Back to Journals » OncoTargets and Therapy » Volume 14
Fibrinogen/Albumin Ratio (FAR) in Patients with Triple Negative Breast Cancer and Its Relationship with Epidermal Growth Factor Receptor Expression
Received 17 September 2021
Accepted for publication 9 November 2021
Published 7 December 2021 Volume 2021:14 Pages 5403—5415
DOI https://doi.org/10.2147/OTT.S339973
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alberto Bongiovanni
Wenbo Gao, Ming Li, Yunhao Zhang
Department of Oncology Surgery, Jiamusi Central Hospital, Jiamusi, 154002, People’s Republic of China
Correspondence: Wenbo Gao
Department of Oncology Surgery, Jiamusi Central Hospital, Jiamusi, 154002, People’s Republic of China
Email [email protected]
Objective: To investigate the potential prognostic significance of fibrinogen/albumin ratio (FAR) in triple negative breast cancer (TNBC) patients and its relationship with epidermal growth factor receptor (EGFR) expression.
Methods: There were 164 patients with TNBC enrolled in this study in our hospital from January 2010 to December 2015. The optimal cutoff value of FAR was evaluated by the receiver operating characteristic curve (ROC). The associations between TNBC and clinicopathological variables by FAR were performed by Chi-square test. Kaplan–Meier method and Log rank test were used for survival analysis. The independent prognostic factors were determined by univariate and multivariate Cox’s proportional hazards regression model. The EGFR expression was analyzed by the immunohistochemistry assay.
Results: One hundred and sixty-four TNBC patients were divided into: low FAR group (FAR < 0.08) and high FAR group (FAR ≥ 0.08) by ROC. The preoperative FAR was associated to BMI, menopause, red blood cell, albumin, fibrinogen (P < 0.05). FAR was an independent prognostic factor for TNBC. In low FAR group, the mean disease-free survival (DFS) and overall survival (OS) were 33.62 months and 52.99 months; in high FAR group, the mean DFS and OS were 30.18 months and 48.27 months, respectively. The DFS and OS survival curve were performed by Log rank assay and were statistically significant (P < 0.05). The mean DFS and OS after operation in patients with EGFR negative expression were longer than that in patients with EGFR positive expression. In EGFR positive group, the mean DFS and OS of low FAR group were higher than that of high FAR group, and the difference was statistically significant (P < 0.05).
Conclusion: Pretreatment FAR is the independent prognostic factor in TNBC, and with low cost, strong repeatability, and high safety. It can be acted as an effective indicator to predict the prognosis of TNBC.
Keywords: fibrinogen/albumin ratio, epidermal growth factor receptor, triple negative breast cancer, prognosis
Introduction
Breast cancer is the most common malignancy in women, and it prove to be an significant cause of cancer deaths worldwide.1 In 2020, the latest global cancer burden data shown that there were 9.23 million new female cancer cases in the world, including 2.26 million breast cancer cases, and 0.68 million cases died of breast cancer.2 Data from China National Cancer Center, there are 272,400 new cases of breast cancer, of which 70,700 patients died of breast cancer.3,4 Triple negative breast cancer (TNBC) is a subtype of breast cancer which is not expressed by estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2), and account for 20% of breast cancer population.5 TNBC has strong invasion ability, easy recurrence or metastasis after treatment, and is not sensitive to traditional endocrine therapy and targeted therapy.6,7 Tumor associated inflammatory response (TAIR) plays an important role in tumor occurrence, development, lymph node metastasis, treatment and prognosis.8,9 The abnormal coagulation function leads to increase the risk of thrombosis, and promote the proliferation, invasion and metastasis of malignant tumor cells.10 Fibrinogen (FIB), as a coagulation factor, participates in coagulation processes such as blood coagulation and platelet aggregation.11,12 Albumin (ALB) is an important indicator to reflect the body nutritional status, and hypoproteinemia is a reliable indicator of malignant tumor cachexia and malnutrition.13 Studies have shown that preoperative fibrinogen/albumin ratio (FAR) were used to predict the prognosis of different malignant tumors.14,15 In a meta-analysis study, high albumin/fibrinogen ratio (FAR) and low fibrinogen/albumin ratio (AFR) were significantly associated with poor OS; and the ratio of fibrinogen and albumin could act as a promising prognostic marker in malignant tumors.16 In Zheng Y’s study, preoperative FAR-PLR score may be a potential new biomarker for predicting survival and prognosis of breast cancer, and may help doctors make better clinical decisions for breast cancer treatment.17 The aim of this study was to investigate the relationship between FAR and the prognosis of TNBC and to provide a reference for the treatment of TNBC.
