Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 10
Fertility intentions of prenatal and postpartum HIV-positive women in primary care in Mpumalanga province, South Africa: a longitudinal study
Authors Peltzer K , Sifunda S, Mandell LN , Rodriguez VJ , Lee TK , Cook R, Weiss SM , Jones DL
Received 4 October 2017
Accepted for publication 28 December 2017
Published 16 February 2018 Volume 2018:10 Pages 9—17
DOI https://doi.org/10.2147/HIV.S153212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Karl Peltzer,1,2 Sibusiso Sifunda,1 Lissa N Mandell,3 Violeta J Rodriguez,3 Tae Kyoung Lee,4 Ryan Cook,5 Stephen M Weiss,3 Deborah L Jones3
1HIV/AIDS/STIs and TB (HAST) Research Programme, Human Sciences Research Council, Pretoria, South Africa; 2Department of Research and Innovation, University of Limpopo, Sovenga, South Africa; 3Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL, USA; 4Department of Public Health Sciences, University of Miami Miller School of Medicine, Miami, FL, USA; 5School of Public Health, University of California, Los Angeles, Los Angeles, CA, USA
Introduction: This study aimed to assess fertility intentions (planning to have more children in the future) and associated factors among pregnant and postpartum HIV positive women in rural South Africa.
Methods: In a longitudinal study, as part of a prevention of mother to child transmission (PMTCT) intervention trial, 699 HIV positive prenatal women, were systematically recruited and followed up at 6 months and 12 months postpartum (retention rate = 59.5%).
Results: At baseline, 32.9% of the women indicated fertility intentions and at 12 months postnatal, 120 (28.0%) reported fertility intentions. In longitudinal analyses, which included time-invariant baseline characteristics predicting fertility intention over time, not having children, having a partner with unknown/HIV-negative status, and having disclosed their HIV status to their partner, were associated with fertility intentions. In a model with time-varying covariates, decreased family planning knowledge, talking to a provider about a future pregnancy, and increased male involvement were associated with fertility intentions.
Conclusion: Results support ongoing perinatal family planning and PMTCT education.
Keywords: fertility intentions, family planning, HIV infection, pregnancy, South Africa, male involvement
Introduction
South Africa had an estimated 6,300,000 people living with HIV (PLHIV) in 20131 and HIV prevalence among pregnant women attending antenatal clinics was 29.7%.1 Antiretroviral therapy (ART)2 and interventions to prevent mother-to-child transmission (PMTCT) of HIV3 have greatly reduced the impact of HIV on health and life expectancy for mothers, children, and sexual partners.4 With the appropriate resources and support, having a healthy pregnancy and HIV-negative child are now attainable goals, and understanding and supporting the fertility intentions, that is, desire to have children, of PLHIV can improve linkages to and effectiveness of these services.
Understanding factors related to motivation for childbearing may guide health care providers as they initiate discussions regarding childbearing intentions and may help them to address topics such as safer conception and pregnancy planning. Discussing reproductive desires with a health care provider is an element of the PMTCT guidelines and has been associated with increased fertility intentions,5,6 though PLHIV have reported that reproductive discussions with their health care providers are infrequent.7–9 Many PLHIV also report a lack of trust that their health care providers will provide unbiased information and support.10–12
Conflicting evidence exists regarding the influence of HIV-specific factors on fertility intentions among PLHIV, that is, the length of time since HIV diagnosis,13–16 time since ART initiation,7,14,17 disclosure of HIV serostatus to partners,16,18,19 the HIV serostatus of partners,7,18,20,21 level of education,15,22,23 income,24–26 and PMTCT knowledge.11,19,27,28 Similarly, concerns regarding welfare of the family, including potential HIV transmission to infants21,29,30 or partners,10,21,31 monetary concerns,29,32,33 and fears regarding maternal mortality10,31,33–35 have been reported as considerations among PLHIV with regard to future pregnancies. Cultural factors, spousal, familial, and societal support, social expectations and attitudes, including stigma, also impact fertility intentions.30 However, these issues underscore the importance of the relationship between fertility intentions and the level of importance the individuals place on these factors.
