbapenem than those against NDM-1-producing strains which suggests that this substitution may have a significant impact on carbapenemase activity even though not being located at the active site, and the mechanism of enhanced activity remains uncertain.8 NDM-5-positive isolates are continuing to be a critical challenge for treatment and the continuous monitoring of infection control policies is critically important.
Out of 56 isolates, 15 colistin-resistant isolates were positive for mcr-1 gene (26.7%) but mcr-2 gene was not detected at all among the isolated K. pneumoniae as colistin is not used in this hospital to treat infections. Moreover, extended spectrum b-lactamase was shown by the tested isolates as 38 isolates were positive for blaCTX-M gene. The present study revealed massive co-existence of different resistance genes among the tested isolates which may greatly contribute to the observed raised variability in resistance genotypes among K. pneumoniae in Egypt.36,37
It was known that efflux pump is one of the mechanisms that is involved in antibiotic resistance in K. pneumoniae,38 and the presence of efflux pump genes (OqxAB) in many Gram-negative pathogens causes an increase in the rates of resistance to many different antibiotic families including beta-lactams, carbapenems, macrolides, fluoroquinolone, tetracyclines that results from their inherent ability to develop resistance.39,40 So, we need to know if efflux pump plays a role in carbapenem and fluoroquinolone resistance or not by testing the effect of CCCP on meropenem and ciprofloxacin resistance. There is a significant decrease in MIC after CCCP is added to ciprofloxacin which indicates that the drug efflux system is involved in resistance to ciprofloxacin. Our study showed no effect of CCCP on meropenem resistance among isolates.
PCR was used to screen for oqxA and oqxB genes. Both OqxA and OqxB were detected in 26 isolates, 13 OqxA only and 11 OqxB only were also detected. Presence of these efflux pump genes seems to contribute to the high resistance of clinical isolates of K. pneumoniae to other antibiotics. Previous research found that OqxAB was detected in all of K. pneumoniae chromosome isolates and suggested the genome of K. pneumoniae is a possible reservoir of oqxAB.41
In the current study we could not observe any effect of CCCP on susceptibility to meropenem as no change in MIC of meropenem after CCCP addition was detected (Table 2). However, the addition of the CCCP decreased resistance to ciprofloxacin (4-fold or more in MIC) in NDM-5- producing K. pneumoniae isolates. Meanwhile only St-26 and St-42 isolates had an MIC fold change of 2 upon CCCP addition. We observed that eight of the nine NDM-5-producing K. pneumoniae had 4-fold decrease in MIC after CCCP exposure. Similarly, other studies reported greater reductions in MIC of ciprofloxacin with the use of efflux pumps inhibitors compared with their initial MIC.42–44 In the present study, the resistance to meropenem could be greatly affected by the presence of beta lactamases, which are not targeted by efflux pump inhibitors45 and the use of CCCP may not improve in vitro susceptibility of K. pneumoniae to meropenem.46
All NDM-5-producing isolates except one isolate could transfer their plasmids carrying blaNDM-5 by conjugation to their corresponding transconjugants (E. coli J53). Our results supported what we had previously reported about the vital role of the plasmid in the transfer of resistant genes.47 This plasmid may promote the rapid dissemination of blaNDM-5 among Gram-negative bacterial pathogens. This may indicate the possible transition of this type (blaNDM-5) between different species by conjugative plasmid and further highlights the importance of carbapenemase genes which could facilitate their dissemination on mobile genetic elements.48
Plasmid replicon typing showed that the FII type was predominant in NDM-5-producing K. pneumonia. It has been reported that blaNDM-5 closely associated with different types of plasmids; among these, the IncF plasmid was found mainly in Enterobacteriaceae and has the ability to transfer its antimicrobial resistance determinants.49 On the other hand, genotyping similarity between strains was found between these isolates (St-37, St-29, St-40) and between two isolates (St-42, St26). This indicated the capability of these NDM-5-producing strains to disseminate between different wards of this pediatric hospital.
Conclusions
The highly resistant K. pneumoniae producing blaNDM-5 type was firstly isolated from pediatric patients. The association of efflux pump genes such as OqxAB is involved in resistance to ciprofloxacin but not to meropenem. This highlights the severity risk of blaNDM-5-positive K. pneumonia as it could disseminate blaNDM-5 to other bacterial cells and has more resistance against carbapenems and other antibiotics. This emphasizes the importance of continuous monitoring of infection control policies, and the urgent need for a national antimicrobial stewardship plan in Egypt.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the faculty of pharmacy, Al-Azhar University, Egypt (9/2022, reference number of ZA-AS/PH/12/C/2022).
Informed Consent Statement
Written informed consent has been obtained from the parents or legal guardians of the pediatric patients to participate in this study.
Funding
This research received no external funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15–21. doi:10.1177/2049936115621709
2. Issakhanian L, Behzadi P. Antimicrobial agents and urinary tract infections. Curr Pharm Des. 2019;25(12):1409–1423. doi:10.2174/1381612825999190619130216
3. Hammoudi Halat D, Ayoub Moubareck C. The current burden of carbapenemases: review of significant properties and dissemination among gram-negative bacteria. Antibiotics. 2020;9(4):1.
