Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Ferulic Acid Activates SIRT1-Mediated Ferroptosis Signaling Pathway to Improve Cognition Dysfunction in Wilson’s Disease
Authors Wang X , Shao N, Zhang X , Chen H , Chang Z, Xie D, Zhang J
Received 27 October 2023
Accepted for publication 21 November 2023
Published 5 December 2023 Volume 2023:19 Pages 2681—2696
DOI https://doi.org/10.2147/NDT.S443278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Xie Wang,1,* Nan Shao,1,* Xiaoyan Zhang,1,* Hong Chen,1,* Ze Chang,2,* Daojun Xie,3 Juan Zhang3
1The First Clinical Medical College of Anhui University of Chinese Medicine, Hefei, 230038, People’s Republic of China; 2Xiyuan Hospital of China Academy of Traditional Chinese Medicine, Beijing, 100091, People’s Republic of China; 3Department of Neurology, the First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, 230031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daojun Xie, Department of Neurology, the First Affiliated Hospital of Anhui University of Chinese Medicine, 117 Meishan Road, Hefei, Anhui, People’s Republic of China, Email [email protected]
Background: Wilson’s disease (WD), an autosomal recessive genetic disease, is characterized by copper metabolism disorder. WD patients may have a series of cognitive deficits in terms of neurological symptoms. Ferroptosis (FPT), a type of programmed cell death, is involved in the pathological progression of various cognitive disorders, and silent information regulator 1 (SIRT1) is considered to be a key factor in FPT. Ferulic acid (FA) is a traditional Chinese medicine monomer, with a remarkable effect in the clinical treatment of cognitive impairment-related disease. However, its intrinsic effect on FPT is still unclear. This study aims to investigate the protective effect of FA on cognitive impairment in animal and cell models of WD, and whether the pharmacological mechanism is related to the SIRT1-mediated FPT signaling pathway.
Methods: Copper-loaded WD rats and PC12 cells WD were used as models of cognitive dysfunction in vivo and in vitro, respectively. Morris Water Maze (MWM) was used to evaluate the spatial exploration and memory abilities of rats. HE staining was used to observe neuronal damage in the CA1 region of the rat hippocampus. Immunofluorescence (IF) was used to detect the expression of GPX4 protein. Transmission electron microscopy (TEM) was used to observe the ultrastructure of neurons. The levels of Fe2+, MDA, SOD, GSH, 4HNE, and ROS were detected. Western blot and qRT-PCR were used to detect the protein and mRNA levels of SIRT1, Nrf2, SCL7A11, and GPX4.
Results: In the WD copper-loaded model rats, MWM, TEM, and IF results showed that FA could promote the repair of learning and memory function, improve the morphological damage to hippocampal neurons, and maintain mitochondria integrity. In the PC12 cell experiment, the MTT method showed that FA increased the viability of copper-overloaded cell models. Western blot and qRT-PCR results confirmed that FA significantly increased the expression of proteins and mRNA in SIRT1, Nrf2, SCL7A11, and GPX4. In addition, FA reversed the expression of oxidative stress-related indicators, including MDA, SOD, GSH, 4HNE, and ROS.
Conclusion: FA alleviates hippocampal neuronal injury by activating SIRT1-mediated FPT, providing a valuable candidate for traditional Chinese medicine monomer for the clinical therapeutics of WD cognitive impairment.
Keywords: ferulic acid, Wilson’s disease cognitive impairment, ferroptosis, SIRT1, PC12 cells
Introduction
Wilson’s disease (WD), an autosomal recessive genetic disease, is characterized by copper metabolism disorder caused by ATP7B mutation.1 WD is one of the few genetic diseases that can be treated by intervention, with a prevalence of 1/30,000 worldwide.2 Due to copper deposits in the liver and brain, WD manifests as hepatic, psychiatric, and neurologic disorders. Almost 100% of WD patients experience psychiatric symptoms throughout the disease process, including affective, behavioral, personality, anxiety, and cognitive disturbances, which can often be reversed to some extent during anti-copper treatment.3 At present, the main treatment is to remove copper with a copper chelator and control the dietary intake of copper. It requires long-term medication and has withdrawal reactions, which seriously affect the life quality and prognosis of patients.4 Therefore, there is an urgent need to find effective drugs for cognitive impairment in Wilson’s disease (WDCI).
Traditional Chinese medicine (TCM) monomers contain rich biological activities. Ferulic acid (FA) is a natural hydroxycinnamic acid widely existing in various Chinese herbal medicines such as Angelica Sinensis, Ligusticum chuanxiong, and Salvia miltiorrhiza.5 Many investigations have shown that FA has multiple physiological characteristics, including antioxidant,6 antibacterial,7 antifibrosis,8 anti-inflammatory,9 and vascular endothelial protection.10 Currently, FA has been confirmed to have good efficacy in cognitive impairment caused by epilepsy, multiple sclerosis, ischemic hypoxia, type 2 diabetes, and high-fat diet.11–15 Therefore, FA may become a potential drug for the treatment of WDCI, but further studies are lacking to confirm it.
The brain of WD patients is usually in a high copper state, and the excessive accumulation of copper in cells can produce large amounts of lipid peroxides. In addition, an MRI study of WD patients confirmed the presence of brain iron deposition, which may together induce ferroptosis (FPT).3,16–18 FPT is a new definition of programmed cell death, characterized by iron overload, accumulation of iron-dependent lipid peroxides, mitochondrial volume reduction, and increased mitochondrial membrane thickness.19 The signaling pathway mediated by silent information regulator 1(SIRT1) is considered to be one of the crucial pathways. The activation of this pathway can reduce lipid peroxidation reaction and inhibit FPT.20 Through the deacetylation of a variety of substrates, SIRT1 can regulate key metabolic processes, including oxidative stress, apoptosis, and aging.21 Additionally, SIRT1 is an important protective transcription factor of nuclear factor E2-related factor 2 (Nrf2) signaling.22,23 Activation of Nrf2 can guard cells from FPT by directly upregulating the transcription of solute carrier family 7 member 11 (SLC7A11, also known as xCT) and glutathione peroxidase 4 (GPX4).24 Several studies have shown that activating the SIRT1-mediated signaling pathway can inhibit FPT of hippocampal neurons in animal models of cognitive impairment such as Alzheimer’s disease (AD) and other age-related diseases.25,26 Besides, FA was confirmed to reverseFPT of dorsal root ganglion.27 Based on these studies, we proposed the hypothesis that FA may activate the SIRT1-mediated FPT signaling pathway to improve cognitive function in WD copper-loaded rats.
