Back to Journals » International Journal of General Medicine » Volume 14
Fibrinogen-to-Albumin Ratio is Associated with All-Cause Mortality in Cancer Patients
Received 1 June 2021
Accepted for publication 9 August 2021
Published 27 August 2021 Volume 2021:14 Pages 4867—4875
DOI https://doi.org/10.2147/IJGM.S322735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yanling Wen, Jingwen Yang, Xiaoyan Han
Bone Marrow Transplantation Center, Department of Hematology, The First Affiliated Hospital, School of Medicine Zhejiang University, Hangzhou, Zhejiang, 310003, People’s Republic of China
Correspondence: Xiaoyan Han Email [email protected]
Background: Past studies have identified fibrinogen-to-albumin ratio (FAR) as a novel prognostic immune biomarker in various diseases. Here, we investigated the prognostic value of FAR in all combined cancer mortality.
Methods: We extracted patient data from the Multiparameter Intelligent Monitoring in Intensive Care Database III. FAR was measured prior to hospital admission. Only first admission data from each patient were used. Baseline data were extracted within 24 h after admission. The clinical endpoints were 90- and 365-day all-cause cancer mortality. Cox proportional hazards models and subgroup analyses were used to determine the relationship between FAR and these clinical endpoints.
Results: A total of 652 eligible patients were enrolled. Upon adjusting for age and gender, multivariate analysis revealed correlation between higher FAR values and increased risk of all-cause mortality. After adjusting for more confounding factors, higher FAR values significantly correlated with 90- and 365-day all-cause mortality relative to low FAR values (tertile 3 vs tertile 1: HR, 95% CI: 1.65, 1.15– 2.39; 1.52, 1.10– 2.10).
Conclusion: Our findings indicate that FAR may predict the risk of cancer mortality and is an independent prognostic indicator of all-cause mortality in cancer patients.
Keywords: fibrinogen-to-albumin ratio, mortality, cancer, biomarker
Introduction
Cancer imposes a serious disease burden worldwide1 and cancer mortality is projected to increase as the global population continues to age.2 The top-10 cancer types are lung, esophageal, liver, cervical, stomach, breast, colorectal, lymphocytes, nasopharyngeal, and ovarian cancer. Five-year survival rates for all-combined cancer were only 30.82%.3 The main cancer treatment methods are surgery, chemotherapy, and radiotherapy. However, despite cancer treatment advances, many cancers are associated with poor prognosis.4,5 Thus, effective and noninvasive prognostic biomarkers are needed to guide personalized treatment and improve cancer outcomes.
Systemic inflammation status has emerged as an indicator of malignancy. More and more reliable evidence shows that cancer-related hypercoagulable state, inflammation and malnutrition are very common in cancer patients, and which are closely associated with cancer initiation, progression, metastasis, and resistance to chemotherapy.6 Albumin and fibrinogen are two commonly used circulating inflammatory proteins. Fibrinogen is an acute-phase protein produced by the liver. The plasma level of fibrinogen increases in hypercoagulable state and inflammatory state.7 A large amount of evidence shows that fibrinogen-related coagulation dysfunction is closely related to tumor angiogenesis, invasion, progression and metastasis.8,9 Likewise, albumin is also produced by hepatocytes. Proinflammatory cytokine tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) inhibit albumin production through hepatocytes.10 The decrease of plasma albumin level indicates high degree of inflammation, poor nutritional status and poor therapeutic effect. Albumin is a well-established prognostic factor for various disorders, including oral cavity cancer,11 metastatic pathological femur fractures,12 and amyotrophic lateral sclerosis.13 The prognostic role of fibrinogen has also been reported in disorders like spontaneous intracerebral hemorrhage.14 Therefore, fibrinogen and albumin better reflect the process of tumor inflammation. Moreover, fibrinogen-to-albumin ratio (FAR) has emerged as a prognostic immune biomarker in various diseases like gallbladder cancer,15 breast cancer,16 and ST-segment elevation myocardial infarction.17
However, the role of FAB in all-combined cancer mortality and its cancer prognostic value is unclear. Here, we examined the relationship between FAB and cancer mortality using data from MIMIC-III V1.3 database and its prognostic value in cancer.
Methods
Study Population
The research was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Data were downloaded from MIMIC-III version 1.4 database and included vital signs, medication, demographic data, and other essential data from patients admitted into intensive care (53,423 distinct admissions) at the Beth Israel Deaconess Medical Center (BIDMC, Boston) from 2001 to 2012.18 Approval was obtained by the Massachusetts Institute of Technology and the Institutional Review Boards before applying the data. Requirement for individual patient consent was waived because the project did not affect clinical care and all protected health information was de-identified. All data accessed complies with relevant data protection and privacy regulations.