4. Ahmadi Z, Noormohammadi Z, Behzadi P, Ranjbar R. Molecular detection of gyrA mutation in clinical strains of Klebsiella pneumoniae. Iran J Public Health. 2022;51(10):2334–2339. doi:10.18502/ijph.v51i10.10992
5. Behzadi P, García-Perdomo HA, Karpiński TM, Issakhanian L. Metallo-ß-lactamases: a review. Mol Biol Rep. 2020;47(8):6281–6294. doi:10.1007/s11033-020-05651-9
6. Karampatakis T, Tsergouli K, Behzadi P. Carbapenem-resistant Klebsiella pneumoniae: virulence factors, molecular epidemiology and latest updates in treatment options. Antibiotics. 2023;12(2):234. doi:10.3390/antibiotics12020234
7. Hornsey M, Phee L, Wareham DW. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother. 2011;55(12):5952–5954. doi:10.1128/AAC.05108-11
8. Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM Metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev. 2019;32(2). doi:10.1128/CMR.00115-18
9. Kazmierczak KM, Rabine S, Hackel M, et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(2):1067–1078. doi:10.1128/AAC.02379-15
10. Zhu YQ, Zhao J-Y, Xu C, Zhao H, Jia N, Li YN. Identification of an NDM-5-producing Escherichia coli sequence type 167 in a neonatal patient in China. Sci Rep. 2016;6(1):29934. doi:10.1038/srep29934
11. Li X, Fu Y, Shen M, et al. Dissemination of bla (NDM-5) gene via an IncX3-type plasmid among non-clonal Escherichia coli in China. Antimicrob Resist Infect Control. 2018;7:59. doi:10.1186/s13756-018-0349-6
12. Algammal A, Hetta HF, Mabrok M, Behzadi P. Editorial: emerging multidrug-resistant bacterial pathogens “superbugs”: a rising public health threat. Front Microbiol. 2023;14:1135614. doi:10.3389/fmicb.2023.1135614
13. Sun J, Deng Z, Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys Res Commun. 2014;453(2):254–267. doi:10.1016/j.bbrc.2014.05.090
14. Hernando-Amado S, Blanco P, Alcalde-Rico M, et al. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist Updat. 2016;28:13–27. doi:10.1016/j.drup.2016.06.007
15. Kim HB, Wang M, Park CH, Kim EC, Jacoby GA, Hooper DC. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Antimicrob Agents Chemother. 2009;53(8):3582–3584. doi:10.1128/AAC.01574-08
16. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
17. Pierce VM, Simner PJ, Lonsway DR, et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol. 2017;55(8):2321–2333. doi:10.1128/JCM.00193-17
18. (EUCAST) TECoAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters.Version 7.1. (EUCAST) TECoAST; 2017.
19. Poirel L, Benouda A, Hays C, Nordmann P. Emergence of NDM-1-producing Klebsiella pneumoniae in Morocco. J Antimicrob Chemother. 2011;66(12):2781–2783. doi:10.1093/jac/dkr384
20. Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi:10.1016/S1473-3099(15)00424-7
21. Liassine N, Assouvie L, Descombes MC, et al. Very low prevalence of MCR-1/MCR-2 plasmid-mediated colistin resistance in urinary tract Enterobacteriaceae in Switzerland. Int J Infect Dis. 2016;51:4–5. doi:10.1016/j.ijid.2016.08.008
22. Pournaras S, Maniati M, Spanakis N, et al. Spread of efflux pump-overexpressing, non-metallo-beta-lactamase-producing, meropenem-resistant but ceftazidime-susceptible Pseudomonas aeruginosa in a region with blaVIM endemicity. J Antimicrob Chemother. 2005;56(4):761–764. doi:10.1093/jac/dki296
23. Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145(3):1365–1373. doi:10.1128/jb.145.3.1365-1373.1981
24. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63(3):219–228. doi:10.1016/j.mimet.2005.03.018
25. Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19(24):6823–6831. doi:10.1093/nar/19.24.6823
26. Pavel AB, Vasile CI. PyElph - a software tool for gel images analysis and phylogenetics. BMC Bioinform. 2012;13(1):9. doi:10.1186/1471-2105-13-9
27. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi:10.1128/CMR.05035-11
28. Abdelaziz NA. Phenotype-genotype correlations among carbapenem-resistant Enterobacterales recovered from four Egyptian hospitals with the report of SPM carbapenemase. Antimicrob Resist Infect Control. 2022;11(1):13. doi:10.1186/s13756-022-01061-7
29. Kotb S, Lyman M, Ismail G, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in Egyptian intensive care units using national healthcare-associated infections surveillance data, 2011–2017. Antimicrob Resist Infect Control. 2020;9(1):2. doi:10.1186/s13756-019-0639-7
30. Mohamed ER, Aly SA, Halby HM, Ahmed SH, Zakaria AM, El-Asheer OM. Epidemiological typing of multidrug-resistant Klebsiella pneumoniae, which causes paediatric ventilator-associated pneumonia in Egypt. J Med Microbiol. 2017;66(5):628–634. doi:10.1099/jmm.0.000473
31. Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(12):1619–1629. doi:10.1099/jmm.0.46747-0
32. El-Sweify M, Gomaa N, El-Maraghy N, Mohamed H. Phenotypic detection of carbapenem resistance among Klebsiella pneumoniae in Suez Canal university hospitals, Ismailiya, Egypt. Int J Curr Microbiol Appl Sci. 2015;4:10–18.