In this study, we constructed a WD copper-loaded rat and a cell model to investigate the therapeutic effect of FA on WD and the effect of the SIRT1-mediated ferroptosis pathway. The purpose of this study is to clarify the potential regulatory mechanism of FA on WDCI at the molecular level and to provide a theoretical basis for the prevention and treatment of WD.
Materials and Methods
Animal and Drug Preparation
Forty male SD rats (SPF, male, weight 180–200 g, 3-month-old), were obtained from Jinan Pengyue Experimental Animal Breeding Co., Ltd., license number SCXK (Lu) 20190003. The experimental protocol was approved by the Experimental Animal Ethics Committee of Anhui University of Chinese Medicine (No. AHUCM-rats-2022052). Ferulic acid (100 mg/box, Cat. S31399), content ≥ 99%, was provided by Shanghai Yuanye Biotechnology Co., Ltd.
Main Reagents and Instruments
Copper sulfate (CuSO4; Sinopharm, Shanghai, China; Cat. 20211105; content ≥ 99%); the determination kit (Jiancheng, Nanjing, China) of copper ion (Cu2+; Cat. E010-1-1), ferrous ion (Fe2+; Cat. E-BC-K773-M), malondialdehyde (MDA; Cat. A003-1), trace reducing glutathione (GSH; Cat. A001-3), and superoxide dismutase (SOD; Cat. A006-2-1); reactive oxygen species (ROS) determination kit (Beibo, Shanghai, China; Cat. BB-460512); 4HNE enzyme-linked immunosorbent assay (ELISA) kit (Elabscience, Wuhan, China; Cat. ELK8373); BCA protein quantitation kit (Biyuntian, Shanghai, China; Cat. P0012); the antibody (Bioss, Beijing, China) of Nrf2 (Cat. bs-1074R), SLC7A11 (Cat. bs-6883R), GPX4 (Cat. bs-6883R); SIRT1 antibody (Santa Cruz, CA, USA; Cat. SC-74504); GAPDH antibody (Immunoway, TX, USA; Cat. YN5585); ferrostatin-1 (MedChemExpress, NJ, USA; Cat. HY-100579); HRP-conjugated goat anti-rabbit IgG (Abbkine, Wuhan, China; Cat. A21021); hematoxylin (HE) staining solution (ebiogo, Hefei, China; Cat. B006); ECL chemiluminescence kit (Biosharp, Hefei, China; Cat. BL520A); PrimeScriptTM RT reagent Kit (TaKaRa, Tokyo, Japan; Cat. RR047A); TB Green®Premix Ex Taq™II Kit (TAKARA, Tokyo, Japan; Cat. RR820A); multifunctional enzyme-linked immunosorbent assay instrument (Peiou, Shanghai, China; Cat. 318C+); Morris water maze (MWM) system (Taimeng, Chengdu, China; Cat. YMT-100); ultrathin sectioning machine (Leica, Wetzlar, Germany; Cat. UC-7); transmission electron microscope (Hitachi, Tokyo, Japan; Cat. HT7800); electrophoresis apparatus (Bio-Rad, CA, USA; Cat. 1645050).
Animal Modeling, Grouping and Administration
Forty SD rats were randomly divided into 4 groups: Control group, Model group, low dose of ferulic acid group (FA-L group), and high dose of ferulic acid group (FA-H group). After adaptive feeding for 1 week, the Model, FA-L, and FA-H groups were fed with copper-containing (1 g/kg) feed and copper-containing (0.185%) water for 12 weeks to establish a copper-loaded rat model.28 From the 9th week, the FA-L, and FA-H groups were given different doses of FA (25, 50 mg/kg/d, respectively) by intragastric administration.29 The Control group was given an equal amount of physiological saline by gavage.
Kit for Detecting Liver Copper and 24 h Urinary Copper Levels in Rats
Fresh liver tissue (0.5 g) was washed with normal saline, homogenized, and centrifuged to obtain the supernatant. The copper content in urine was detected 24 h before sacrifice. According to the instructions of the copper ion reagent kit, the liver supernatant or 24 h of urine collected was added to a 96-well plate and incubated at 37°C. Finally, the optical density (OD) values were measured at 600 nm in a microplate reader.
Morris Water Maze (MWM) for Evaluating the Spatial Exploration and Memory Ability of Rats
On day 22 after administration, MWM test was performed to detect the spatial exploration and memory ability of rats. The device consisted of a circular water tank (diameter 170 cm, height 50 cm) divided into four quadrants with a water temperature of 25 ± 1°C. A circular platform with a radius of 6 cm was placed in the middle of the target quadrant. Spatial training was performed during the first 4 days. Rats were placed in each quadrant successively to start swimming. On day 5, rats were placed in the contralateral quadrant of the platform, and the time to reach the platform considered to be the escape latency. On day 6, the platform was taken out, and the swimming time and distance in the platform quadrant and the times of crossing platform were recorded.
HE Staining for Observing the Neuronal Injury in CA1 Region of Rat Hippocampus
After fixation with 4% paraformaldehyde, the brain tissue was embedded in paraffin and sliced (thickness 5 μm). After gradient dewaxing and dehydration with xylene and ethanol, HE staining was performed to observe the morphological changes of hippocampal CA1 neurons under an optical microscope.
Immunofluorescence (IF) for Detecting the Expression of GPX4 Protein in Rat Hippocampus
The paraffin sections were baked to dewax, penetrated with 0.3% Triton X-100 for 5 min, blocked with 5% BSA at room temperature for 1 h, and incubated with GPX4 antibody (1:100) overnight at 4°C. After washing, the slides were incubated with fluorescent secondary antibody for 1 h at room temperature in the dark and sealed with anti-fluorescence quencher. Finally, the expression of GPX4 protein in the hippocampal CA1 region of rats was observed by fluorescence microscopy.
Cell Culture
To investigate the effect of FA on high copper-induced neuronal death, we used CuSO4 to induce the excitotoxicity of PC12 cells (from the rat adrenal pheochromocytoma cell line, purchased by the Chinese Academy of Sciences Shanghai Cell Bank) to establish copper-loaded cell model. Resuscitated PC12 cells were inoculated into culture bottles, and DMEM high glucose medium containing 10% fetal bovine serum was added. The cells were incubated in a cell incubator at 37°C and 5% CO2. After the cells grew stably, they were used for experiments.
