Back to Journals » Infection and Drug Resistance » Volume 16
FibroScan Predicts Liver Fibrosis Progression in Chronic HBV Infection Patients with No Clear Indication for Antiviral Therapy: A Retrospective Cohort Study
Authors Xu W, Hu Q, Chen C, Li W, Li Q , Chen L
Received 9 February 2023
Accepted for publication 22 March 2023
Published 27 March 2023 Volume 2023:16 Pages 1777—1785
DOI https://doi.org/10.2147/IDR.S402990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wei Xu,1,* Qiankun Hu,1,* Chong Chen,2,* Weixia Li,2 Qiang Li,1 Liang Chen1
1Department of Liver Disease, Shanghai Public Health Clinical Center, Fudan University, Shanghai, People’s Republic of China; 2Department of Infectious Disease, Shanghai Public Health Clinical Center, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Li; Liang Chen, Email [email protected]; [email protected]
Background and Aims: Chronic hepatitis B virus (HBV) infection patients who do not fulfill the typical treatment indications should be followed up. This study aimed to evaluate the risk of liver fibrosis progression (LFP) and assess the role of noninvasive tests (NITs) of liver fibrosis in monitoring LFP in these patients.
Methods: A total of 116 patients with active HBV replication, persistently normal or minimally elevated alanine aminotransferase (ALT) levels, and no or mild hepatic necroinflammation or fibrosis based on liver biopsy tests at baseline and followed by a repeated liver biopsy assessment during follow-up. LFP was defined as increase in METAVIR fibrosis score by 1 score or more.
Results: Among 116 patients, 40 (34.5%) progressed by at least one fibrosis stage, 16 (13.8%) progressed by at least two fibrosis stages at a median follow-up interval of 27 months (IQR: 12– 36). Multivariate analysis confirmed the significant association of an increase in liver stiffness measurement (LSM) value with LFP on histology (p =0.005). The AUROC of LSM value increase rate is significantly higher than that of serum-based NITs of liver fibrosis for the prediction of LFP (p < 0.05). An increase in LSM by 20% is the optimal cutoff for the prediction of LFP.
Conclusion: LFP is non-negligible in patients with active HBV replication, persistently normal or minimally elevated ALT, and initially no or minimal hepatic necroinflammation or fibrosis. Serial LSM tests would be more reliable in identifying LFP than serum-based NITs, and easier to obtain than serial liver biopsy tests.
Keywords: liver fibrosis progression, noninvasive tests, transient elastography, liver stiffness measurement, chronic hepatitis B
Introduction
Hepatitis B virus (HBV) infection remains a global public health problem.1 WHO estimates that 296 million people were living with chronic HBV infection in 2019, with 1.5 million new infections each year.2 All patients with chronic HBV infection are at increased risk of progression to cirrhosis and hepatocellular carcinoma (HCC).1 Between 1990 and 2013, global viral hepatitis deaths increased from 0.89 million to 1.45 million, and the number of HBV-related deaths increased by 33%.3
The goal of therapy for chronic HBV infection is to improve survival of the infected person by preventing progression of the disease to cirrhosis, HCC and death.4 The indications for antiviral therapy are generally based on the combination of HBV DNA levels, alanine aminotransferase (ALT) levels, and severity of liver disease (assessed by liver biopsy). For patients with active HBV replication and persistently normal or minimally elevated ALT levels, liver biopsy may be needed to assess the necroinflammatory grade and determine the fibrotic stage as a guide for consideration of antiviral treatment.4 Treatment should be instituted if moderate-to-severe hepatic necroinflammation or significant fibrosis is found. If patients are not considered for treatment (no or mild hepatic necroinflammation and/or fibrosis), they should be followed up regularly at least every 3–6 months. If they do not fulfill any treatment indication within the first 3 years of follow-up, they should be consequently followed for life.