Relationship factors, such as intimate partner violence (IPV) and male involvement during pregnancy planning and perinatal care, play a potential role in fertility intentions due to their impact on relationship quality. Depression, which has been associated with IPV,36 may also impact fertility intentions, which require future-oriented thinking.37 In addition, HIV and family planning knowledge may influence fertility intentions as concerns with regard to the risk of HIV transmission during pregnancy have been reported.21,29 Factors related to prior pregnancies, including unplanned pregnancy and HIV diagnosis during pregnancy, appear likely to also influence attitudes and plans with regard to future pregnancy.
Despite the volume of research on fertility intentions among PLHIV, studies have neglected the potential changes that occur in intentions following delivery. Rather, the few longitudinal studies of intentions among PLHIV have focused on comparisons between HIV-infected and HIV-uninfected women, for example,38 or the relative influence of ART. This study seeks to address this gap in research by exploring factors associated with existing or changing fertility intentions during the antenatal and/or postnatal period, addressing understudied factors associated with relationships, health, and knowledge. Care during the perinatal period provides a window of opportunity for linkage to and uptake of HIV treatment services,39 and information, therefore, regarding intentions assessed throughout the perinatal period may be especially important.
Patients and methods
Study design
This study is drawn from an ongoing longitudinal clinic-randomized PMTCT-controlled trial with 2 assessments prenatally (8–24 and 32 weeks pregnant) and 2 assessments during the postnatal period (6 and 12 months). The trial is aimed at increasing PMTCT uptake, family planning, and male partner participation in the antenatal and postnatal process in 12 randomly selected community health centers in Gert Sibande and Nkangala districts in Mpumalanga province, South Africa. Further details about the study design, staff training, subject recruitment, and procedures have been previously reported.40
Ethical approval
Ethical approval was granted by the Human Sciences Research Council (HSRC) Research Ethics Committee (REC), protocol approval number REC4/21/08/13, and the University of Miami Miller School of Medicine Institutional Review Board (IRB ID: 20130238) (CR00006122). Study approval was also obtained from the Department of Health and Welfare, Mpumalanga Provincial Government, South Africa. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants.
Sample and procedure
Eligible women were HIV-seropositive pregnant women with partners, between 8 and 24 weeks pregnant, the typical time of entry into antenatal care, and aged ≥18 years. Candidates agreeing to participate were enrolled following provision of written informed consent. There were no exclusions based on literacy as all assessments were administered using an audio computer-assisted self-interview (ACASI) system.
After enrollment, all women completed study measures in their preferred language (English, isiZulu, or seSotho) using ACASI to enhance disclosure, accommodate all levels of literacy, and reduce interviewer bias. To familiarize participants with the computer system, assessors completed the demographic component of the questionnaire with participants prior to completion of all other assessments. In addition, an on-site assessor was available to assist where necessary and answer any questions.
Measures
Fertility intentions were assessed with the question, “Are you planning to have more children in the future?” (response option, yes, no). Sociodemographic factors assessed included age, education, income, partner status, and number of children. Reproductive issues assessed included planning of the current pregnancy, talking to a health care provider about future pregnancy, and an 8-item measure of family planning knowledge.40 Family planning knowledge items assessed perception of risk of transmission to the partner during pregnancy as well as knowledge of the fertility cycle and ideal time to conceive, with heterogeneous response options. Knowledge of safer conception practices was also assessed.41 HIV-specific issues assessed included date of HIV diagnosis, a 12-item measure on HIV knowledge (Cronbach’s α=0.69, 0.64, 0.52, and 0.65 at the 2 prenatal and postnatal assessment points, respectively) using an adaptation of the AIDS-Related Knowledge Test to South African context; items reflect information about HIV transmission, reinfection with resistant virus, and condom use knowledge.42 Partner-specific issues assessed included disclosure of HIV status to partner, HIV status of partner, consistency of condom use, and an 11-item male involvement index40 (Cronbach’s α=0.83, 0.82, 0.84, and 0.82 at the 2 prenatal and postnatal assessment points, respectively). IPV was assessed using an adaptation of the Conflict Tactics Scale 18,43 which included a 9-item partner psychological victimization subscale (Cronbach’s α=0.76, 0.66, 0.83, and 0.83 at the 2 prenatal and postnatal assessment points, respectively), and a 9-item partner physical violence subscale (Cronbach’s α=0.92, 0.89, 0.94, and 0.94 at the 4 assessment points). Emotional status was assessed at baseline with the Edinburgh Postnatal Depression Scale 10 (EPDS-10).44 The EPDS-10 is a 10-item instrument asking participants to rate how often they have experienced different symptoms associated with depression in the past 7 days. Scores range from 0 through 30; the validated cut-off score for South African populations is 12.45 Cronbach’s α for the EPDS-10 scale ranged from 0.66 to 0.70 at the different assessment points in this study sample.