Cell Viability Detection
(1) The PC12 cells were treated with different concentrations of CuSO4 (50, 100, 150, 200, 250, and 300 μM) and cultured for 12 h, 24 h, and 48 h, respectively. MTT assay was used to detect cell viability and screen for the optimal modeling concentration of CuSO4. (2) The PC12 cells were treated with different concentrations of FA (0, 2.5, 5, 10, 20, 40, 60, and 80 μM) and cultured for 24 h. The MTT method was used to determine the cell viability and detect the safe concentration of FA. (3) PC12 cells were divided into 5 groups: the Control group, Model group, FA low-dose group (FA-L group), FA medium-dose group (FA-M group), and FA high-dose group (FA-H group). Except for the Control group, all other groups used CuSO4 to construct a copper-load cell model. The FA group was given different concentrations of FA to culture cells for 24 h. MTT assay was used to determine cell viability and screen for the optimal concentration of FA. (4) Copper-loaded cells were treated with different concentrations of Fer-1 (2.5, 5, 10, 20, 40 μM) and incubated for 24 h. MTT assay was used to determine cell viability and screen for the optimal inhibitor concentration. (5) According to the screening results of the optimal concentrations of FA and Fer-1, subsequent experiments were divided into 5 groups: the Control group, Model group, FA group, Fer-1 group, and FA+Fer-1 group. Except for the Control group, all other groups used CuSO4 to construct a copper-load model. The administration group was given FA or Fer-1 to culture cells for 24 h.
Observation of Neuronal Ultrastructure with Transmission Electron Microscopy (TEM)
TEM was used to examine the mitochondria ultrastructure of neurons in the rat hippocampus CA1 area and PC12 cells. The isolated samples were immediately fixed in 2.5% glutaraldehyde. After dehydrated, soaked, embedded, sliced, and stained, the samples were examined and photographed by transmission electron microscope.
Determination of Fe2+, MDA, SOD, GSH, 4HNE and ROS Levels
To determine the levels of MDA, SOD, Fe2+, GSH, and ROS in rat hippocampus or PC12 cells, we used Fe2+, MDA, SOD, GSH, and ROS assay kits according to the manufacturer’s instructions. To assess the level of the lipid peroxidation marker 4HNE, we used the ELISA kit of 4HNE according to the manufacturer’s instructions.
Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) for Detecting the mRNA Levels of SIRT1, Nrf2, SCL7A11, and GPX4
Total mRNA was extracted from mice hippocampal neurons and PC12 cells using Trizol method. Then, the mRNA was converted to cDNA by reverse transcription. Applied Biosystems 7500 Real-Time PCR System and TB Green®Premix Ex Taq™II Kit were used for RT-qPCR experiments. The primer sequences used in this study were designed by Shenggong Bioengineering Co., Ltd. (Shanghai, China) as shown in Table 1. By using the 2−ΔΔCt method, the relative expression level of mRNA was determined.
 |
Table 1 Primer Sequences and Product Lengths of SIRT1, Nrf2, SCL7A11, GPX4 and β-Actin |
Results
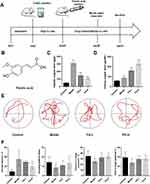
FA Alleviated Learning and Memory Dysfunction in WD Copper-Loaded Model Rats
We established a WD model with copper loading in rats (Figure 1A). The FA-L and FA-H groups were given different concentrations of FA (Figure 1B). Compared with the Control group, the 24 h urinary copper and liver copper levels in the Model group were increased (P < 0.001) (Figure 1C and D), which is consistent with the characteristics of WD copper metabolism. In addition, the MWM experiment showed that compared with the Control group, in the model group, the trajectory was long and chaotic (Figure 1E), the escape latency increased (P < 0.001), the number of crossing platforms reduced (P < 0.05), and the distance and time in the target quadrant decreased (P < 0.05) (Figure 1F). These results proved the existence of cognitive dysfunction in the WD copper-loading rats. After FA intervention, the 24 h urinary copper level increased (P < 0.001) and the liver copper level decreased (P < 0.001) in WD copper-loaded rats, especially in the FA-H group (Figure 1C and D). In the MWM test, the FA-H group had decreased escape latency time (P < 0.01), increased the number of crossing platform (P < 0.05), and increased distance and time in the target quadrant (P < 0.05) (Figure 1E and F). The above results indicated that FA could alleviate learning and memory dysfunction in copper-loaded model rats.
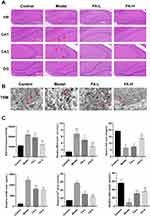
FA Rescued FPT in Hippocampal Neurons of WD Copper-Loaded Model Rats
HE staining was used to explore the protective effect of FA on hippocampal neurons in WD copper-loaded model rats. The hippocampal neurons in the Control group had normal morphology, regular and orderly arrangement, and plump and round nuclei. However, the cells in the Model group were disordered, with deep staining and pyknosis of nuclei and irregular shape, especially in the area of CA1 and CA3. Although the cells in the FA-L group were neatly arranged, there were still many nuclei with deep staining and pyknosis. After FA-H treatment, the cells were significantly improved compared with the model group, and the cell morphology tended to be normal (Figure 2A). In order to further explore the mechanism of hippocampal neuron damage in copper-loaded model rats, we used TEM to detect the neurons ultrastructure in the CA1 region of the rat hippocampus. It was found that the nuclei in the Control group were regular, the cytoplasmic contents were plump, and the mitochondrial structure was clear. In the Model group, the cytoplasmic contents were reduced, the mitochondrial volume was reduced, the cristae were broken, and the membrane was thickened, which were the morphological changes unique to FPT. However, the cell structure in the FA-H group was more complete, the mitochondrial structure was clear, the volume was basically normal, and the membrane contents were normal (Figure 2B). These results suggested that the role of FA in rescuing learning and memory dysfunction in copper-loaded model rats might be related to the FPT pathway. In addition, we measured the levels of Fe2+, MDA, SOD, GSH, and 4HNE in hippocampal tissues of rats. Compared with the Control group, the levels of Fe2+, MDA, 4HNE, and ROS in the Model group were significantly increased (P < 0.001), while the levels of GSH and SOD were significantly decreased (P < 0.001). Compared with the Model group, the FA-H and FA-L groups showed that FA intervention could significantly reverse the levels of Fe2+, MDA, SOD, GSH, 4HNE, and ROS (P < 0.05 or P < 0.001). The improvement in the FA-H group was more obvious than that in the FA-L group (Figure 2C). The process of lipid peroxidation level is an important mechanism for the occurrence and development of FPT in neurons. FA pretreatment could significantly reverse the expressions of lipid peroxidation-related indicators.