Liver biopsy is the reference procedure for liver fibrosis assessment, but its limitation (sampling error, inter-observers variability, cost, adverse events) makes it unsuitable as first-line procedure for the follow-up of liver fibrosis state. Therefore, non-invasive tests (NITs) of liver fibrosis have been an alternative to follow up the progression of liver fibrosis.5 Although previous studies demonstrated that liver stiffness is a promising predictor for prognosis of HBV patients, they evaluated the performances of liver stiffness for the diagnosis of liver fibrosis in a cross section, or in patients following antiviral therapy.6,7 At present, there is still a lack of the researches on the risk of liver fibrosis progression (LFP) in patients with active HBV replication, persistently normal or minimally elevated ALT levels, and initially no or minimal hepatic necroinflammation or fibrosis. In addition, there is also a lack of the researches on the role of liver stiffness in monitoring LFP in chronic HBV infection patients without antiviral therapy.
In this study, using paired liver biopsies as the “gold standard”, we aimed to evaluate the risk of LFP in chronic HBV infection patients with active HBV replication, persistently normal or minimally elevated ALT levels, and initially no or minimal hepatic necroinflammation or fibrosis. In addition, we assessed the role of NITs of liver fibrosis such as the liver stiffness measurement (LSM), the aspartate aminotransferase-to-platelet ratio index (APRI), the fibrosis index based on four factors (FIB-4), and the γ-glutamyl transpeptidase-to-platelet ratio (GPR) in monitoring LFP.
Methods
Patients
From June 2013 to August 2020, we performed liver biopsy tests and NITs of liver fibrosis for 452 untreated chronic HBV infection patients with active HBV replication and persistently normal or minimally elevated ALT levels who hospitalized at the Department of Liver Disease, Shanghai Public Health Clinical Center, Shanghai, China. The inclusion criteria were the following: 1) HBsAg positive >6 months (the cutoff of HBsAg positivity is >0.05 IU/mL), 2) persistently normal or minimally elevated ALT levels, which defined as ALT ≤ 2 upper limit of normal (ULN) (the ULN of ALT is 40 IU/L), 3) no or minimal hepatic necroinflammation or fibrosis at the first liver biopsy test (METAVIR necroinflammation score A0 or A1 and METAVIR fibrosis score F0 or F1), 4) NITs of liver fibrosis were measured contemporaneously with their baseline liver biopsy, and had a subsequent liver biopsy and contemporaneous NITs of liver fibrosis, 5) no previous antiviral treatment. The exclusion criteria were the following: 1) in addition to HBV infection, the patient had other liver diseases, 2) co-infection with human immunodeficiency virus, 3) alcohol consumption ≥20g/day for more than 5 years, 4) the absence of simultaneous and interpretable liver biopsy and NITs of liver fibrosis, 5) incomplete clinical data. Finally, this retrospective study included 116 chronic HBV infection patients who underwent a paired liver biopsy tests and NITs of liver fibrosis at two-points.
Liver Biopsy
Ultrasound-guided percutaneous liver biopsy was performed with 16-G Menghini needle. The biopsy specimens were fixed with 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. A minimum of 15mm of liver tissue with at least 6 portal tracts were considered suitable for liver histopathological analysis.8 The staging of hepatic necroinflammation and fibrosis refers to the METAVIR scoring system.9 The grade of liver necroinflammatory activity is assessed on a 4-point scale (A0 means no necroinflammatory activity, A1 means mild necroinflammatory activity, A2 means moderate necroinflammatory activity, A3 means severe necroinflammatory activity).9 The stage of liver fibrosis is evaluated on a 5-point scale (F0 means no fibrosis, F1 means portal fibrosis without septa, F2 means few septa, F3 means numerous septa without cirrhosis, F4 means cirrhosis).9 No or mild hepatic necroinflammation is defined as METAVIR activity score A0 or A1, and significant hepatic necroinflammation is defined as METAVIR activity score A2 or A3.4 No or mild liver fibrosis is defined as METAVIR fibrosis score <F2, and significant fibrosis means METAVIR fibrosis score ≥F2.4
Liver Transient Elastography
Liver transient elastography was performed using the FibroScan@ apparatus (Echosens, Paris, France) on a patient lying supine.10 The tip of the probe is contacted to the intercostal skin with coupling gel in the 9th to 11th intercostal space. The operator locates a liver portion at least 6 cm deep and free of large vascular structures, and then presses the probe button to start the measurements. The software determines whether each measurement is successful or not. The final result of a transient elastography session can be regarded as valid if the following criteria are fulfilled8: (1 a number of valid shots of at least 10, (2 a success rate (the ratio of valid shots to the total number of shots) above 60%, and (3 an interquartile range (IQR) less than 30% of the median LSM value (IQR/M ≤0.30%).