Population Selection Criteria
Of the patients recorded in the MIMIC-III database, we selected cancer patients aged >18, who were first admitted into hospital for >1 day. Those lacking >5% of individual data or whose biopsies revealed hematological malignancy were excluded.
Data Extraction
Patient demographic data, including age, gender, ethnicity, vital signs, laboratory characteristics, comorbidities, and scoring systems were retrieved. Vital signs within 24 h after ICU admission included systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate, respiratory rate, temperature, and SPO2. Comorbidities included congestive heart failure (CHF), coronary artery disease (CAD), atrial fibrillation (AFIB), stroke, renal disease, liver disease, pneumonia, respiratory failure, chronic obstructive pulmonary disease (COPD), and acute respiratory distress syndrome (ARDS). Laboratory measurements included fibrinogen, albumin, bicarbonate, anion gap, creatinine, bilirubin, chloride, glucose, hematocrit, hemoglobin, platelet, sodium, potassium, blood urea nitrogen (BUN), white blood cell (WBC), lactate, prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR) in the first 24 h. The outcomes of our study were 90- and 1-year mortality rates. Patient day of admission was considered the start day of follow-up, and all participants were followed up for at least a year. Date of death was obtained from the Social Security Death Index records.
Statistical Analysis
Three subgroups were developed based on FAR. Continuous results were presented as the average value ± standard deviation (SD). Categorical data were presented as percentage or frequency. Kruskal–Wallis H-test and χ2 tests were used to compare baseline feature differences between the FAR subgroups for continuous variables and categorical variables. COX regression analysis was used to determine the association between FAR and cancer outcomes. The matched hazard ratio was changed by >10% upon adding covariances to the model.19 There was no covariate adjustment in Model 1. Age and gender were adjusted in Model 2. Confounders like age, gender, anion gap, SBP, respiratory rate, hemoglobin, INR, temperature, SPO2, chronic conditions including AFIB (yes/no), renal disease, respiratory failure, ARDS (yes/no), and pneumonia were adjusted in Model 3. All analyses were done on R version 3.6.1. P values were two-sided and p=<0.05 was considered statistically significant.
Results
Subject Characteristics
A total of 652 cancer patients were included. Baseline clinical, laboratory, and demographic data are shown in Table 1. This analysis showed that the proportion of renal disease, pneumonia, respiratory failure, heart rate, respiratory rate, platelet count, and BUN level were elevated in the high-FAR group. SBP, MBP, serum albumin levels, bicarbonate, bilirubin, glucose, hemoglobin, potassium, lactate, and APTT were decreased (p=<0.05 for all).
 |
Table 1 Characteristics of the Study Patients According to Neutrophil Percentage-to-Albumin Ratios |
Association Between FAR and Cancer Outcomes
Here, different models were established to evaluate the independent effects of FAR and cancer outcomes after adjusting for other potential confounders. Effect sizes (HR) and 95% Cis are displayed in Table 2. We stratified FAR levels by tertiles to assess if FAR was associated with 90- and 365-day all-cause mortality. In model I, after adjusting for age and gender, higher FAR values correlated with elevated risk of all-cause mortality. In model II, after adjusting for age, gender, anion gap, diastolic blood pressure, respiratory rate, hemoglobin, INR, temperature, SPO2, atrial fibrillation, renal disease, respiratory failure, ARDS, and pneumonia, higher FAR values significantly correlated with high risk of 90- and 365-day all-cause mortality relative to low FAR levels (tertile 3 vs tertile 1: HR, 95% CI: 1.65, 1.15–2.39; 1.52, 1.10–2.10).
 |
Table 2 HRs (95% CIs) for All-Cause Mortality Across Groups of Neutrophil Percentage-to-Albumin Ratios |
Subgroup Analyses
Subgroup analysis of the association between FAR and 90-day all-cause mortality (Table 3) revealed no interactions in most strata (p=0.0506–0.9372). Patients with a DBP ≥60 mmHg, MBP ≥76mmHg, hemoglobin ≥11.2g/dl, bicarbonate ≥24 mg/dl, and glucose ≥180mg/dl had significantly higher risk of 90-day mortality with FAR ≥0.192 (HR, 95% CI: 3.47, 1.91–6.31; 3.43, 1.95–6.02; 3.78, 2.16–6.62; 3.20, 1.77–5.78; 3.02, 1.81–5.04, respectively). Similarly, patients with a chloride <109 mmol/l and respiratory failure (no) showed increased risk with a FAR ≥0.192 (HR, 95% CI: 1.91, 1.18–3.10 and 2.51, 1.55–4.06).