Materials and Methods
Patient Selection
A total of 164 cases with TNBC were enrolled this study in our hospital from January 2010 to December 2015. This retrospective study received approval from Institutional Review Board of Jiamusi central hospital and was performed in accordance with the Declaration of Helsinki. All patients signed informed consent forms.
Inclusion Criteria and Exclusion Criteria
The inclusion criteria of this study were as follows: 1) patients with TNBC were confirmed by histopathology; 2) no cancer treatment such as chemotherapy, radiotherapy, or targeted therapy prior to operation at our hospital; 3) complete clinicopathological data; and 4) complete follow-up information. The exclusion criteria of this study were as follows: 1) with unresectable or metastatic breast cancer, or other malignant tumors; 2) chronic inflammatory systemic diseases were are difficult to control, such as hypertension, diabetes, immune system diseases; 3) the liver and kidney function is abnormal and cannot tolerate the surgery; 4) take anti-inflammatory or immunosuppressive drugs; and 5) received blood product transfusion or peripheral blood tests were not available before treatment.
Peripheral Venous Blood Parameters
All laboratory and hematological data of patients were collected and tested before operation. Albumin (ALB) and fibrinogen (FIB) were collected in the peripheral venous blood, and analyzed by automatic blood analyzer. The FAR was defined as follows: FAR = F/A, (the units were F (g/L), A (g/L) where F and A are pretreatment peripheral fibrinogen (F) and albumin (A).
Follow-Up
All patients were followed regularly after operation by outpatient and telephone. The follow-up included postoperative recurrence, metastasis, and death information. The postoperative schedule was reexamined every three months for the first and second year after operation, every half a year for the third through the fifth year, and then every year after fifth year.
Statistical Analysis
SPSS software 21.0 (Chicago, IL, USA) and GraphPad prism software 8.0 (La Jolla, CA, USA) were used for all statistical analyses. All data in this study were conformed to normal distribution. The optimal cutoff value of FAR was determined according to the receiver operating characteristic curve (ROC). The associations between TNBC subtype breast cancer and enumeration variables by FAR were performed by Chi-square test. The clinical outcomes of DFS and OS were analyzed by Kaplan–Meier survival analysis and compared the survival curve using the Log rank test. The univariate and multivariate Cox proportional hazard regression analyses were used to analyze prognostic factors. A two-tailed P<0.05 was considered to indicate statistical significance.
Results
General and Clinicopathologic Characteristics
The general and clinicopathological characteristics of 164 patients are shown in Table 1. The optimal cutoff value of FAR was 0.08, and the area under curve (AUC) was 0.668 (Figure 1). And 85 cases (51.8%) in low FAR group, 79 cases (48.2%) in high FAR group, respectively. Compared to the high FAR group, the low FAR group was significantly associated with body mass index (BMI) (χ2= 8.610, P = 0.003) and menopause (χ2= 4.572, P = 0.033) (Table 1).
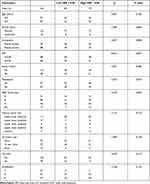 |
Table 1 General and Clinicopathologic Characteristics |
 |
Figure 1 The receiver operating characteristic (ROC) curve for FAR. (A) Survival condition of FAR, (B) AUC of ROC. |
Relationship Between FAR and Clinicopathological Characteristics of TNBC
Compared to the high FAR group, the low FAR group was significantly improved the characteristics of clinical T stage (χ2= 12.129, P = 0.016) and type of surgery (χ2=4.512, P = 0.034) (Table 2).
 |
Table 2 Relationship Between FAR and Clinicopathological Characteristics of TNBC |
Associations Between FAR and Inflammation Indexes
The enrolled blood parameters were analyzed by the median value. Compared with the high FAR group, the low FAR group was significantly associated with red blood cell (χ2= 3.982, P = 0.046), ALB (χ2= 24.785, P < 0.001) and FIB (χ2= 47.146, P < 0.001) (Table 3).