Serum-Based NITs of Liver Fibrosis
The APRI, FIB-4 and GPR were evaluated for comparison with LSM.
Definitions
LFP was defined as increase in METAVIR fibrosis score by 1 score or more from baseline at the second assessment of liver biopsy. NITs1 means the NITs value synchronized with the first liver biopsy. NITs2 means the NITs value synchronized with the second liver biopsy. The increase of 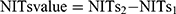 . The increase rate of
. The increase rate of  . For example, the increase of LSM value = LSM 2-LSM 1, and the increase rate of
. For example, the increase of LSM value = LSM 2-LSM 1, and the increase rate of 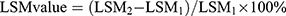 .
.
Statistical Analysis
Quantitative variables were presented as mean ± SD or median [IQR]. Categorical variables were presented as number (percentage). The differences between two groups were compared using T-test (for normal distribution variables), Mann–Whitney U-test (for non-normal distribution variables), or Chi-square test (for categorical variables). Univariable and multivariable analyses were performed to identify the independent predictors of LFP. Receiver operating characteristic (ROC) curves were used to assess the performances of NITs. The comparison of the area under ROC curves (AUROCs) was performed using the DeLong test.11 p < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS V 0.19.0 (SPSS Inc., IL, and USA), GraphPad Prism 8.0 (Inc., La Jolla, and CA), and Origin 2022b (Origin Lab Inc., and USA).
Results
Patients Characteristics at Baseline and Follow-Up Visit
The characteristics of the patients at baseline (the first liver biopsy) and follow-up visit (the second liver biopsy) are summarized in Table 1. The median time between the first and the second liver biopsy was 27 months (IQR 12–36). The majority of patients were male (79 patients, 68.1%), HBeAg positive (67 patients, 57.7%), and their median age at baseline was 36 years (IQR, 31–42). Among 116 patients, 40 (34.5%) progressed by at least one METAVIR fibrosis stage, 16 (13.8%) progressed by at least two METAVIR fibrosis stages, and 76 (65.5%) had no LFP. None of the clinical parameters, namely HBV DNA (median, 5.0 vs 5.2 log10 copies/mL, p =0.873), platelet, ALT, aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (γ-GT), albumin, globulin, and LSM value were statistically different between baseline and follow-up visit (all p>0.05).
 |
Table 1 Patient Characteristics at Baseline and Follow-Up Visit |
Comparison of Patients with and without LFP
Comparison of patients with and without LFP is shown in Table 2. Patients with LFP had significantly higher age (median, 37 vs 35 years, p=0.009) compared with those without LFP. The results supported that age is associated with liver fibrosis and its progression, and older age is associated with faster fibrosis progression. No significant differences were found between patients with LFP and those without LFP in sex, HBeAg positive rate, HBV DNA (median, 5.1 vs 4.8 log10 copies/mL, p =0.747), platelet, ALT, AST, ALP, γ-GT, albumin, globulin, LSM value, APRI, FIB-4, and GPR (all p > 0.05).
 |
Table 2 Comparison of Patients with and without Liver Fibrosis Progression |
Variables Associated with LFP
The variables associated with LFP are shown in Table 3. Univariate analysis showed that age [OR (95% CI): 1.05 (1.00–1.11), p=0.035], the increase of LSM value [OR (95% CI): 1.28 (1.07–1.52), p=0.007], the increase rate of LSM value [OR (95% CI): 1.03 (1.02–1.05), p < 0.001] were associated with LFP. Multivariate analysis confirmed the significant association of the increase rate of LSM with LFP [OR (95% CI): 1.04 (1.01–1.06), p = 0.005].