33. Gamal D, Fernández-Martínez M, Salem D, et al. Carbapenem-resistant Klebsiella pneumoniae isolates from Egypt containing blaNDM-1 on IncR plasmids and its association with rmtF. Int J Infect Dis. 2016;43:17–20. doi:10.1016/j.ijid.2015.12.003
34. Morsi SS. Comparative evaluation of phenotypic and genotypic methods for detection of carbapenemases in clinically significant Klebsiella pneumoniae isolates. Egypt J Med Microbiol. 2016;38(87):1–8.
35. Gamal D, Fernández-Martínez M, El-Defrawy I, Ocampo-Sosa AA, Martínez-Martínez L. First identification of NDM-5 associated with OXA-181 in Escherichia coli from Egypt. Emerg Microbes Infect. 2016;5(4):e30–e30. doi:10.1038/emi.2016.24
36. Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:4. doi:10.3389/fcimb.2018.00004
37. Ahmadi M, Ranjbar R, Behzadi P, Mohammadian T. Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella pneumoniae. Expert Rev Anti Infect Ther. 2022;20(3):463–472. doi:10.1080/14787210.2022.1990040
38. Impey RE, Hawkins DA, Sutton JM, Soares da Costa TP. Overcoming intrinsic and acquired resistance mechanisms associated with the cell wall of Gram-negative bacteria. Antibiotics. 2020;9(9):623. doi:10.3390/antibiotics9090623
39. Sanchez-Carbonel A, Mondragón B, López-Chegne N, et al. The effect of the efflux pump inhibitor Carbonyl Cyanide m-Chlorophenylhydrazone (CCCP) on the susceptibility to imipenem and cefepime in clinical strains of Acinetobacter baumannii. PLoS One. 2021;16(12):e0259915. doi:10.1371/journal.pone.0259915
40. Ahmadi Z, Noormohammadi Z, Ranjbar R, Behzadi P. Prevalence of tetracycline resistance genes tet (A, B, C, 39) in Klebsiella pneumoniae isolated from Tehran, Iran. J Iran J Med Microbiol. 2022;16(2):141–147. doi:10.30699/ijmm.16.2.141
41. Yuan J, Xu X, Guo Q, et al. Prevalence of the oqxAB gene complex in Klebsiella pneumoniae and Escherichia coli clinical isolates. J Antimicrob Chemother. 2012;67(7):1655–1659. doi:10.1093/jac/dks086
42. Ardebili A, Talebi M, Azimi L, Lari AR. Effect of efflux pump inhibitor carbonyl cyanide 3-chlorophenylhydrazone on the minimum inhibitory concentration of ciprofloxacin in Acinetobacter baumannii clinical isolates. Jundishapur J Microbiol. 2014;7(1). doi:10.5812/jjm.8691
43. Rajamohan G, Srinivasan VB, Gebreyes WA. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J Antimicrob Chemother. 2010;65(2):228–232. doi:10.1093/jac/dkp427
44. Lin L, Ling B-D, Li X-Z. Distribution of the multidrug efflux pump genes, adeABC, adeDE and adeIJK, and class 1 integron genes in multiple-antimicrobial-resistant clinical isolates of Acinetobacter baumannii–Acinetobacter calcoaceticus complex. Int J Antimicrob Agents. 2009;33(1):27–32. doi:10.1016/j.ijantimicag.2008.06.027
45. Vrancianu CO, Gheorghe I, Czobor IB, Chifiriuc MC. Antibiotic resistance profiles, molecular mechanisms and innovative treatment strategies of Acinetobacter baumannii. Microorganisms. 2020;8(6):935. doi:10.3390/microorganisms8060935
46. Osei Sekyere J, Amoako DG. Carbonyl cyanide m-chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug-resistant Enterobacteriaceae. Front Microbiol. 2017;8:228. doi:10.3389/fmicb.2017.00228
47. Ramadan Mohamed E, Ali MY, Waly NG, Halby HM, Abd El-Baky RM. The Inc FII plasmid and its contribution in the transmission of blaNDM-1 and blaKPC-2 in Klebsiella pneumoniae in Egypt. Antibiotics. 2019;8(4):266. doi:10.3390/antibiotics8040266
48. Loqman S, Soraa N, Diene SM, Rolain JM. Dissemination of carbapenemases (OXA-48, NDM and VIM) producing Enterobacteriaceae isolated from the Mohamed VI University Hospital in Marrakech, Morocco. Antibiotics. 2021;10(5). doi:10.3390/antibiotics10050492
49. Huang L, Hu H, Xu C, et al. Characterization of NDM-5-producing Escherichia coli strains isolated from pediatric patients with bloodstream infections in a Chinese hospital. Genes. 2023;14(2):520. doi:10.3390/genes14020520
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