Data analysis
Statistical analyses included descriptive statistics (such as means, SDs, frequencies, and percentages), Student’s t-tests or its non-parametric alternative (Mann–Whitney Z-test), as well as chi-square or Fisher’s exact tests. The dependent variable consisted of women living with HIV and whether they had a desire to have more children at baseline, 32 weeks assessment, and 6 and 12 months assessments.
To investigate patterns the participants’ binary responses (yes/no) for fertility intention between 2 adjacent time points (time and time +1), we used the autoregressive model (also known as “Markov chain model”),46 which tested how fertility intention at one time point predicted fertility intention at the next time point. Then, using multigroup test approach, we investigated the effects of intervention on response changes in fertility intention. The hypothesized autoregressive model for fertility intention continuity is presented in Figure 1. According to this approach, the same autoregressive model was estimated using 2 groups (standard of care vs. enhanced intervention) simultaneously. Multigroup analysis is useful to test for differences (Δ) using the “parameter invariance” method.47 Given the categorical (binary) outcomes, logistic regression coefficients were estimated with odds-ratios (ORs, as effect sizes of logistic coefficients).48 To estimate unique autoregressive associations of fertility intention, the current model used several time-invariant (baseline demographic variables) and varying covariates as control variables. In addition, to account for lack of independence between observations within multiple sites, we used a sandwich estimator to adjust for underestimated standard errors and bias in chi-square computation.49 Missing data were handled using multiple imputation50 after comparing it with inverse-probability treatment weighting, specifying 10 imputed datasets. All data analyses were conducted using Mplus (version 7.4).51
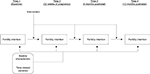  | Figure 1 Hypothesized autoregressive model for fertility intention continuity. |
Results
Sample characteristics at baseline
In all, 699 women living with HIV were enrolled during pregnancy (8–24 weeks) and completed assessments at baseline; 61.7% of the sample completed assessments at 32 weeks pregnant; 50.6% postnatal at 6 months; and 59.5% at 12 months. At baseline, 224 women (32.9%) indicated that they were “planning to have more children in the future” (fertility intentions).
Attrition analyses indicated that women with more education, those who already had children, and those who had an HIV-infected infant (OR=0.64, p<0.10) were less likely to drop out of the study over time, and these variables were accounted for in all subsequent analyses. Other demographic and psychosocial variables, such as age, income, HIV-infected partner, disclosure of HIV status to partner, depression, and relationship status were not found to predict missing data over time.
Table 1 describes baseline characteristics, overall and by fertility intention. Women were, on average, aged 28.4 years, the majority (71.1%) had grade 10–11 education, 51.1% had a household income of ≥600 Rand a month, 20.2% had no children, and 53.0% reported that the current pregnancy had been unplanned. Almost half (47.1%) had talked to their health care provider about trying to get pregnant in the future. Slightly more than half (54.1%) of women had been diagnosed with HIV during this pregnancy. As a requirement of eligibility, all women had a partner; 41.1% were married or cohabiting, 59.0% had disclosed their HIV status to their partners, and 25.1% of their partners were known to be infected with HIV. Fertility intentions were more likely to be reported by younger women, those with more education, those having lower income, those with no children or only one child, those having been diagnosed with HIV during this pregnancy, those having a partner and family with high-fertility desires, and those having less HIV and family planning knowledge (Table 1).
