Back to Journals » International Journal of General Medicine » Volume 16
Fibrinogen-to-Lymphocyte Ratio Was an Independent Predictor of Lymph Node Metastasis in Patients with Clinically Node-Negative Advanced-Stage Gastric Cancer
Received 9 February 2023
Accepted for publication 11 April 2023
Published 17 April 2023 Volume 2023:16 Pages 1345—1354
DOI https://doi.org/10.2147/IJGM.S407833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Pei Hu, Wei Wang, Chiyi He
Department of Gastroenterology, Yijishan Hospital of Wannan Medical College, Wuhu, Anhui, People’s Republic of China
Correspondence: Wei Wang, Department of Gastroenterology, Yijishan Hospital of Wannan Medical College, No. 2, Zheshan West Road, Wuhu, Anhui, 241000, People’s Republic of China, Tel +86-0553-5739316, Email [email protected]
Purpose: Various hematological indicators have been reported to predict lymph node metastasis (LNM) in gastric cancer (GC) patients, but the relationship between FLR and LNM has not been studied. Therefore, the aim of this study was to evaluate the role of preoperative fibrinogen-to-lymphocyte ratio (FLR) in predicting LNM in patients with clinically node-negative (cN0) advanced gastric cancer (AGC).
Patients and Methods: We retrospectively reviewed 571 eligible patients with primary AGC adenocarcinoma who underwent radical gastrectomy (discovery cohort). Patients were divided into high and low FLR groups according to the optimal cutoff value determined by Youden index. FLR is an independent predictor of LNM determined by logistic regression and validated in the validation cohort of 207 patients. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive value of FLR for LNM. The nonlinear relationship between FLR and LNM risk was assessed using restricted cubic spline. Sensitivity analyses were performed according to FLR quartiles to further assess the robustness of the results. The nomogram was built based on FLR and clinicopathological characteristics, and was evaluated by calibration curves, ROC curve analysis and decision curve analysis.
Results: In the discovery cohort, the area under the curve (AUC) value for FLR to predict LNM was 0.592. There is a linear relationship between the FLR value and the risk of LNM, and the risk of LNM increased with FLR value. High FLR level is an independent risk factor for LNM, and the results of sensitivity analysis robust this finding. The nomogram for individual risk assessment performed well. Furthermore, we verified the FLR was an independent predictor of LNM in the validation cohort.
Conclusion: FLR was an independent predictor of LNM in patients with cN0 AGC.
Keywords: advanced gastric cancer, clinically node-negative, lymph node metastasis, fibrinogen-to-lymphocyte ratio, nomogram
Introduction
Gastric cancer (GC) is one of the most common malignant tumors worldwide, with more than 1 million new cases and 769,000 deaths recorded in 2020.1 GC accounts for 7.7% of all global deaths and ranks fifth and fourth in terms of morbidity and mortality, respectively.1 Although there has been significant progress in the early diagnosis and treatment of GC in recent years, advanced-stage gastric cancer (AGC) still accounts for approximately 70% of all cases, and its 5-year survival rate is only approximately 30%.2 Lymph node metastasis (LNM) is an independent indicator of poor prognosis in GC, and the incidence rate of LNM in patients with AGC patients can be as high as 80%.3 Currently, surgical resection is the preferred treatment option for AGC. Previous papers indicated that neoadjuvant chemotherapy is beneficial for AGC, and it can reduce tumor stage, particularly among patients with LNM, thereby improving the rates of complete (R0) resection.4,5 Therefore, the early and accurate prediction of LNM can help establish preoperative individualized treatment plans and improve prognosis. Currently, contrast-enhanced abdominal computed tomography (CT) scan is the most commonly used method in the preoperative assessment of LNM. However, there are still issues in terms of accuracy and consistency rates, which generally vary from 40% to 70%.6,7 In addition, several protein markers that can be used for predicting LNM in GC are still assessed. However, these are expensive and complex and are challenging to perform clinically.8,9 Therefore, it is necessary to actively search for convenient and effective biomarkers for predicting LNM before surgery.