 |
Table 3 Associations Between FAR and Inflammation Indexes |
Univariate and Multivariate Cox Regression Survival Analyses
The univariate and multivariate analysis performed that FAR, ALB, FIB, L, clinical T stage, histologic type, positive lymph nodes, and postoperative chemotherapy were the prognostic factors for DFS (Table 4); and FAR, ALB, FIB, N, L, B, US-LNM, clinical T stage, histologic type, positive lymph nodes (PLN), and postoperative chemotherapy were the prognostic factors for OS (Table 5).
 |
Table 4 Univariate and Multivariate Analyses of Disease-Free Survival in Triple Negative Breast Cancer (TNBC) |
 |
Table 5 Univariate and Multivariate Analyses of Overall Survival in Triple Negative Breast Cancer (TNBC) |
All patients were followed regularly after operation. The last follow-up time was March 2021. In the low FAR group, the mean DFS and OS were 33.62 months, 52.99 months; in the high FAR group, the mean DFS and OS were 30.18 months, 48.27 months. The survival time of DFS and OS in the low FAR group were higher than that in the high FAR group (P < 0.05). Kaplan–Meier survival curves for DFS and OS for the FAR of all patients are shown in Figure 2.
Construction and Validation of the Predictive Nomogram
The independent prognostic factors for DFS and OS were evaluated by univariate and multivariate Cox regression model. On the basis of the above identified prognostic factors, a nomogram was constructed for predicting the 1-year, 3-year and 5-year DFS and OS for breast cancer patients (Figure 3A and B). The c-index was calculated for the current standard of OS prediction (FAR classification) as well as for the proposed nomogram. The c-index for the nomogram of FAR alone was 0.767 [95% confidence interval (CI): 0.696–0.838].
Chemotherapy After Operation
Chemotherapy was the prognostic factor by the univariate and multivariate analysis on DFS (P < 0.001, HR: 6.285, 95% CI: 2.309–17.106; P < 0.001, HR: 4.502, 95% CI: 2.142–9.463) and OS (P = 0.007, HR: 4.283, 95% CI: 1.498–12.246; P < 0.001, HR: 3.840, 95% CI: 1.800–8.190). After operation, 110 patients were received with chemotherapy, 54 patients were not received with chemotherapy. In patients received chemotherapy, patients with low FAR were survival longer than those with high FAR (χ2= 7.861, P = 0.005; χ2= 8.830, P = 0.003). In patients not received chemotherapy, patients with low FAR were survival longer than those with high FAR (χ2=1.026, P = 0.311; χ2=1.455, P = 0.228) (Figure 4).
Relationship Between FAR and EGFR Expression
In this study, 86 cases were negative expression of EGFR, and 78 cases were positive expression of EGFR. In patients with positive expression of EGFR, the mean DFS and OS in the low FAR group were survival longer than those in the high FAR group (χ2= 6.800, P = 0.009; χ2= 7.447, P = 0.006). In patients with negative expression of EGFR, the mean DFS and OS in the low FAR group were survival longer than those in the high FAR group (χ2= 1.319, P = 0.251; χ2= 1.088, P = 0.297) (Figure 5).
Discussion
TNBC is characterized by high histological grade, strong invasiveness and high malignancy, and with no effective treatment.5,18 Chronic inflammation is considered an important factor to promote tumor occurrence and development, affect the proliferation, invasion, apoptosis and angiogenesis of tumor cells, also inhibit anti-tumor immune response.19,20 Studies point out that FIB is a multifunctional protein, which is accompanied by abnormalities in pathophysiological processes such as tumor, infection and inflammation.21 Moreover, the activation of coagulation system and the release of coagulation factors play an important role in the occurrence and progression of tumor.21 Patients with high fibrinogen will aggravate the risk of tumor progression, and promote the further development of tumor by inhibiting tumor cells from cytotoxicity mediated by natural killer cells.22 Nevertheless, patients with hypoproteinemia will aggravate the occurrence of tumor cachexia and further deteriorate their nutritional status.23 Inflammatory immune parameters, such as SIRI, SII and C-reactive protein, were used to judge the prognosis of a variety of tumors.24–26 In recent years, FAR has been proved to be related to prognosis in a variety of malignant tumors.27,28 Therefore, it is necessary to make a profound study on FAR for the clinical prognosis of TNBC.