Transition patterns of fertility intention and moderating effects of intervention across 4 time points
Transition patterns showed that overall; women who reported planning to have children at Time 1 were more likely to report planning to have children in all future time points (see ORs in Table 2). Similar response patterns were detected even after adjusting the effects of all time-invarying (baseline characteristics) and varying covariates (see AORs in Table 2). Next, to examine the intervention effects on the transition frequencies for fertility intention, these ORs were compared by condition. After adjusting for time-varying covariates, participants in the experimental group were less likely to plan to have children in the future from time points 1–2 (AORs=13.17 (standard of care) vs 10.26 (enhanced intervention); Wald-test=6.42, p<0.05) and from time points 3–4 (AORs=4.74 [standard of care] vs 3.99 [enhanced intervention]; Wald-test=4.88, p<0.05) compared with those in control group.
Effects of baseline characteristics and time-varying covariates on fertility intention across time
In longitudinal analyses, in Model 1, which included time-invariant predictors (at baseline) of fertility intention assessed over 4 time periods, not having children (AOR=0.61, p<0.001), having a partner with unknown/HIV-negative status (AOR=0.76, p<0.01), and having disclosed their HIV status to their partner (AOR=1.25, p<0.05) were associated with fertility intentions. In Model 2, which included time-varying covariates, decreased family planning knowledge (AOR=0.84, p<0.001), talking to a provider about a future pregnancy (AOR=1.34, p<0.01), and increased male involvement (AOR=1.01, p<0.01) were associated with fertility intentions (Table 3).
Discussion
The study examined fertility intentions among women living with HIV during the pre- and postnatal periods, and found less than one-third of women intended to have more children, as previously found.30 As in earlier studies,14,19,23,30,52 younger age and having fewer or no children were associated with intentions to conceive again in the future.
In contrast with previous research, neither educational level15,22,53 nor income24,26 was associated with reproductive desires. This may have been due to a lack of educational and socioeconomic variability in this rural sample, with a largely low-income sample with the majority having attained 10 or 11 years of education. As found in previous studies,20,21,54,55 partner’s HIV-positive status was negatively associated with fertility intentions. Disclosure of HIV serostatus to partners at the baseline assessment, as found previously,16,18,19 was positively associated with fertility intentions. Male involvement was associated with fertility intentions, showing the important role of male partners in fertility desires and the promotion of male involvement in the mother and child care continuum.56 Inconsistent condom use was not associated with fertility intentions in this study, as seen in previous studies.21,57–59 This finding may have been associated with local beliefs that women are less likely to conceive following childbirth or due to concerns about insisting on condom use being associated with HIV, as noted previously.
Furthermore, the study found an association between discussing family planning with a health care provider and fertility intentions, as in previous research.60 These findings have been confirmed in several other studies,5,6 and show the importance of the South African Department of Health guidelines in helping facilitate the initiation of discussions about fertility intentions by health care providers with PLHIV. In contrast, family planning knowledge and participation in an enhanced PMTCT intervention, which included information on family planning, were associated with decreased fertility intention. It is possible that better family planning knowledge and having received information on family planning from interventionists may have heightened awareness of risks of HIV transmission during pregnancy or consideration of other issues associated with pregnancy, such that knowledge may be necessary but not sufficient to arrive at fertility intentions.
Surprisingly, IPV during the perinatal period, unplanned pregnancy, time since HIV diagnosis, and depression (analysis not shown) were not associated with fertility intentions, contrary to previous research.60 Future research should examine the relative trade-offs in reproductive decision making among this vulnerable population.
Study limitations
Study follow-up rates were lower than the original target and those previously achieved in our pilot studies, and results may have been influenced by self-selection among women who were followed to 12 months postpartum. The high level of attrition and low clinic attendance may have been related to the need for many women to travel long distances to reach the health facility, migration arising from economic necessity, and culturally condoned migration of women during the perinatal period to their mothers’ homes.61 The inclusion criteria for the study participants were limited to women who had a partner, preventing generalization to women without partners. The content of discussions about fertility and pregnancy between couples and with health care providers was not assessed, and should be considered in future studies to gain more insight in fertility and reproductive intentions and their influence by providers.