Inflammation plays an important role in tumor pathogenesis, and systemic inflammation contributes to tumor progression and metastasis by inhibiting apoptosis and promoting angiogenesis.10 Various hematological indicators have been reported to predict LNM in GC, such as prognostic nutritional index, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and lymphocyte-monocyte ratio cell ratio.11–13
Fibrinogen is a 340-kDa glycoprotein produced by hepatocytes that regulate the process of coagulation and thrombosis. Further, it plays an important role in hemostasis, cell adhesion, and systemic inflammatory responses.14 Hyperfibrinogenemia can increase the risk of LNM in GC.15 Recently, fibrinogen-to-lymphocyte ratio (FLR) was found to be associated with prognosis in different types of cancers. Moreover, it could be a predictor of peritoneal metastasis in GC.16–20 However, there are no relevant reports focusing on the association between FLR and LNM in patients with GC. Therefore, the current study aimed to assess the predictive value of FLR in LNM in patients with clinically node-negative (cN0) AGC.
Materials and Methods
Patients
Patients with primary AGC (T2-T4) with adenocarcinoma pathologically who underwent curative R0 gastrectomy at our hospital from January 2018 to January 2021 were reviewed in this study (n = 1207, discovery cohort). The exclusion criteria were as follows: 1) patients with a history of other types of malignant tumors or gastrectomy, 2) those who had distant metastases or who received neoadjuvant chemotherapy, 3) those with < 15 surgically cleared lymph nodes, 4) those who did not undergo CT scan at our institution, 5) those with emergency admission, 6) those with diseases or oral medications that can affect preoperative hematological parameters, 7) those with clinically positive lymph node (cN+), and 8) those with incomplete clinicopathological and laboratory data. Based on the same criteria, we recruited patients as the validation cohort between February 2021 and January 2022. Figure 1 shows the specific screening process. This study was approved by the institutional review board of Yijishan Hospital of Wannan Medical College (2022–027). The need for informed consent was waived given the retrospective nature of this study. Identifying information was removed to protect patient confidentiality.
 |
Figure 1 Screening flowchart. |
Data Collection
Data on the following clinicopathological features were obtained from the medical records: sex, age, presence of hypertension and diabetes mellitus, tumor size and site, T stage, tumor grade, Lauren type, number of dissected and positive lymph nodes, and preoperative hematological parameters. Tumor size was measured as the largest diameter of the tumor, and tumor sites were classified as upper (cardia and fundus), middle (body and angle), lower (antrum), and overlap. According to the American Joint Committee on Cancer/International Union Against Cancer TNM staging system (8th edition), the T stage was divided into T2 (muscularis propria), T3 (subserosal and serosal layers), and T4 (invasion of adjacent organs). Tumor grade was categorized into high/moderate differentiation and low differentiation, and the least differentiated components were used for analysis among patients with multiple differentiation types.13 The preoperative hematological parameters included fibrinogen, lymphocyte, and carbohydrate antigen 19–9 levels. These parameters were measured within 1 week before surgery, and the results of the first blood test after admission were considered the main results.21
Definition
FLR was defined as fibrinogen level (g/L) divided by lymphocyte level (109/L).17 The patients were divided into the high and low groups based on the optimal cutoff value of FLR determined using the Youden index.22 Clinical N stage (cN) was identified using a contrast-enhanced abdominal CT scan. Based on previous studies, cN+ was defined as lymph nodes with a short-axis diameter of ≥ 8 mm or a long-axis diameter of ≥ 10 mm23,24 and cN0 as the absence of LNM.