FA Improved FPT of Hippocampal Neurons in WD Copper-Loaded Model Rats Through SIRT1/Nrf2/SLC7A11/GPX4 Signaling Pathway
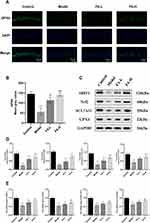
In order to further determine the FPT of WD copper-loaded model rats, IF was used to observe the expression of GPX4, a key protein of FPT. Compared with the Control group, the hippocampus in the Model group showed a decrease in GPX4 green fluorescence (P < 0.001). However, FA treatment increased the fluorescence expression of GPX4 in the hippocampus of WD rat models, with high-dose FA having the best effect (P < 0.001) (Figure 3A and B). In addition, we compared the expressions of SIRT1/Nrf2/SCL7A11/GPX4 signaling pathway related proteins. Compared with the Control group, the protein expressions of SIRT1, Nrf2, SCL7A11, and GPX4 in the Model group were decreased (P < 0.001). Compared with the Model group, the expressions of these proteins in the FA-H group were increased (P < 0.001) (Figure 3C and D). In addition, we compared the mRNA contents of SIRT1, Nrf2, SCL7A11, and GPX4 in the hippocampus of rats in each group. The Model group had significantly lower mRNA levels of SIRT1, Nrf2, SCL7A11, and GPX4 compared to the Control group (P < 0.001). Whereas, the FA-H group had significantly higher mRNA levels of SIRT1, Nrf2, SCL7A11, and GPX4 compared to the Model group (P < 0.001) (Figure 3E). These results found that FA might improve the FPT of hippocampal neurons in WD copper-loaded model rats through the SIRT1/Nrf2/SLC7A11/GPX4 pathway.
FA Had a Neuroprotective Effect on the FPT of Copper-Loaded Neurons
We established a copper-loaded cell model by intervening with CuSO4 in PC12 cells. MTT method was used to evaluate the effect of CuSO4 on PC12 cells. The results showed that the cell viability decreased to 50% after incubation with 200 µM CuSO4 for 24 h, indicating that 200 μM was the optimal concentration and 24 h was the optimal time for modeling (Figure 4A). By detecting the vitality of PC12 cells treated with different concentrations of FA, it was found that the concentration of FA at 60 μM was toxic to cells. Therefore, 10, 20, and 40 μM were selected as the drug concentrations for subsequent experiments to explore the effect of FA on the vitality of the copper-loaded cell model (Figure 4B). The results showed that FA treatment improved the viability of WD copper-loaded cells in a dose-dependent manner. About 40 μM was selected as the optimal therapeutic concentration for FA for subsequent studies (Figure 4C). Moreover, we screened the optimal concentration of FPT inhibitor Fer-1. Fer-1 at 10 μM had the most significant effect on improving the viability of WD copper-loaded cells and was used for subsequent experiments (Figure 4D). MTT assay showed that the cell viability of Model group was significantly reduced compared to the Control group (P < 0.01). Both FA and Fer-1 had significant neuroprotective effects, which were enhanced after combined treatment (P < 0.001) (Figure 4E). Therefore, we speculated that the neuroprotective effect of FA on copper-loaded neurons was related to the inhibition of FPT pathway. TEM detection further verified our hypothesis. Neurons in the Model group showed mitochondrial shrinkage, cristae rupture, and membrane thickening, which were typical characteristics of FPT. The FA group, Fer-1 group, and FA+Fer-1 group were significantly improved (Figure 4F). In addition, compared with the Control group, the levels of Fe2+, MDA, 4HNE, and ROS were significantly increased in the Model group, while the levels of GSH and SOD were significantly decreased (P < 0.001). Compared with the Model group, the levels of Fe2+, MDA, ROS, and 4HNE in the FA, Fer-1, and FA+Fer-1 groups were significantly reduced, while the levels of GSH and SOD were significantly increased. (P < 0.001) (Figure 4G). These results suggested that similar to the effect of Fer-1, FA can significantly reverse the changes in lipid peroxidation-related indicators, rescue FPT in copper-loaded neurons, and play a role in protecting neurons.
FA Activated SIRT1/Nrf2/SLC7A11/GPX4 Signaling Pathway to Improve FPT of Copper-Loaded Neuronal Cells
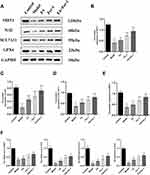
To investigate if the neuroprotective impact of FA on copper-loaded neuronal FPT is through the SIRT1-mediated pathway, we detected the protein and mRNA expressions of SIRT1, Nrf2, SCL7A11, and GPX4 in each group. Compared with the Control group, the protein expression of SIRT1, Nrf2, SCL7A11, and GPX4 in the Model group decreased (P < 0.001). After the administration of FA, Fer-1, and Fer-1 + FA, the expression of these proteins increased significantly (P < 0.01 or P < 0.001) (Figure 5A–E). Furthermore, the mRNA content of SIRT1, Nrf2, SCL7A11, and GPX4 in the Model group was significantly decreased compared to the Control group (P < 0.01). However, the mRNA levels were higher in the FA, Fer-1, and Fer-1 + FA group than in the Model group (P < 0.01 or P < 0.001) (Figure 5F). These results were consistent with the results in vivo, further confirming that FA inhibited the FPT of neuron through the SIRT1/Nrf2/SLC7A11/GPX4 pathway.