Conclusion
This study of pregnant women living with HIV demonstrated declining fertility intentions from the prenatal to postnatal period. PMTCT and family planning knowledge, along with sociodemographic factors, were associated with fertility intentions, and individual discussions between patients, providers, and interventionists were associated with diminished desire for more children. Factors identified related to motivation for childbearing may be useful in helping health care providers to initiate and explore fertility-related discussions, and in developing and providing effective and suitable strategies for contraception, safer conception, and pregnancy planning. Future studies should explore the role of culture in fertility intentions, and potential moderation of its influence in the uptake of clinical guidance by providers.
Acknowledgments
This study was funded by a collaborative NIH/PEPFAR Grant, R01HD078187-S. Activities were conducted with the support of the University of Miami Miller School of Medicine Center for AIDS Research, funded by NIH grants, P30AI073961 and K23HD074489.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
National Department of Health. The 2013 National Antenatal Sentinel HIV Prevalence Survey, South Africa. Pretoria: National Department of Health; 2015. | ||
Mahy M, Stover J, Stanecki K, Stoneburner R, Tassie JM. Estimating the impact of antiretroviral therapy: regional and global estimates of life-years gained among adults. Sex Transm Infect. 2010;86 (Suppl 2): ii67–ii71. | ||
Sturt AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database Syst Rev. 2010(3):CD008440. | ||
Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. | ||
Abbawa F, Awoke W, Alemu Y. Fertility desire and associated factors among clients on highly active antiretroviral treatment at finoteselam hospital Northwest Ethiopia: a cross sectional study. Reprod Health. 2015;12:69. | ||
Steiner RJ, Black V, Rees H, Schwartz SR. Low receipt and uptake of safer conception messages in routine HIV Care: findings from a prospective cohort of women living with HIV in South Africa. J Acquir Immune Defic Syndr. 2016;72(1):105–113. | ||
Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions of HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2007;21(4):278–285. | ||
Sherr L, Barry N. Fatherhood and HIV-positive heterosexual men. HIV Med. 2004;5(4):258–263. | ||
West N, Schwartz S, Phofa R, et al. “I don’t know if this is righ …but this is what I’m offering”: healthcare provider knowledge, practice, and attitudes towards safer conception for HIV-affected couples in the context of Southern African guidelines. AIDS Care. 2016;28(3):390–396. | ||
Cooper D, Harries J, Myer L, Orner P, Bracken H. “Life is still going on”: reproductive intentions among HIV-positive women and men in South Africa. Soc Sci Med. 2007;65(2):274–283. | ||
Nobrega AA, Oliveira FA, Galvao MT, et al. Desire for a child among women living with HIV/AIDS in northeast Brazil. AIDS Patient Care STDS. 2007;21(4):261–267. | ||
Sowell RL, Misener TR. Decisions to have a baby by HIV-infected women. West J Nurs Res. 1997;19(1):56–70. | ||
Gosselin JT, Sauer MV. Life after HIV: examination of HIV serodiscordant couples’ desire to conceive through assisted reproduction. AIDS Behav. 2011;15(2):469–478. | ||
Kaida A, Laher F, Strathdee SA, et al. Childbearing intentions of HIV-positive women of reproductive age in Soweto, South Africa: the influence of expanding access to HAART in an HIV hyperendemic setting. Am J Public Health. 2011;101(2):350–358. | ||
Kawale P, Mindry D, Stramotas S, et al. Factors associated with desire for children among HIV-infected women and men: a quantitative and qualitative analysis from Malawi and implications for the delivery of safer conception counseling. AIDS Care. 2014;26(6):769–776. | ||