Statistical Analysis
The median and interquartile ranges were used to describe non-normally distributed quantitative data. The chi-square test and Mann–Whitney U-test were used to compare categorical and continuous variables between the two groups, respectively. Receiver operating characteristic (ROC) curves were drawn to assess the predictive power of preoperative FLR for LNM, and the Youden index was utilized to determine the optimal cutoff value of FLR. Variables with P values of < 0.05 in the univariate logistic regression analysis were included in the multivariate analysis to identify the independent indicators of LNM. The association between FLR and the risk of LNM was evaluated on a continuous scale using restricted cubic spline curves based on the multivariate analysis.25 Sensitivity analysis was performed according to FLR quartiles to further assess the robustness of the results. A nomogram was established based on independent indicators and was then assessed via area under the curve (AUC), calibration curve, and decision curve analysis.26 The bootstrap method was used to resample 1000 times to obtain the corrected concordance index (C-index) for internal validation.27 The Delong test was used to compare the AUC value.28 The Statistical Package for the Social Sciences software for Windows (version 26.0) and R version 4.0.2 were used in this study. All analyses were two-sided, and a P value of < 0.05 was considered statistically significant.
Results
Characteristics of the Patients
The current study enrolled 571 eligible patients in the discovery cohort. Table 1 shows the baseline characteristics of the participants. The incidence rate of LNM in patients with cN0 AGC was 63.0%. The lower portion of the stomach (35.2%) was the common site, followed by the middle portion (31.9%) and the upper portion (21.7%). Approximately 56.9% of the patients presented with T3 stage disease. Meanwhile, 23.8% and 19.3% of patients had T2 and T4 stage disease, respectively. In terms of tumor grade and Lauren type, the patients mainly presented with low differentiation (72.3%) and intestinal type (71.6%), respectively. The positivity rate of LNM was significantly high in the subgroups with a tumor size of ≥ 5 cm (71.7%), CA199 level of ≥ 37 (74.2%), low differentiation (70.2%), and high FLR group (68.9%).
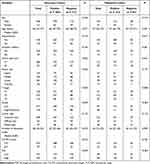 |
Table 1 Clinicopathological Variables of Patients with cN0 Advanced Gastric Cancer |
ROC Curve Analysis
As depicted in Figure 2A, based on the ROC curve, the AUC value of FLR for predicting LNM in patients with cN0 AGC was 0.592. The sensitivity and specificity of FLR for predicting LNM were 66.9% and 48.3%, respectively. Further, patients were divided into the high and low groups based on the optimum FLR cutoff value (1.78). In addition, the Delong test analysis showed that the FLR had comparable predictive ability with the CA199 (p value > 0.05) (Figure 2B).
Univariate and Multivariate Logistic Regression Analyses
According to the results of the univariate analysis, T stage, grade, tumor size, number of dissected nodes, CA199 level, and FLR were significantly correlated with LNM (all P < 0.05). The multivariate analysis showed that T stage (P < 0.001), grade (P < 0.001), and FLR (P = 0.009) were independent risk indicators of LNM (Table 2). Furthermore, the sensitivity analysis confirmed the robustness of these findings (P for trend = 0.013) (Supplementary Table 1). As shown in Supplementary Figure 1, based on the restricted cubic spline curve, there was a linear association between FLR and the risk of LNM (P for non-linearity > 0.05). Moreover, the risk of LNM increased with a higher FLR.
 |
Table 2 Logistic Analyses the Predictors of Lymph Node Metastasis in Patients with cN0 Advanced Gastric Cancer |
Comparison of Clinicopathological Characteristics Between the High and Low FLR Group
As shown in Supplementary Table 2, there were no significant differences in terms of sex, presence of hypertension and diabetes mellitus, tumor site and grade, Lauren type, and the number of dissected nodes between the high and low FLR groups. However, age, tumor size, T stage, CA199 levels, and incidence rates of LNM significantly differed between the two groups. The distribution of FLR value significantly differed among the subgroups classified according to T stage (P = 0.027) and tumor size (P < 0.001) (Figure 3).