Discussion
WDCI was first proposed in the early 20th century. Twenty percent of patients with neuromotor disorders as the first symptom had cognitive decline in the early stage of onset.30–32 Previous studies confirmed that WDCI patients mainly manifested as subcortical dementia and frontal lobe syndrome, usually characterized by memory loss, executive dysfunction, behavior and personality changes, and emotional and mental disorders.33–35 Copper and iron are trace metal elements essential for biological processes, which enter the brain parenchyma through the blood–brain barrier under the action of blood circulation and various enzymes.36,37 WD patients suffer from brain involvement and neuropsychiatric dysfunction due to copper metabolism disorders caused by ATP7B gene mutations, of which cognitive dysfunction is one of the neuropsychiatric symptoms.38,39 Studies have also shown that there is an excessive deposition of copper and iron in the brains of patients with WD, and the deposition of copper and iron in the brain can also be detected by 3 Tesla MRI quantitative susceptibility mapping. The deposition of these metal substances may be significantly correlated with the occurrence of cognitive dysfunction.40 This view was also verified by a 7 Tesla MRI plain scan of 9 WD cadaveric brain tissues.16 Some studies have confirmed that the deposition of brain iron may promote oxidative stress, leading to damage of nerve cells, which in turn affects neural transmission and exacerbates the development of cognitive impairment.41 In addition, many scholars have conducted research on copper homeostasis imbalance related to cognitive disorders. The study found that the increased copper level in the hippocampus may be one of the pathogenesis of cognitive dysfunction in AD and type 2 diabetes.42,43 In addition, high copper in the hippocampus reduces the learning and memory ability of mice, and the mechanism may be related to the impaired function of ROS.44–46 Therefore, the WD copper-loaded rat model was established in this study. Compared with the control group, the 24 h urinary copper and liver copper levels of rats in the Model group were increased, indicating that the modeling of WD copper-loaded rats was successful.47 In the PC12 cell experiment, high copper concentration affected cell viability in a dose-dependent manner. When the cells were treated with 200 μM CuSO4 for 24 h, the cell viability decreased significantly. Morris water maze experiment evaluated the spatial exploration and memory ability of WD copper-loaded rats. The results showed that compared with the Control group, the spatial exploration and memory ability of rats in the Model group decreased, indicating that the WD copper-loaded rats model had cognitive impairment.
The latest guidelines pointed out that no drugs have been approved for the treatment of WDCI up to now.48 At present, the main drugs used in WDCI are copper chelators such as D-penicillamine and trientine, and metal ion antagonists such as zinc salts. However, about 10% of patients experience neuropsychiatric deterioration during treatment. Secondly, about one-third of WD patients experience drug-related adverse events. Therefore, the exploration of new treatment methods for WD in the future is becoming increasingly urgent. Bischoline tetrathiomolybdate (TTM), once-daily trientine, and methanobactin are considered to be potential therapeutic agents for WD, but they are still in the animal and clinical trial stages.38 The discussion of gene therapy and liver cell transplantation is increasingly becoming a hot topic. Some studies have confirmed that the autologous reprogramming technology of ATP7B gene therapy can repair the function and efficacy of liver cells in WD model mice, which provides a new direction for the treatment of WD.49 In addition, traditional Chinese medicine (TCM) monomers have rich biological activity. FA, 4-hydroxy-3-methoxy cinnamic acid (C10H10O4, relative molecular mass 194.19), was first discovered in Angelica sinensis in 1986. It is a phenolic acid widely existing in the plant kingdom, commonly found in grains, spinach, parsley, and grapes, and also in a variety of Chinese herbal medicines, such as Gramineae, buttercups, and Umbelliferae.50,51 FA is easy to be absorbed by the human body, can be excreted with urine, and has low toxicity, safety, and strong antioxidant effects.52 Therefore, more and more attention has been paid to the medicinal value of FA. Multiple studies showed that FA had a strong neuroprotective effect and improved cognitive dysfunction complicated by various causes. FA has been confirmed to improve Aβ plaque deposition, and spatial memory defects, and protects hippocampal neurons in AD model rats and mice.14,53 A recent study confirmed that FA has a certain effect on cognitive impairment complicated by epilepsy, which may be related to inhibiting neuronal oxidative stress.12 In addition, FA can improve the nerve injury in mice with cognitive impairment induced by high-fat diet, inhibit oxidative stress and cell apoptosis, which has positive significance for the prevention of age-related diseases.15 However, there are currently no research reports on the treatment of WDCI with FA. Therefore, this study aims to investigate the neuroprotective effect of FA in a rat and cell model of WD copper-loaded. The results showed that FA could promote the excretion of copper in the body of WD copper-loaded rats, improve spatial exploration and memory ability, and alleviate hippocampal neuronal damage, with the most significant effect at a high dose (50mg/kg/d). In addition, cell experiments confirmed that when the concentration of FA reached 40 μM, it could most obviously improve the neuronal damage in a copper-loaded cell model. Therefore, we speculate that the monomer FA of TCM may be a potential drug for the treatment of WDCI.
Previous studies have shown that copper accumulation can lead to the occurrence of FPT. Since the ATP7B gene encodes a copper-transporting P-type ATPase, mutations in this gene lead to copper transport disorders in circulation and biliary excretion, further causing a decrease in ceruloplasmin (CP).38 CP has ferroxidase and antioxidant effect, which can oxidize Fe2+ into Fe3+. When CP is reduced, a large amount of Fe2+ in nerve cells of brain tissue cannot be oxidized into Fe3+. Fe2+ has extremely strong oxidation and is prone to cause Fenton reaction in nerve cells, which can produce hydroxyl radicals that cause oxidative damage to DNA, protein and membrane lipid.54 The results of TEM in this study showed that a large amount of FPT neurons occurred in WD rats and cells models with copper load. The oxidative stress-related indicators suggested that Fe2+ accumulation and Fenton reaction had occurred in animal and cell models, leading to changes in the levels of MDA, SOD, GSH, 4HNE, and ROS and inducing the occurrence of FPT. In addition, several studies have shown that GPX4 is a key regulatory factor for FPT.55,56 The IF results of GPX4 in rat hippocampus showed that GPX4 was highly expressed in the model group, which was consistent with the expressions of mRNA and protein and also indicated the occurrence of FPT. However, FA-H treatment significantly reduced oxidative stress injury in neurons, improved mitochondrial FPT, and increased the expression of key regulator GPX4. In summary, FPT was activated in the hippocampus of WD copper-loaded rats and cells, and FA treatment significantly inhibited neuronal FPT.