Our results indicated that FAR was associated with the prognosis of TNBC and was a key prognostic factor for poor prognosis of breast cancer. The mean DFS and OS in the low FAR group were survival longer than those in the high FAR group, and with statistically significant. This was generally consistent with Zheng’s study.17 Other study indicated that patients with high FAR value had shorter DFS, and could be used as an effective prognostic marker for breast cancer patients.29 In Zheng’s study, they found that AFR was an independent prognostic factor for improving DFS in breast cancer, and the predictive model was effective.30 There are some potential mechanisms can explain these results. Fibrinogen is an acute phase reactive protein reflecting systemic inflammatory response, and it is also an important factor involved in hemostasis. According to directly bind to members of fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), fibrinogen has been reported to play a critical role in angiogenesis, epithelial-to-mesenchymal transition (EMT), and hematogenous metastasis.31,32 Low concentration albumin reflect poor nutritional status and performance status, participates in the systemic inflammatory response. Malnutrition may weaken the immune system and have a negative impact on the prognosis of cancer patients.33 Since albumin and fibrinogen are synthesized by hepatocytes, impaired liver function will influence the accuracy of evaluation based on albumin or fibrinogen alone, and in predicting the prognosis for patients with TNBC.15
EGFR is a tyrosine protein kinase receptor located on human chromosome 7p13-q22, and is the expression product of protooncogene c-erbB1. It is activated by gene mutation or gene amplification to regulate tumor cell proliferation and angiogenesis, promote tumor cell invasion and metastasis.34 Patients were prone to recurrence and metastasis by positive expression of EGFR, and DFS and OS were significantly shortened.35 Other study indicated that EGFR was expressed in different molecular subtypes of breast cancer, especially in HER2 overexpression subtype and TNBC subtype. It also can be used as an independent prognostic factor for breast cancer.36 Our results also shown that the EGFR was related to the prognosis of TNBC, and the survival time of DFS and OS after operation with negative expression was shorter than that with EGFR positive expression. Not surprisingly, the survival time of DFS and OS in the low FAR group was significantly higher than that in the high FAR group.
However, this study has several limitations, such as single-center retrospective study, a small number of patients. And further comparative studies should determine the best predictors of prognosis in patients, and in order to provide more powerful evidence for the prevention and treatment of TNBC.
Conclusions
The peripheral blood routine examination has the advantages of low cost, strong repeatability, and high safety. Preoperative FAR can be used as an independent prognostic factor for TNBC, and EGFR expression is also related to the prognosis of breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Rongshou Z, Kexin S, Siwei Z, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. 2019;41(1):19–28. doi:10.3760/cma.j.issn.0253-3766.2019.01.005
5. Yin L, Duan JJ, Bian XW, et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61. doi:10.1186/s13058-020-01296-5
6. Denkert C, Liedtke C, Tutt A, et al. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389(10087):2430–2442. doi:10.1016/S0140-6736(16)32454-0
7. Lyons TG, Traina TA. Emerging novel therapeutics in triple-negative breast cancer. Adv Exp Med Biol. 2019;1152:377–399. doi:10.1007/978-3-030-20301-6_20
8. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
9. Del Prete A, Allavena P, Santoro G, et al. Molecular pathways in cancer-related inflammation. Biochem Med. 2011;21(3):264–275. doi:10.11613/bm.2011.036
10. Zacharski LR, Meehan KR, Algarra SM, et al. Clinical trials with anticoagulant and antiplatelet therapies. Cancer Metastasis Rev. 1992;11(3–4):421–431. doi:10.1007/BF01307191