Establishment and Validation of the Nomogram
T stage, grade, and FLR are the independent indicators in the multivariate analysis. These parameters were selected to establish a nomogram for predicting LNM in patients with cN0 AGC (Figure 4A). According to the calibration curve, the predicted probabilities of the model were consistent with the actual probabilities (Hosmer−Lemeshow test, P = 0.503) (Figure 4B). In addition, the C-index of internal validation was 0.755. The AUC of the nomogram was 0.741 (95% confidence interval: 0.703–0.777), and it was significantly better than that of T stage (0.696; 95% confidence interval: 0.656–0.0.733, P < 0.001) or grade (0.611; 95% confidence interval: 0.570–0.652, P < 0.001) alone (Figure 4C). Decision curve analysis revealed that the model had higher clinical usefulness than T stage, grade, or FLR alone (Figure 4D).
 |
Figure 4 Development and evaluation of the nomogram for predicting LNM in patients with cN0 AGC. (A) Nomogram. (B) Calibration curves. (C) ROC curves. (D) Decision curve analysis. |
FLR is a Independent Risk Indicator in the Validation Cohort
In the validation cohort, we divided patients into high and low FLR groups according to the cutoff value of discovery cohort. The multivariate analysis validated that FLR (P < 0.001) was an independent indicator of LNM (Table 2).
Discussion
The current study reported the use of FLR, a novel indicator, for independently predicting LNM in patients with cN0 AGC. Results showed that patients with a high FLR level are more likely to develop LNM, and the risk of LNM increases with high FLR. In addition, a nomogram for individual risk assessment based on FLR and clinicopathological characteristics showed good performance.
Tumor cells can activate the hemostatic system via different mechanisms, including the release of procoagulant tissue factors, cancer procoagulants and microparticles, soluble factors, and direct adhesive contact to activate the host’s hemostatic cells (endothelial cells and platelets).29 Fibrinogen, a hemostatic factor, is an important determinant of the metastatic potential of tumor cells, and its mechanisms of action are as follows: acts as a bridge to support the adhesion of tumor cells to the vascular endothelium; combines with other proteins and deposited on the extracellular matrix to form a stable scaffold; and promotes tumor proliferation and stimulates angiogenesis by supporting the binding of various growth factors to tumor cells.30,31 As early as 2005, Yamashita et al retrospectively analyzed the preoperative plasma fibrinogen levels of 649 patients with GC undergoing surgery. Results showed that hyperfibrinogenemia may provide a favorable environment for cancer cell metastasis via the lymphatic system.15 Previous studies have shown that fibrinogen can increase the metastatic potential by disrupting the clearance of tumor cells by natural killer cells, and it also reflects the connection between fibrinogen and the immune system in the process of cancer metastasis.32 Recently, a multivariate analysis evaluated 136 patients with GC undergoing surgery. Results showed that fibrinogen levels were significantly associated with lymph node involvement in patients with GC.33
Lymphocytes are an important component of the immune system, can destruct tumor cells, and can participate in mediating anti-tumor host immune responses in the tumor microenvironment.34 Tumor-infiltrating lymphocytes (TILs) mainly include T cells, B cells, and natural killer cells, and the low expression of TILs will promote tumor invasion and metastasis.35 Kim et al found that low TILs and submucosal invasion were independent predictors of LNM in early-stage GC.34 In recent years, lymphocytes are often selected to construct some novel predictive biomarkers and are widely used in the prediction of LNM in patients with GC. Kosuga et al found that the preoperative neutrophil-to-lymphocyte ratio may be a useful complementary diagnostic tool for predicting LNM in AGC.36 Du et al showed that a low preoperative lymphocyte-to-monocyte ratio was positively correlated with LNM in patients with GC.13 Tong et al used the derived monocyte-to-lymphocyte ratio for predicting LNM in patients with gastric signet ring cell carcinoma.21
FLR, combined with hemagglutination and inflammation markers, have a potential prognostic value in different types of cancers, such as non-small cell lung cancer, esophageal cancer, liver cancer, and head and neck adenoid cystic carcinoma.16–19 In addition, Huang et al found that a high FLR level was a risk factor for peritoneal metastasis in GC.20 The current study first reported the association between FLR and LNM in patients with AGC.