The regulatory mechanism of FPT is relatively complex, mainly involved in biological processes such as iron metabolism disorder, imbalance of amino acid antioxidant system, accumulation of lipid peroxide and non-classical mechanisms.56 Among the many signaling pathways regulating FPT, the SIRT1-mediated signaling pathway is receiving increasing attention from researchers. SIRT1 is a member of the deacetylase protein family, widely distributed in the hippocampus, cerebral cortex, hypothalamus, and cerebellum, and is mainly expressed by neurons and microglia.57 In addition, SIRT1 is an important neuroprotective and repair molecule with a powerful antioxidant stress effect, which has been proved to be necessary for the regulation of synaptic plasticity and cognitive function.58,59 Multiple studies showed that its function played a large extent depends on the activation of Nrf2.60,61 In recent years, some studies confirmed that activation of Nrf2 was involved in the inhibition of FPT and played a protective role in a variety of cognitive disorders related diseases.62,63 SLC7A11 is a cystine/glutamate transporter gene, a key gene regulating “iron overload-mediated FPT”, and also an important part of the reverse amino acid transport system. The expression of SLC7A11 was significantly reduced in a variety of cognitive disorders related diseases, and its knockout could induce FPT in hippocampal neurons.64 GPX4 can prevent lipid peroxidation through antioxidant activity, maintain the homeostasis of membrane lipid bilayer, and inhibit FPT.65 In addition, the inhibition of Nrf2 and SLC7A11 can also reduce the expression of GPX4.66,67 At present, relevant studies on a variety of diseases confirmed that SIRT1-mediated signaling pathway was closely related to FPT.20,67–70 Therefore, we speculated that the inhibitory effect of FA on neurons FPT in WD copper-loaded rats and cells models might be related to SIRT1/Nrf2/SCL7A11/GPX4 signaling pathway. In this study, compared with the Control group, the protein and mRNA expressions of SIRT1, Nrf2, SCL7A11, and GPX4 in the Model group were decreased, suggesting that the SIRT1-mediated signaling pathway was involved in the activation of FPT in WD copper-loaded rats and cells models. After FA treatment, the protein and mRNA expressions of SIRT1, Nrf2, SCL7A11, and GPX4 in WD copper-loaded models were increased. In addition, the inhibition experiment of FPT in vitro found that FA had a similar effect to Fer-1, indicating that FA might inhibit FPT by regulating the SIRT1-mediated signaling pathway in WD copper-loaded models (Figure 6).
 |
Figure 6 The mechanism of FA improving WD cognitive impairment by activating SIRT1-mediated FPT signaling pathway. |
In conclusion, FA has neuroprotective effects on WD rats and cells models in a dose–response relationship, which may be related to inhibiting FPT by regulating SIRT1/Nrf2/SCL7A11/GPX4 signaling pathway. However, this study lacks positive controls and clinical efficacy studies. In the future, we will further explore the regulatory mechanism of FPT and its upstream and downstream signaling pathways involved in WD with copper overload, so as to provide a basis for the development of new therapies for WD cognitive impairment.
Data Sharing Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Ethical Approval and Consent to Participate
All the animals were obtained from Jinan Pengyue Experimental Animal Breeding Co., Ltd., license number SCXK (Lu) 20190003. All animal experiments were approved by the Experimental Animal Ethics Committee of AnHui University of Chinese Medicine (Hefei, Anhui, China; animal ethics number: AHUCM-rats-2022052) and were in complete with the ethical guidelines of the NIH Guide for the Care and Use of Laboratory Animals. All rats were housed under the following housing conditions: temperature controlled at 21–23°C, 55%, 12 hours of light, and 12 hours of dark cycles, with free access to food and water.
Consent for Publication
All authors agree to publish this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas, took part in drafting, revising or critically reviewing the article, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Natural Foundation of China under the National Science Foundation Program (Grant No. 81874389).
Disclosure
The authors have no known competing financial interests or personal relationships that may influence the work reported here.
References
1. Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103–113. doi:10.1016/S1474-4422(14)70190-5
2. Sandahl TD, Laursen TL, Munk DE, Vilstrup H, Weiss KH, Ott P. The prevalence of Wilson’s disease: an Update. Hepatology. 2020;71(2):722–732. doi:10.1002/hep.30911
3. Litwin T, Dusek P, Szafrański T, Dzieżyc K, Członkowska A, Rybakowski JK. Psychiatric manifestations in Wilson’s disease: possibilities and difficulties for treatment. Ther Adv Psychopharmacol. 2018;8(7):199–211. doi:10.1177/2045125318759461
4. Roberts EA. Update on the diagnosis and management of Wilson disease. Current Gastroenterol Reports. 2018;20(12):56. doi:10.1007/s11894-018-0660-7
5. Chaudhary A, Jaswal VS, Choudhary S, et al. Ferulic acid: a promising therapeutic phytochemical and recent patents advances. Recent Pat Inflamm Allergy Drug Discov. 2019;13(2):115–123. doi:10.2174/1872213X13666190621125048
6. Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol. 2018;31(6):332–336. doi:10.1159/000491755
7. Alim Z, Kilinç N, Şengül B, Beydemir Ş. Inhibition behaviours of some phenolic acids on rat kidney aldose reductase enzyme: an in vitro study. J Enzyme Inhib Med. 2017;32(1):277–284. doi:10.1080/14756366.2016.1250752
8. Ali SA, Saifi MA, Pulivendala G, Godugu C, Talla V. Ferulic acid ameliorates the progression of pulmonary fibrosis via inhibition of TGF-β/smad signalling. Food and Chemical Toxicology. 2021;149:111980. doi:10.1016/j.fct.2021.111980
9. Mahmoud AM, Hussein OE, Abd El-Twab SM, Hozayen WG. Ferulic acid protects against methotrexate nephrotoxicity via activation of Nrf2/ARE/HO-1 signaling and PPARγ, and suppression of NF-κB/NLRP3 inflammasome axis. Food Funct. 2019;10(8):4593–4607. doi:10.1039/C9FO00114J
10. Zhou ZY, Xu JQ, Zhao WR, et al. Ferulic acid relaxed rat aortic, small mesenteric and coronary arteries by blocking voltage-gated calcium channel and calcium desensitization via dephosphorylation of ERK1/2 and MYPT1. Eur J Pharmacol. 2017;815:26–32. doi:10.1016/j.ejphar.2017.10.008
11. Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R. Ferulic acid exhibits antiepileptogenic effect and prevents oxidative stress and cognitive impairment in the kindling model of epilepsy. Life Sci. 2017;179:9–14. doi:10.1016/j.lfs.2016.08.011
12. Ghobadi M, Arji B, Yadegari M, Esmailidehaj M, Homayouni Moghadam F, Rezvani ME. Ferulic acid ameliorates cell injuries, cognitive and motor impairments in cuprizone-induced demyelination model of multiple sclerosis. Cell J. 2022;24(11):681–688. doi:10.22074/cellj.2022.8261
13. Wang H, Sun X, Zhang N, et al. Ferulic acid attenuates diabetes-induced cognitive impairment in rats via regulation of PTP1B and insulin signaling pathway. Physiol Behav. 2017;182:93–100. doi:10.1016/j.physbeh.2017.10.001
14. Mei Z, Hong Y, Yang H, et al. Ferulic acid alleviates high fat diet-induced cognitive impairment by inhibiting oxidative stress and apoptosis. Eur J Pharmacol. 2023;946:175642.