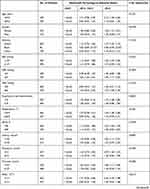 | 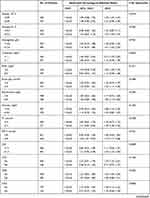 |  |
Table 3 Subgroup Analysis of the Associations Between the Neutrophil Percentage-to-Albumin Ratios and 30-Day All-Cause Mortality |
Discussion
A positive association between FAR and cancer mortality was expressed within our study. Our findings show that FAR may predict risk of cancer mortality. The results showed that higher FAR values correlated with increased all-cause mortality in 90- and 365-day after adjusting several variables. Similar observations were found in the model that adjusted for more confounding factors, indicating that FAR is still an independent and effective tumor prognostic marker. Although several previous studies have shown that FAR was associated with mortality in certain cancers, such as stage IB-IIA cervical cancer and resectable gastric cancer,20,21 evidence of this association is limited. The majority of prior studies focused on FAR’s single-type cancer association. As the association between FAR and all-cancer mortality is unclear at this moment, we focused on this relationship.
Fibrinogen, a protein used in blood coagulation, is also responsible for the mediation between hemostatic components and cancer biology. Fibrinogen has been shown via mechanisms, such as angiogenesis stimulation, platelet adhesion promotion, and tumor cell proliferation/migration via growth factor bondage to promote tumor growth.22 An analysis of 1196 patients with GC revealed that elevated fibrinogen positively correlates with poor survival.23 Circulation of albumin and prealbumin can be used to evaluate nutritional and immunity status. Albumin may restrain tumor progression stabilizing DNA replication and enhancing immune response.24 Albumin, which accumulates at inflammation and tumor sites, can be used to deliver anti-inflammatory and anticancer drugs.25,26 Additionally, albumin levels are correlated with poor prognosis in cancer due to malnutrition and postoperative complications.27 FAR, which is mechanistically important for nutrition, coagulation, and systemic inflammation, is strongly correlated with the tumor cell survival, intraversion and adhesiveness leading to increased metastatic potential.28 This may explain why FAR can be a strong prognostic tool in cancer patients.
FAR’s prognostic role was previously applied to several cancers, ranging from esophageal squamous cell carcinoma29 to metastatic colorectal cancer.30 Liu et al recently identified FAR as an inflammatory factor, illustrating the status of ankylosing spondylitis.31 Observations of FAR’s prognostic role were expressed proceeding percutaneous coronary intervention in patients with non-ST elevation acute coronary syndrome.32 Via meta-analysis Zhang et al discovered a strong correlation between FAR and positive lymph node metastasis, distant metastasis, deeper infiltration, and advanced clinical stage.33 Yu et al were recently able to independently predict resistance to chemotherapeutic drugs and advanced epithelial ovarian cancer prognosis via a biomarker constructed by and albumin-to-fibrinogen ratio. Similarly, within our study, the results showed that higher FAR values were independently associated with increased all-cause mortality at 90 and 365 days of all-cancer, which is consistent with above research results.
The main strengths of our study are its large sample size and in-depth analysis. In addition, this is the first study of the association between FAR and risk of mortality in ICU cancer patients. However, some limitations of the study were worth noting. First, due to its retrospective nature, the study cannot prove a causal relationship between mortality and cancer. Second, although we adjusted for possible risk factors, additional confounders like proinflammatory factors, and unknown factors cannot be ruled out. Third, FAR was assessed for only the first 24 h of admission and the relationship between subsequent FAR and prognosis was not evaluated. The baseline assessment used may increase the risk of misclassification bias.
Conclusions
Our data indicated that FAR is an independent prognostic indicator of all-cause mortality in cancer. However, the prospective cohort studies are needed to validate our conclusions.