 |
Table 3 Variables Associated with Liver Fibrosis Progression |
Correlation Between LSM Value and LFP
At the second liver biopsy, the median LSM value of patients with F1, F2, F3, and F4 was 4.8 kPa (IQR, 4.2–6.2), 7.8 kPa (IQR, 6.3–8.1), 9.2 kPa (IQR, 8.1–11.4), and 23.9 kPa (IQR, 20.0–31.3), respectively. A significant association of the increase rate of LSM value with LFP (r=0.47, p <0.001) is shown in Figure 1. The METAVIR fibrosis score was positively correlated with LSM value, resulting in higher median LSM value with increasing METAVIR fibrosis score (Figure 2A). Patients in LFP group showed higher LSM increase rate compared with those without LFP (28.6% vs 0.5%, p < 0.001) (Figure 2B).
Performances of NITs for Assessing LFP
Using repeated liver biopsies as the gold standard, we compared the performance of LSM value and serum-based NITs in predicting LFP (Table 4 and Figure 2C). The AUROC of LSM value increase rate is significantly higher than that of APRI, FIB-4 and GPR for the prediction of LFP (0.79, 0.67, 0.65, and 0.63, respectively; all p < 0.05) (Table 4). Maximizing the Youden index, an increase in LSM by 20% or more is the optimal cutoff value for the prediction of LFP (the sensitivity, specificity, positive predictive value, and negative predictive value was 75%, 80%, 67%, and 86%, respectively).
 |
Table 4 Performances of Noninvasive Fibrosis Tests for Assessing Liver Fibrosis Progression |
Discussion
Estimatation of LFP represents an important surrogate end point that may facilitate treatment decisions by clarifying the vulnerability of an individual patient to progression.12 The concept of LFP estimation was first developed and applied to patients with chronic hepatitis C.13 However, LFP estimation is equally important for patients with other common liver diseases. In this study, using repeated liver biopsies as the gold standard, we observed non-negligible LFP (40/116, 34.5%) in chronic HBV infection patients with active HBV replication, persistently normal or minimally elevated ALT levels, and initially no or minimal hepatic necroinflammation or fibrosis. In addition, we found that serial LSM over time would be more reliable in identifying LFP than serum-based NITs of liver fibrosis, and easier to obtain than serial liver biopsies.
Previous study reported that among 271 untreated patients with chronic hepatitis C who having initial liver biopsy showing no or mild liver fibrosis, 75.6% progressed by at least one stage, and 45% progressed by at least two stages in 5 years.14 A prospective cohort study reported that during an average follow-up period of 35.3 months, cirrhosis occurred 6 to 64 months after entry in 35 HBeAg-positive and 7 anti-HBe positive patients with a calculated annual incidence of 2.4% and 1.3%, respectively.15 In this study, 40 patients (34.5%) progressed by at least one stage and 16 patients (13.8%) progressed by at least two stages. Our results show that, although there is no typical indications for antiviral therapy according to current guidelines,1,4,16 patients with active HBV replication, persistently normal or minimally elevated ALT levels, and initially no or minimal hepatic necroinflammation or fibrosis are at an increased risk of LFP.
As liver biopsy has a risk of 3/1000 severe adverse events and 1/10,000 mortality rate, repeated liver biopsies were less often performed.12 Using LSM, it is possible to perform repeated liver fibrosis assessments on a large number of patients. Previous studies had validated the diagnostic performance of LSM value for assessing the dynamic changes of liver fibrosis.17–19 Chon et al reported that annual LSM revealed that fibrosis improvement during treatment in CHB patients with advanced fibrosis receiving antiviral treatment, and LSM value <12.0 kPa at baseline was a significant predictor for 5-year fibrosis improvement.17 Kim et al reported that LSM can be used to assess liver fibrosis regression after antiviral treatment using nucleos(t)ide analogs in patients with CHB.18 Enomoto et al also reported that LSM can be useful for monitoring regression of liver fibrosis during entecavir treatment in patients with CHB.19 However, these studies only focused on monitoring of liver fibrosis regression in patients following anti-HBV therapy.17–19 As a supplement to previous studies, we validated LSM is an accurate and reproducible method to predict LFP in chronic HBV infection patients without antiviral therapy. LFP could be predicted using an increase in LSM value by 20% or more in patients with active HBV replication AND persistently normal or minimally elevated ALT levels.