An accurate assessment of preoperative lymph node status in patients with AGC is important for determining whether to provide neoadjuvant chemotherapy and the extent of surgical lymph node dissection. Due to the low accuracy of imaging assessment of cN, in patients with cN0 stage, the use of FLR to assist imaging examinations, such as contrast-enhanced abdominal CT scan, to identify unsuspected LNM involvement has clinical value. Moreover, this indicator can be obtained by collecting blood samples from the peripheral veins, which has the advantages of simple procedures, less invasiveness and low cost.37
The current study had several limitations. First, this was a retrospective study, and selection bias could not be avoided. Second, the study only included patients from a single hospital, which limits the generalisability of our findings. Therefore, prospective large-scale multicenter studies should be performed to validate our findings. Third, this study only included patients who underwent radical gastrectomy, which may not be representative of the overall population of AGC patients. Fourth, in the current study, the predictors of T stage and grade were determined via postoperative pathological examination. Although preoperative ultrasound gastroscopy and gastroscopic biopsy have good power to obtain preoperatively evaluation parameters in terms of them, there are still certain differences between preoperative diagnosis and pathological results.38 The inclusion of postoperative parameters in the preoperative assessment might have caused some bias.
Conclusion
We found that FLR is a low-cost, accessible, and potentially novel serological marker that independently predicts LNM in patients with cN0 AGC.
Ethical Statement
This study was approved by the institutional review board of Yijishan Hospital of Wannan Medical College (2022-027). The need for informed consent was waived given the retrospective nature of this study. Identifying information was removed to protect patient confidentiality. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol. 2015;7:39–48.
3. Deng J, Zhang R, Pan Y, et al. N stages of the seventh edition of TNM Classification are the most intensive variables for predictions of the overall survival of gastric cancer patients who underwent limited lymphadenectomy. Tumour Biol. 2014;35:3269–3281.
4. Chen ZD, Zhang PF, Xi HQ, et al. Recent Advances in the Diagnosis, Staging, Treatment, and Prognosis of Advanced Gastric Cancer: a Literature Review. Front Med. 2021;8:744839.
5. Xiong B, Ma L, Cheng Y, et al. Clinical effectiveness of neoadjuvant chemotherapy in advanced gastric cancer: an updated meta-analysis of randomized controlled trials. Eur J Surg Oncol. 2014;40:1321–1330.
6. Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22.
7. Fairweather M, Jajoo K, Sainani N, et al. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111:1016–1020.
8. Li W, Ye F, Wang D, et al. Protein predictive signatures for lymph node metastasis of gastric cancer. Int J Cancer. 2013;132:1851–1859.
9. Huang KH, Lan YT, Fang WL, et al. The correlation between miRNA and lymph node metastasis in gastric cancer. Biomed Res Int. 2015;2015:543163.
10. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi:10.1097/MCO.0b013e32832a7902
11. Kosuga T, Konishi T, Kubota T, et al. Value of Prognostic Nutritional Index as a Predictor of Lymph Node Metastasis in Gastric Cancer. Anticancer Res. 2019;39(12):6843–6849. doi:10.21873/anticanres.13901
12. Zhang L-X, Wei Z-J, Xu A-M, et al. Can the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio be beneficial in predicting lymph node metastasis and promising prognostic markers of gastric cancer patients? Tumor maker retrospective study. Int J Surg. 2018;56:320–327. doi:10.1016/j.ijsu.2018.06.037
13. Du D, Han Z, Lian D, et al. The value of preoperative lymphocytes-to-monocytes ratio in predicting lymph node metastasis in gastric cancer. Transl Cancer Res. 2019;8(5):2053–2058. doi:10.21037/tcr.2019.09.17
14. Lee SE, Lee JH, Ryu KW, et al. Preoperative plasma fibrinogen level is a useful predictor of adjacent organ involvement in patients with advanced gastric cancer. Journal of Gastric Cancer. 2012;12(2):81–87. doi:10.5230/jgc.2012.12.2.81
15. Yamashita H, Kitayama J, Nagawa H. Hyperfibrinogenemia is a useful predictor for lymphatic metastasis in human gastric cancer. Jpn J Clin Oncol. 2005;35(10):595–600. doi:10.1093/jjco/hyi150
16. Liu M, Yang J, Wan L, et al. Elevated Pretreatment Fibrinogen-to-Lymphocyte Percentage Ratio Predict Tumor Staging and Poor Survival in Non-Small Cell Lung Cancer Patients with Chemotherapy or Surgery Combined with Chemotherapy. Cancer Manag Res. 2021;13:4921–4933. doi:10.2147/CMAR.S308659
17. Fan N, Chen D, Zheng J, et al. A novel preoperative plasma indicator to predict prognoses for patients with esophageal squamous cell carcinoma after radical esophagectomy: fibrinogen-to-lymphocyte ratio. Cancer Manag Res. 2019;11:4719–4728.