Oladapo OT, Daniel OJ, Odusoga OL, Ayoola-Sotubo O. Fertility desires and intentions of HIV-positive patients at a suburban specialist center. J Natl Med Assoc. 2005;97(12):1672–1681. | ||
Cooper D, Moodley J, Zweigenthal V, Bekker LG, Shah I, Myer L. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009;13(Suppl 1):38–46. | ||
Melaku YA, Zeleke EG, Kinsman J, Abraha AK. Fertility desire among HIV-positive women in Tigray region, Ethiopia: implications for the provision of reproductive health and prevention of mother-to-child HIV transmission services. BMC Womens Health. 2014;14:137. | ||
Peltzer K, Chao LW, Dana P. Family planning among HIV positive and negative prevention of mother to child transmission (PMTCT) clients in a resource poor setting in South Africa. AIDS Behav. 2009; 13(5):973–979. | ||
Demissie DB, Tebeje B, Tesfaye T. Fertility desire and associated factors among people living with HIV attending antiretroviral therapy clinic in Ethiopia. BMC Pregnancy Childbirth. 2014;14:382. | ||
Haddad LB, Machen LK, Cordes S, et al. Future desire for children among women living with HIV in Atlanta, Georgia. AIDS Care. 2016;28(4):455–459. | ||
Asfaw HM, Gashe FE. Fertility intentions among HIV positive women aged 18-49 years in Addis Ababa Ethiopia: a cross sectional study. Reprod Health. 2014;11:36. | ||
Berhan Y, Berhan A. Meta-analyses of fertility desires of people living with HIV. BMC Public Health. 2013;13:409. | ||
Kimani J, Warren C, Abuya T, et al. Family planning use and fertility desires among women living with HIV in Kenya. BMC Public Health. 2015;15:909. | ||
Santos N, Ventura-Filipe E, Paiva V. HIV positive women, reproduction and sexuality in São Paulo, Brazil. Reprod Health Matters. 1998;6(12):31–40. | ||
Wekesa E, Coast E. Fertility desires among men and women living with HIV/AIDS in Nairobi slums: a mixed methods study. PLoS One. 2014;9(8):e106292. | ||
Finocchario-Kessler S, Sweat MD, Dariotis JK, et al. Understanding high fertility desires and intentions among a sample of urban women living with HIV in the United States. AIDS Behav. 2010;14(5):1106–1114. | ||
Nakayiwa S, Abang B, Packel L, et al. Desire for children and pregnancy risk behavior among HIV-infected men and women in Uganda. AIDS Behav. 2006;10(4 Suppl):S95–S104. | ||
Aska ML, Chompikul J, Keiwkarnka B. Determinants of fertility desires among HIV positive women living in the Western Highlands Province of Papua New Guinea. World J AIDS. 2011;01(04):198–207. | ||
Nattabi B, Li J, Thompson SC, Orach CG, Earnest J. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav. 2009;13(5):949–968. | ||
Richter DL, Sowell RL, Pluto DM. Factors affecting reproductive decisions of African American women living with HIV. Women Health. 2002;36(1):81–96. | ||
Akelo V, McLellan-Lemal E, Toledo L, et al. Determinants and experiences of repeat pregnancy among HIV-positive kenyan women–a mixed-methods analysis. PLoS One. 2015;10(6):e0131163. | ||
Feldman R, Maposhere C. Safer sex and reproductive choice: findings from “Positive Women: Voices and Choices” in Zimbabwe. Reprod Health Matters. 2003;11(22):162–173. | ||
Kanniappan S, Jeyapaul MJ, Kalyanwala S. Desire for motherhood: exploring HIV-positive women’s desires, intentions and decision-making in attaining motherhood. AIDS Care. 2008;20(6):625–630. | ||
Sowell RL, Phillips KD, Misener TR. HIV-infected women and motivation to add children to their families. J Fam Nurs. 1999;5(3):316–331. | ||
Peltzer K, Rodriguez VJ, Jones D. Prevalence of prenatal depression and associated factors among HIV-positive women in primary care in Mpumalanga province, South Africa. SAHARA J. 2016;13(1):60–67. | ||
Bjärehed J, Sarkohi A, Andersson G. Less Positive or more negative? Future-directed thinking in mild to moderate depression. Cogn Behav Ther. 2010;39(1):37–45. | ||