11. Lebreton A, Casini A. Diagnosis of congenital fibrinogen disorders. Ann Biol Clin. 2016;74(4):405–412. doi:10.1684/abc.2016.1167
12. Kwaan HC, Lindholm PF. Fibrin and fibrinolysis in cancer. Semin Thromb Hemost. 2019;45(4):413–422. doi:10.1055/s-0039-1688495
13. Usama SM, Park GK, Nomura S, et al. Role of albumin in accumulation and persistence of tumor-seeking cyanine dyes. Bioconjug Chem. 2020;31(2):248–259. doi:10.1021/acs.bioconjchem.9b00771
14. Xu WY, Zhang HH, Xiong JP, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24(29):3281–3292. doi:10.3748/wjg.v24.i29.3281
15. Xu Q, Yan Y, Gu S, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts Prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. 2018;2018:4925498. doi:10.1155/2018/4925498
16. Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manag Res. 2019;11:3381–3393. doi:10.2147/CMAR.S198419
17. Zheng Y, Wu C, Yan H, et al. Prognostic value of combined preoperative fibrinogen-albumin ratio and platelet-lymphocyte ratio score in patients with breast cancer: a prognostic nomogram study. Clin Chim Acta. 2020;506:110–121. doi:10.1016/j.cca.2020.03.011
18. Bergin ART, Loi S. Triple-negative breast cancer: recent treatment advances. F1000Res. 2019;8:F1000. doi:10.12688/f1000research.18888.1
19. Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi:10.4103/aam.aam_56_18
20. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. doi:10.1186/s12199-018-0740-1
21. Wang H, Gao J, Bai M, et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25(5):382–387. doi:10.3109/09537104.2013.827782
22. Suzuki T, Shimada H, Nanami T, et al. Hyperfibrinogenemia is associated with inflammatory mediators and poor prognosis in patients with gastric cancer. Surg Today. 2016;46(12):1394–1401. doi:10.1007/s00595-016-1339-z
23. Zhou L, Ma S, Balde AI, et al. A retrospective propensity score matched study of the preoperative C-reactive protein to albumin ratio and prognosis in patients with resectable non-metastatic breast cancer. Med Sci Monit. 2019;25:4342–4352. doi:10.12659/MSM.913684
24. Wang L, Zhou Y, Xia S, et al. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. 2020;28(4):537–547. doi:10.3233/CBM-201682
25. Jomrich G, Gruber ES, Winkler D, et al. Systemic Immune-Inflammation Index (SII) predicts poor survival in pancreatic cancer Patients undergoing resection. J Gastrointest Surg. 2020;24(3):610–618. doi:10.1007/s11605-019-04187-z
26. Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–170. doi:10.3109/10408363.2011.599831
27. Sun F, Tan YA, Gao QF, et al. Circulating fibrinogen to pre-albumin ratio is a promising biomarker for diagnosis of colorectal cancer. J Clin Lab Anal. 2019;33(1):e22635. doi:10.1002/jcla.22635
28. Zhang J, Ruan J, Wang W, et al. Prognostic value of the combination of CEA and fibrinogen/albumin ratio in resectable Gastric cancer. Cancer Manag Res. 2020;12:2767–2775. doi:10.2147/CMAR.S246566
29. Cao X, Yidong Z, Feng M, et al. Effect of fibrinogen to albumin ratio on prognosis of operable breast cancer patients. Chin J Breast Dis. 2020;14(4):213–220.
30. Lihua ZHENG, Feng LIU, Wei LI, et al. Relation between albumin to fibrinogen ratio and overall survival of breast cancer. Zhong Liu Fang Zhi Yan Jiu. 2020;47(2):102–107.
31. Martino MM, Briquez PS, Ranga A, et al. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110(12):4563–4568. doi:10.1073/pnas.1221602110
32. Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology. 2010;25(2):85–101. doi:10.1152/physiol.00045.2009
33. Virizuela JA, Camblor-álvarez M, Luengo-Pérez LM, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol. 2018;20(5):619–629. doi:10.1007/s12094-017-1757-4
34. Talukdar S, Emdad L, Das SK, et al. EGFR: an essential receptor tyrosine kinase-regulator of cancer stem cells. Adv Cancer Res. 2020;147:161–188. doi:10.1016/bs.acr.2020.04.003
35. Jr RR. Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers. Pharmacol Res. 2019;139:395–411. doi:10.1016/j.phrs.2018.11.014
36. Xiaoli W, Deying X, Zhenzhen S, et al. Expression and prognostic significance of EGFR, p53, Ki-67 and AR in breast cancer. J Henan Univ Sci Tech. 2019;37(2):86–90.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