18. Li Y, Li Z, Deng K, et al. Fibrinogen/Lymphocyte Count Ratio Can Be Used as a New Indicator of Prognosis in Patients with Hepatocellular Carcinoma After Radical Resection. Cancer Manag Res. 2020;12:9057–9066.
19. Brkic FF, Stoiber S, Friedl M, et al. The Potential Prognostic Value of a Novel Hematologic Marker Fibrinogen-to-Lymphocyte Ratio in Head and Neck Adenoid-Cystic Carcinoma. J Pers Med. 2021;2:11.
20. Huang C, Liu Z, Xiao L, et al. Clinical Significance of Serum CA125, CA19-9, CA72-4, and Fibrinogen-to-Lymphocyte Ratio in Gastric Cancer With Peritoneal Dissemination. Front Oncol. 2019;9:1159.
21. Tong C, Wang W, Xia Y, et al. A potential novel biomarker in predicting lymph node metastasis of gastric signet ring cell carcinoma: a derived monocyte to lymphocyte ratio. Am J Surg. 2022;223:1144–1150.
22. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458–472.
23. Fukagawa T, Katai H, Mizusawa J, et al. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer. 2018;21:68–73.
24. Kubota K, Suzuki A, Shiozaki H, et al. Accuracy of Multidetector-Row Computed Tomography in the Preoperative Diagnosis of Lymph Node Metastasis in Patients with Gastric Cancer. Gastrointest Tumors. 2017;3:163–170.
25. Harrell FE, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202.
26. Wang T, Wu Y, Zhou H, et al. Development and validation of a novel competing risk model for predicting survival of esophagogastric junction adenocarcinoma: a SEER population-based study and external validation. BMC Gastroenterol. 2021;21:38.
27. Huang YQ, Liang CH, He L, et al. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol. 2016;34:2157–2164.
28. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845.
29. Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223–233.
30. Steinbrecher KA, Horowitz NA, Blevins EA, et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70:2634–2643.
31. Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309.
32. Palumbo JS, Talmage KE, Massari JV, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141.
33. Palaj J, Kečkéš Š, Marek V, et al. Fibrinogen Levels Are Associated with Lymph Node Involvement and Overall Survival in Gastric Cancer Patients. Anticancer Res. 2018;38:1097–1104.
34. Kim JY, Kim CH, Lee Y, et al. Tumour infiltrating lymphocytes are predictors of lymph node metastasis in early gastric cancers. Pathology. 2017;49:589–595.
35. Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4:84–95.
36. Kosuga T, Konishi T, Kubota T, et al. Clinical significance of neutrophil-to-lymphocyte ratio as a predictor of lymph node metastasis in gastric cancer. BMC Cancer. 2019;19:1187.
37. Yin X, Fang T, Wang Y, et al. Prognostic significance of serum inflammation indexes in different Lauren classification of gastric cancer. Cancer Med. 2021;10:1103–1119.
38. Oda I, Gotoda T, Sasako M, et al. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95:1495–1500.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