Lu et al reported that serum-based NITs of liver fibrosis such as APRI and FIB-4 indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection.20 Al-Mohri et al reported that the APRI may be a useful marker for longitudinal evaluation of the progression of liver disease in HIV-HCV coinfection.21 However, the data derived from a large, well-characterized cohort of 426 CHB patients demonstrate that prediction of hepatic fibrosis using APRI and FIB-4 scores are not an adequate replacement for liver biopsy in clinical practice, nor do they reflect improvements in liver fibrosis during therapy.7 The present study also evaluated the role of serum-based NITs of liver fibrosis in assessing LFP in chronic HBV infection patients. We found that serum-based NITs are not an adequate replacement for liver biopsy in assessing LFP (AUROC < 0.68). Therefore, although APRI or FIB-4 scores were reported are adequate replacement for liver biopsy for longitudinal evaluation of the progression of liver disease in HCV infection patients and patients with HIV-HCV coinfection,20,21 practitioners utilizing APRI or FIB-4 need to be aware of their limitations in HBV patients.
In previous study, we found that serum HBV RNA levels were a more accurate noninvasive test than APRI and FIB-4 for the diagnosis of significant liver fibrosis in treatment-naive patients with chronic HBV infection.22 However, the previous study is a cross-sectional study.22 In this study, we performed a follow-up study, and assessed the role of noninvasive tests of liver fibrosis in monitoring LFP during follow-up. Certainly, HBV RNA may be a great prognostic marker in monitoring LFP. However, HBV RNA is not routinely tested in clinical settings, and the present study was a retrospective study which included patients from June 2013 to August 2020. In this retrospective cohort, we could not get the serum for HBV RNA tests for all patients. Therefore, HBV RNA not included as a readout in the present study.
According to the Asian-Pacific clinical practice guidelines on the management of hepatitis B, patients with active HBV replication and persistently normal or minimally elevated ALT levels, liver biopsy may be needed to assess the necroinflammatory grade and determine the fibrotic stage as a guide for consideration of antiviral treatment.4 Therefore, liver inflammation and fibrosis stage were evaluated in the participants underwent liver biopsy. In the present study, the staging of hepatic necroinflammation and fibrosis refers to the METAVIR scoring system. We provided the information about the METAVIR inflammation vs fibrosis score in Table 1. In fact, for untreated patients with CHB, liver fibrosis is often accompanied by liver inflammation. However, liver inflammation is not necessarily accompanied by liver fibrosis. However, persistent liver inflammation is an important cause of liver fibrosis.
Our study has a few limitations. First, the sample size was small. Although patients with active HBV replication and minimally elevated or persistently normal ALT should undergo liver histological evaluation, repeated liver biopsies were less often performed because of its invasiveness, explaining the small sample size of the study. Second, this study was a retrospective cohort study. Third, this study was a single-center study.