15. Yao K, Yang Q, Li Y, Lan T, Yu H, Yu Y. MicroRNA-9 mediated the protective effect of ferulic acid on hypoxic-ischemic brain damage in neonatal rats. PLoS One. 2020;15(5):e0228825. doi:10.1371/journal.pone.0228825
16. Dusek P, Bahn E, Litwin T, et al. Brain iron accumulation in Wilson disease: a post mortem 7 Tesla MRI - histopathological study. Neuropathol Appl Neurobiol. 2017;43(6):514–532.
17. Belyaeva EA, Sokolova TV, Emelyanova LV, Zakharova IO. Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. TheScientificWorldJournal. 2012;2012:136063. doi:10.1100/2012/136063
18. Xue Q, Yan D, Chen X, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19(7):1982–1996.
19. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
20. Qiongyue Z, Xin Y, Meng P, et al. Post-treatment With Irisin Attenuates Acute Kidney Injury in Sepsis Mice Through Anti-Ferroptosis via the SIRT1/Nrf2 Pathway. Front Pharmacol. 2022;13:857067. doi:10.3389/fphar.2022.857067
21. Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev. 2017;2017:7543973. doi:10.1155/2017/7543973
22. Do MT, Kim HG, Choi JH, Jeong HG. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med. 2014;74:21–34. doi:10.1016/j.freeradbiomed.2014.06.010
23. Zhang X, Yu Y, Lei H, et al. The Nrf-2/HO-1 signaling axis: a ray of hope in cardiovascular diseases. Cardiol Res Pract. 2020;2020:5695723. doi:10.1155/2020/5695723
24. Dai C, Chen X, Li J, Comish P, Kang R, Tang D. Transcription factors in ferroptotic cell death. Cancer Gene Ther. 2020;27(9):645–656. doi:10.1038/s41417-020-0170-2
25. Yang Y, Wang X, Xiao A, Han J, Wang Z, Wen M. Ketogenic diet prevents chronic sleep deprivation-induced Alzheimer’s disease by inhibiting iron dyshomeostasis and promoting repair via Sirt1/Nrf2 pathway. Front Aging Neurosci. 2022;14:998292. doi:10.3389/fnagi.2022.998292
26. Liu J, Huang J, Zhang Z, et al. Mesenchymal stem cell-derived exosomes ameliorate delayed neurocognitive recovery in aged mice by inhibiting hippocampus ferroptosis via activating SIRT1/Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2022;2022:3593294. doi:10.1155/2022/3593294
27. Liu T, Wang R, Qi W, et al. Methyl ferulic acid alleviates neuropathic pain by inhibiting nox4-induced ferroptosis in dorsal root ganglia neurons in rats. Mol Neurobiol. 2023;60(6):3175–3189. doi:10.1007/s12035-023-03270-6
28. Xu J, Jiang H, Li J, Cheng KK, Dong J, Chen Z. 1H NMR-based metabolomics investigation of copper-laden rat: a model of Wilson’s disease. PLoS One. 2015;10:4.
29. Liu G, Nie Y, Huang C, et al. Ferulic acid produces neuroprotection against radiation-induced neuroinflammation by affecting NLRP3 inflammasome activation. Int J Radiat Biol. 2022;98(9):1442–1451. doi:10.1080/09553002.2022.2055798
30. Compston A. Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver, by S. A. Kinnier Wilson, (From the National Hospital, and the Laboratory of the National Hospital, Queen Square, London) Brain 1912: 34; 295–509. Brain. 2009;132(Pt 8):1997–2001. doi:10.1093/brain/awp193
31. Walshe JM, Yealland M. Wilson’s disease: the problem of delayed diagnosis. J Neurol Neurosurg Psychiatr. 1992;55(8):692–696. doi:10.1136/jnnp.55.8.692
32. Lauterbach EC, Cummings JL, Duffy J, et al. Neuropsychiatric correlates and treatment of lenticulostriatal diseases: a review of the literature and overview of research opportunities in Huntington’s, Wilson’s, and Fahr’s diseases. A report of the ANPA Committee on Research. American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 1998;10(3):249–266. doi:10.1176/jnp.10.3.249
33. Machado A, Chien HF, Deguti MM, et al. Neurological manifestations in Wilson’s disease: report of 119 cases. Mov Disord. 2006;21(12):2192–2196. doi:10.1002/mds.21170
34. Fonseca LM, Yokomizo JE, Bottino CM, Fuentes D. Frontal lobe degeneration in adults with down syndrome and alzheimer’s disease: a review. Dement Geriatr Cogn Disord. 2016;41(3–4):123–136. doi:10.1159/000442941
35. Scheiber IF, Brůha R, Dušek P. Pathogenesis of Wilson disease. Handb Clin Neurol. 2017;142:43–55.
36. Li Y. Copper homeostasis: emerging target for cancer treatment. IUBMB Life. 2020;72(9):1900–1908. doi:10.1002/iub.2341
37. Ge EJ, Bush AI, Casini A, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22(2):102–113. doi:10.1038/s41568-021-00417-2
38. Litwin T, Dzieżyc K, Członkowska A. Wilson disease-treatment perspectives. Ann Transl Med. 2019;7(Suppl 2):S68. doi:10.21037/atm.2018.12.09
39. Li WJ, Wang JF, Wang XP. Wilson’s disease: update on integrated Chinese and Western medicine. Chin J Integr Med. 2013;19(3):233–240. doi:10.1007/s11655-012-1089-8
40. Li G, Wu R, Tong R, et al. Quantitative measurement of metal accumulation in brain of patients with Wilson’s disease. Mov Disord. 2020;35(10):1787–1795. doi:10.1002/mds.28141
41. Gao G, You L, Zhang J, Chang YZ, Yu P. Brain iron metabolism, redox balance and neurological diseases. Antioxidants. 2023;12(6):1289.