Taulo F, Berry M, Tsui A, et al. Fertility intentions of HIV-1 infected and uninfected women in Malawi: a longitudinal study. AIDS Behav. 2009;13 (Suppl 1):20–27. | ||
Watson-Jones D, Balira R, Ross DA, Weiss HA, Mabey D. Missed opportunities: poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One. 2012;7(7):e40091. | ||
Jones DL, Peltzer K, Villar-Loubet O, et al. Reducing the risk of HIV infection during pregnancy among South African women: a randomized controlled trial. AIDS Care. 2013;25(6):702–709. | ||
Kaye K, Suellentrop K, Sloup C. The Fog Zone: How Misperceptions, Magical Thinking, and Ambivalence Put Young Adults at Risk for Unplanned Pregnancy. Washington, DC: The National Campaign to Prevent Teen and Unplanned Pregnancy; 2009. | ||
Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14(2):172–182. | ||
Straus MA. Measuring intrafamily conflict and violence: the conflict tactics (CT) scales. J Marriage Fam. 1979;41:75–88. | ||
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. | ||
Lawrie TA, Hofmeyr GJ, de Jager M, Berk M. Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. S Afr Med J. 1998;88(10):1340–1344. | ||
Kaplan D. An overview of Markov chain methods for the study of stage-sequential developmental processes. Dev Psychol. 2008;44(2):457–467. | ||
Lubke GH, Muthen BO. Applying multigroup confirmatory factor models for continuous outcomes to Likert scale data complicates meaningful group comparisons. Struct Equ Modeling. 2004;11(4):514–534. | ||
Allen J, Le H. An additional measure of overall effect size for logistic regression models. J Educ Behav Stat. 2008;33(4):416–441. | ||
Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73(1):13–22. | ||
Asparouhov T, Muthén B. Multiple Imputation with Mplus. MPlus Web Notes; 2010. | ||
Mplus [computer program]. Version 7.4. Los Angeles, CA; 2014. | ||
Antelman G, Medley A, Mbatia R, et al. Pregnancy desire and dual method contraceptive use among people living with HIV attending clinical care in Kenya, Namibia and Tanzania. J Fam Plann Reprod Health Care. 2015;41(1):e1. | ||
Olowookere SA, Abioye-Kuteyi EA, Bamiwuye SO. Fertility intentions of people living with HIV/AIDS at Osogbo, Southwest Nigeria. Eur J Contracept Reprod Health Care. 2013;18(1):61–67. | ||
Sufa A, Wordofa MA, Wossen BA. Determinants of fertility intention among women living with hiv in western Ethiopia: implications for service delivery. Afr J Reprod Health. 2014;18(4):54–60. | ||
Jose H, Madi D, Chowta N, et al. Fertility Desires and Intentions among People Living with HIV/AIDS (PLWHA) in Southern India. J Clin Diagn Res. 2016;10(6):OC19–OC22. | ||
Gutin SA, Namusoke F, Shade SB, Mirembe F. Fertility desires and intentions among HIV-positive women during the post-natal period in Uganda. Afr J Reprod Health. 2014;18(3):67–77. | ||
Deering KN, Shaw SY, Thompson LH, et al. Fertility intentions, power relations and condom use within intimate and other non-paying partnerships of women in sex work in Bagalkot District, South India. AIDS Care. 2015;27(10):1241–1249. | ||
Finger JL, Clum GA, Trent ME, Ellen JM, Adolescent Medicine Trials Network for HIV/AIDS Interventions. Desire for pregnancy and risk behavior in young HIV-positive women. AIDS Patient Care STDS. 2012;26(3):173–180. | ||
Wagner GJ, Wanyenze R. Fertility Desires and intentions and the relationship to consistent condom use and provider communication regarding childbearing among HIV clients in Uganda. ISRN Infect Dis. 2013;2013:7. | ||
Rodriguez VJ, Cook RR, Weiss SM, Peltzer K, Jones DL. Psychosocial correlates of patient–provider family planning discussions among HIV-infected pregnant women in South Africa. Open Access J Contracept. 2017;8:25. | ||
Rodriguez VJ, LaCabe RP, Privette CK, et al. The Achilles’ heel of prevention to mother-to-child transmission of HIV: Protocol implementation, uptake, and sustainability. SAHARA J 2017;14(1):38–52. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.