Despite these limitations, we observed non-negligible LFP in more than a third of chronic HBV infection patients with active HBV replication, persistently normal or minimally elevated ALT, and initially no or minimal hepatic necroinflammation or fibrosis who had no received antiviral therapy. Serial FibroScan tests over time would be more reliable in predicting LFP than serum-based NITs of liver fibrosis (APRI, FIB-4, or GPR), and easier to obtain than serial liver biopsies.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethics Guidelines
The study protocol was approved by the Clinical Research Ethics Committee of Shanghai Public Health Clinical Center (No 2023-S002-01). The procedures were in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association. The informed consents were obtained from the study participants prior to study commencement.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grant 21S11905600 from the Shanghai Association for Science and Technology, grant SHDC12020109 from the Shanghai Shenkang Hospital Development Center, and grant 2022YQ027 from the Shanghai Municipal Health Commission. The rapid service fee was funded by the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lampertico P, Agarwal K, Berg T. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021
2. Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi:10.1016/S0140-6736(15)61412-X
3. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388(10049):1081–1088. doi:10.1016/S0140-6736(16)30579-7
4. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi:10.1007/s12072-015-9675-4
5. Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet. 2010;375(9724):1419–1420. doi:10.1016/S0140-6736(09)62195-4
6. Wu SD, Liu LL, Cheng JL, et al. Longitudinal monitoring of liver fibrosis status by transient elastography in chronic hepatitis B patients during long-term entecavir treatment. Clin Exp Med. 2018;18(3):433–443. doi:10.1007/s10238-018-0501-x
7. Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64(4):773–780. doi:10.1016/j.jhep.2015.11.012
8. European Association for the Study of the Liver. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi:10.1016/j.jhep.2015.04.006
9. Bedossa P, Poynard T; The METAVIR Cooperative Study Group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24(2):289–293. doi:10.1002/hep.510240201
10. de Ledinghen V, Wong VW, Vergniol J, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R). J Hepatol. 2012;56(4):833–839. doi:10.1016/j.jhep.2011.10.017
11. Delong ER, Delong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
12. Poynard T, Munteanu M, Deckmyn O, et al. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: proof of concept and first application in a large population. J Hepatol. 2012;57(3):541–548. doi:10.1016/j.jhep.2012.04.025
13. Poynard T, Bedossa P, Opolon P; The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349(9055):825–832. doi:10.1016/S0140-6736(96)07642-8
14. Marcolongo M, Young B, Dal Pero F, et al. A seven-gene signature (cirrhosis risk score) predicts liver fibrosis progression in patients with initially mild chronic hepatitis C. Hepatology. 2009;50(4):1038–1044. doi:10.1002/hep.23111
15. Liaw YF, Tai DI, Chu CM, et al. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8(3):493–496. doi:10.1002/hep.1840080310
16. Terrault NA, Lok A, Mcmahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
17. Chon YE, Park JY, Myoung SM, et al. Improvement of liver fibrosis after long-term antiviral therapy assessed by fibroscan in chronic hepatitis B patients with advanced fibrosis. Am J Gastroenterol. 2017;112(6):882–891. doi:10.1038/ajg.2017.93
18. Kim SU, Park JY, Kim DY, et al. Non-invasive assessment of changes in liver fibrosis via liver stiffness measurement in patients with chronic hepatitis B: impact of antiviral treatment on fibrosis regression. Hepatol Int. 2010;4(4):673–680. doi:10.1007/s12072-010-9201-7
19. Enomoto M, Mori M, Ogawa T, et al. Usefulness of transient elastography for assessment of liver fibrosis in chronic hepatitis B: regression of liver stiffness during entecavir therapy. Hepatol Res. 2010;40(9):853–861. doi:10.1111/j.1872-034X.2010.00687.x
20. Lu M, Li J, Zhang T, et al. Serum biomarkers indicate long-term reduction in liver fibrosis in patients with sustained virological response to treatment for HCV infection. Clin Gastroenterol Hepatol. 2016;14(7):1044–1055. doi:10.1016/j.cgh.2016.01.009
21. Al-Mohri H, Murphy T, Lu Y, et al. Evaluating liver fibrosis progression and the impact of antiretroviral therapy in HIV and hepatitis C coinfection using a noninvasive marker. J Acquir Immune Defic Syndr. 2007;44(4):463–469. doi:10.1097/QAI.0b013e318030ff8e
22. Huang C, Li Q, Xu W, et al. Serum HBV RNA levels predict significant liver fibrosis in patients with chronic HBV infection. Discov Med. 2020;29(157):119–128.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.