42. Philbert SA, Schönberger SJ, Xu J, Church SJ, Unwin RD, Cooper GJS. Elevated hippocampal copper in cases of type 2 diabetes. EBioMedicine. 2022;86:104317.
43. Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273(21):12817–12826. doi:10.1074/jbc.273.21.12817
44. Gu L, Yu J, He Y, Fan Y, Sheng J. Blood copper excess is associated with mild cognitive impairment in elderly Chinese. Aging Clin Exp Res. 2022;34(5):1007–1019. doi:10.1007/s40520-021-02034-3
45. Pal A, Prasad R. Regional distribution of copper, zinc and iron in brain of Wistar rat model for non-Wilsonian brain copper toxicosis. Indian J Clin Biochem. 2016;31(1):93–98. doi:10.1007/s12291-015-0503-3
46. Pal A, Prasad R. Recent discoveries on the functions of astrocytes in the copper homeostasis of the brain: a brief update. Neurotox Res. 2014;26(1):78–84. doi:10.1007/s12640-013-9453-9
47. Wang N, Cheng M, Zhang X, et al. Gandou decoction decreases copper levels and alleviates hepatic injury in copper-laden hepatolenticular degeneration model rats. Front Pharmacol. 2020;11:582390. doi:10.3389/fphar.2020.582390
48. Lucena-Valera A, Ruz-Zafra P, Ampuero J. Wilson’s disease: overview. Med Clin. 2023;160(6):261–267. doi:10.1016/j.medcli.2022.12.016
49. Cai H, Cheng X, Wang XP. ATP7B gene therapy of autologous reprogrammed hepatocytes alleviates copper accumulation in a mouse model of Wilson’s disease. Hepatology. 2022;76(4):1046–1057. doi:10.1002/hep.32484
50. Mathew S, Abraham TE. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004;24(2–3):59–83. doi:10.1080/07388550490491467
51. Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem. 2008;109(4):691–702. doi:10.1016/j.foodchem.2008.02.039
52. Picone P, Nuzzo D, Di Carlo M. Ferulic acid: a natural antioxidant against oxidative stress induced by oligomeric A-beta on sea urchin embryo. Biol Bull. 2013;224(1):18–28. doi:10.1086/BBLv224n1p18
53. Wang NY, Li JN, Liu WL, et al. Ferulic acid ameliorates alzheimer’s disease-like pathology and repairs cognitive decline by preventing capillary hypofunction in APP/PS1 mice. Neurotherapeutics. 2021;18(2):1064–1080. doi:10.1007/s13311-021-01024-7
54. Hilton JB, White AR, Crouch PJ. Endogenous Cu in the central nervous system fails to satiate the elevated requirement for Cu in a mutant SOD1 mouse model of ALS. Metallomics. 2016;8(9):1002–1011. doi:10.1039/C6MT00099A
55. Yagoda N, von Rechenberg M, Zaganjor E, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–868. doi:10.1038/nature05859
56. Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi:10.1038/s41419-020-2298-2
57. Ramadori G, Lee CE, Bookout AL, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28(40):9989–9996. doi:10.1523/JNEUROSCI.3257-08.2008
58. Zhang F, Wang S, Gan L, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95(3):373–395. doi:10.1016/j.pneurobio.2011.09.001
59. Zhang XS, Wu Q, Wu LY, et al. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016;7(10):e2416. doi:10.1038/cddis.2016.292
60. Koronowski KB, Khoury N, Saul I, et al. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) regulates glycolysis and mediates resveratrol-induced ischemic tolerance. Stroke. 2017;48(11):3117–3125. doi:10.1161/STROKEAHA.117.018562
61. Li D, Liu X, Pi W, et al. Fisetin attenuates doxorubicin-induced cardiomyopathy in vivo and in vitro by inhibiting ferroptosis through SIRT1/Nrf2 signaling pathway activation. Front Pharmacol. 2021;12:808480. doi:10.3389/fphar.2021.808480
62. Lane DJR, Metselaar B, Greenough M, Bush AI, Ayton SJ. Ferroptosis and NRF2: an emerging battlefield in the neurodegeneration of Alzheimer’s disease. Essays Biochem. 2021;65(7):925–940. doi:10.1042/EBC20210017
63. Yang T, Zhang F. Targeting transcription factor Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) for the intervention of vascular cognitive impairment and dementia. Arterioscler Thromb Vasc Biol. 2021;41(1):97–116. doi:10.1161/ATVBAHA.120.314804
64. Guan X, Li Z, Zhu S, et al. Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 2021;264:118660. doi:10.1016/j.lfs.2020.118660
65. Miotto G, Rossetto M, Di Paolo ML, et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020;28:101328. doi:10.1016/j.redox.2019.101328
66. Lu H, Xiao H, Dai M, Xue Y, Zhao R. Britanin relieves ferroptosis-mediated myocardial ischaemia/reperfusion damage by upregulating GPX4 through activation of AMPK/GSK3β/Nrf2 signalling. Pharm Biol. 2022;60(1):38–45. doi:10.1080/13880209.2021.2007269
67. Xie R, Zhao W, Lowe S, et al. Quercetin alleviates kainic acid-induced seizure by inhibiting the Nrf2-mediated ferroptosis pathway. Free Radic Biol Med. 2022;191:212–226. doi:10.1016/j.freeradbiomed.2022.09.001
68. Dang R, Wang M, Li X, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19(1):41. doi:10.1186/s12974-022-02400-6
69. Yuan B, Zhao XD, Shen JD, et al. Activation of SIRT1 alleviates ferroptosis in the early brain injury after subarachnoid hemorrhage. Oxid Med Cell Longev. 2022;2022:9069825. doi:10.1155/2022/9069825
70. Chen H, Lin X, Yi X, et al. SIRT1-mediated p53 deacetylation inhibits ferroptosis and alleviates heat stress-induced lung epithelial cells injury. Int J Hyperthermia. 2022;39(1):977–986. doi:10.1080/02656736.2022.2094476
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.