Acknowledgments
Thanks for Wang Jie’s help and support.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chine J Cancer. 2017;36(1):66. doi:10.1186/s40880-017-0234-3
2. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18(12):1579–1589. doi:10.1016/S1470-2045(17)30677-0
3. Lan L, Zhao F, Cai Y, Wu RX, Meng Q. [Epidemiological analysis on mortality of cancer in China, 2015]. Zhonghua Liu Xing Bing Xue Za Zh. 2018;39(1):32–34. Chinese
4. Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK. Darling GE: surgery Versus Chemotherapy and Radiotherapy For Early and Locally Advanced Small Cell Lung Cancer: a Propensity-Matched Analysis of Survival. Lung Cancer. 2017;109:78–88. doi:10.1016/j.lungcan.2017.04.021
5. Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized Phase III trial. Ann Oncol. 2015;26(4):696–701. doi:10.1093/annonc/mdu560
6. Galdiero MR, Marone G, Mantovani A. Cancer Inflammation and Cytokines. Cold Spring Harbor Perspectives Biol. 2018;10(8):548. doi:10.1101/cshperspect.a028662
7. Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(10):1494–1503. doi:10.1016/j.ejso.2018.07.052
8. Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–3309. doi:10.1182/blood.V96.10.3302
9. Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64(23):8613–8619. doi:10.1158/0008-5472.CAN-04-2078
10. Pfensig C, Dominik A, Borufka L, et al. Application for Albumin Dialysis in Extracorporeal Organ Support: characterization of a Putative Interaction Between Human Albumin and Proinflammatory Cytokines IL-6 and TNFα. Artif Organs. 2016;40(4):397–402. doi:10.1111/aor.12557
11. Valero C, Zanoni DK, Pillai A, et al. Host Factors Independently Associated With Prognosis in Patients With Oral Cavity Cancer. JAMA otolaryngol. 2020;146(8):699–707. doi:10.1001/jamaoto.2020.1019
12. Sim DS, Zainul-Abidin S, Sim EY, et al. Serum albumin level predicts survival after surgical treatment of metastatic femur fractures: a retrospective study. J Orthopaedic Surg Res. 2020;15(1):128. doi:10.1186/s13018-020-01632-7
13. Sun J, Carrero JJ, Zagai U, et al. Blood biomarkers and prognosis of amyotrophic lateral sclerosis. Eur J Neurol. 2020;27(11):2125–2133. doi:10.1111/ene.14409
14. Song X, Zhang Q, Cao Y, Wang S, Zhao J. Antiplatelet therapy does not increase mortality of surgical treatment for spontaneous intracerebral haemorrhage. Clin Neurol Neurosurgery. 2020;196:105873. doi:10.1016/j.clineuro.2020.105873
15. Xu WY, Zhang HH, Xiong JP, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24(29):3281–3292. doi:10.3748/wjg.v24.i29.3281
16. Hwang KT, Chung JK, Roh EY, et al. Prognostic Influence of Preoperative Fibrinogen to Albumin Ratio for Breast Cancer. J Breast Cancer. 2017;20(3):254–263. doi:10.4048/jbc.2017.20.3.254
17. Zhao Y, Yang J, Ji Y, et al. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels. 2019;34(10):1600–1607. doi:10.1007/s00380-019-01399-w
18. Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi:10.1038/sdata.2016.35
19. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi:10.1093/oxfordjournals.aje.a116813
20. An Q, Liu W, Yang Y, Yang B. Preoperative fibrinogen-to-albumin ratio, a potential prognostic factor for patients with stage IB-IIA cervical cancer. BMC Cancer. 2020;20(1):691. doi:10.1186/s12885-020-07191-8
21. Tang S, Lin L, Cheng J, et al. The prognostic value of preoperative fibrinogen-to-prealbumin ratio and a novel FFC score in patients with resectable gastric cancer. BMC Cancer. 2020;20(1):382. doi:10.1186/s12885-020-06866-6
22. Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann New York Acad Sci. 2017;1404(1):27–48. doi:10.1111/nyas.13454
23. Yu X, Hu F, Yao Q, Li C, Zhang H, Xue Y. Serum fibrinogen levels are positively correlated with advanced tumor stage and poor survival in patients with gastric cancer undergoing gastrectomy: a large cohort retrospective study. BMC Cancer. 2016;16:480. doi:10.1186/s12885-016-2510-z
24. Bağırsakçı E, Şahin E, Atabey N, Erdal E, Guerra V, Carr BI. Role of Albumin in Growth Inhibition in Hepatocellular Carcinoma. Oncology. 2017;93(2):136–142. doi:10.1159/000471807
25. Sleep D. Albumin and its application in drug delivery. Expert Opinion Drug Delivery. 2015;12(5):793–812. doi:10.1517/17425247.2015.993313
26. Bern M, Sand KM, Nilsen J, Sandlie I, Andersen JT. The role of albumin receptors in regulation of albumin homeostasis: implications for drug delivery. J Controlled Release. 2015;211:144–162.
27. Pacelli F, Bossola M, Rosa F, Tortorelli AP, Papa V, Doglietto GB. Is malnutrition still a risk factor of postoperative complications in gastric cancer surgery?. Clin Nutrition. 2008;27(3):398–407.
28. Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302–3309.
29. Tan Z, Zhang M, Han Q, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017;8(6):1025–1029. doi:10.7150/jca.16491
30. Zhang L, Zhang J, Wang Y, et al. Potential prognostic factors for predicting the chemotherapeutic outcomes and prognosis of patients with metastatic colorectal cancer. J Clin Lab Analysis. 2019;33(8):e22958. doi:10.1002/jcla.22958
31. Liu M, Huang Y, Huang Z, et al. The role of fibrinogen to albumin ratio in ankylosing spondylitis: correlation with disease activity. Clin Chimica Acta. 2020;505:136–140. doi:10.1016/j.cca.2020.02.029
32. Nouwen A, Adriaanse M, van Dam K, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabetic Med. 2019;36(12):1562–1572.
33. Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Management Res. 2019;11:3381–3393. doi:10.2147/CMAR.S198419
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
