Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Fecal Microbiota Transplantation in Autism Spectrum Disorder
Authors Li Y, Wang Y , Zhang T
Received 15 July 2022
Accepted for publication 6 December 2022
Published 15 December 2022 Volume 2022:18 Pages 2905—2915
DOI https://doi.org/10.2147/NDT.S382571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Youran Li,1 Yizhong Wang,1,2 Ting Zhang1,2
1Department of Gastroenterology, Hepatology and Nutrition, Shanghai Children’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China; 2Institute of Pediatric Infection, Immunity and Critical Care Medicine, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China
Correspondence: Ting Zhang; Yizhong Wang, Department of Gastroenterology, Hepatology and Nutrition, Shanghai Children’s Hospital, School of medicine, Shanghai Jiao Tong University, Shanghai, 200062, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders that begin in infancy. In recent years, the incidence of ASD in the world is increasing year by year. At present, the etiology and pathogenesis of ASD are not clear, and effective treatments are still lacking. In addition to neurobehavioral symptoms, children with ASD often have obvious gastrointestinal symptoms. Gut microbiota is a large microbial community in the human gut, which is closely related to the nervous system and can affect brain development and behavior through the neuroendocrine, neuroimmune and autonomic nervous systems, forming a microbiota-gut-brain axis connection. Recent studies have shown that children with ASD have significant gut microbiota and metabolic disorders, and fecal microbiota transplantation (FMT) is expected to improve ASD-related symptoms by regulating gut microbiota and metabolism. This review paper will therefore focus on FMT in the treatment of ASD, and FMT is effective in improving gastrointestinal and neurobehavioral symptoms in children with ASD.
Keywords: autism spectrum disorder, fecal microbiota transplantation, gastrointestinal symptoms, gut microbiota, microbiota-gut-brain axis
Introduction
Autism spectrum disorder (ASD) is a group of multifactorial neurodevelopmental disorders characterized by verbal communication deficiency, social interaction impairment, restricted interests and repetitive behaviors.1 At present, the etiology and pathogenesis of ASD are not clear.2 In recent years, the incidence of ASD in the world is increasing year by year. For the one in 100 children affected worldwide,3 progress in the treatments of ASD has been frustratingly slow and effective treatments are still lacking.4 Children with ASD are at least three times more likely to experience co-occurring gastrointestinal symptoms, including constipation, diarrhea and abdominal pain, than neurotypical children.5
Recent studies have confirmed differences in the composition of gut microbiota between ASD patients and neurotypical people.6 Microbial communities play a central role in the maturation and development of the immune, neural and gastrointestinal systems and are also responsible for important metabolic pathways.7 A bidirectional connection exchanges information between gut microbiota and central nervous system. Therefore, the microbiota-gut-brain axis plays an important role in ASD and has been proposed as a target for treatment of ASD.8,9 Fecal microbiota transplantation (FMT) refers to the introduction of fecal microbiota from a healthy donor into the gastrointestinal tract of a patient. In recent years, FMT is a novel and adequate approach to alter the host gastrointestinal microbial ecosystem.10 Evidence indicates that gut microbiota are involved in several neurological diseases, for including ASD, parkinson’s disease, alzheimer’s disease, epilepsy, multiple sclerosis, Tourette’s syndrome, Guillain-Barre syndrome and diabetic neuropathy, and FMT may be a potential treatment option.11–13
Although FMT had been used in ASD, the available evidence is still insufficient, with limited studies conducted or ongoing in human studies. Large double-blind randomized controlled trials are needed to further elucidate the effect of FMT in ASD.14–17 This review paper will therefore focus on FMT in the treatment of the core behavioral symptoms and gastrointestinal symptoms of ASD, by beginning with describing the gut microbiota changes observed in ASD patients and the involvement of microbiota-gut-brain axis in several neurological diseases, then examine the studies of FMT conducted in animals and patients with ASD, and determine that FMT is effective in improving gastrointestinal and neurobehavioral symptoms in children with ASD.
Autism Spectrum Disorder and Gut Microbiota
Gastrointestinal symptoms are common in individuals with ASD, but the underlying mechanisms remain unclear.18 Changes in the gut microbiota may be involved in the pathogenesis of gastrointestinal symptoms in children with ASD, including significant increases in the relative abundance of Actinobacteria and Proteobacteria.19 Compared with neurotypical children, the development of gut microbiota in children with ASD was relatively stagnant and gradually deviated from the normal track. There was no significant change in alpha diversity with age in children with ASD, the early microbiota was unstable and immature and the common bacteria was difficult in colonization. The microbiome relationship significantly altered before 3 years old in children with ASD, which is consistent with their nodes at which behavioral deficits occur. The changes of microbial function and bacterial relationship were correlated with the severity of neurobehavioral and gastrointestinal symptoms.20
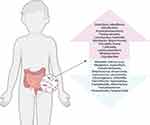
The changes of gut microbiota and metabolites in the blood of children with ASD are summarized (Table 1). The most striking finding was the complete absence of non-sporogenic anaerobes and microaerophiles in the neurotypical children, while the children with ASD had significant numbers of these bacteria.21 Compared with the neurotypical children, the phylum level of Verrucomicrobia decreased in autistic children.22 Betaproteobacteria and Clostridiales increased from the class level and order level respectively. Selenomonadales and Bacteroidales decreased from the order level.23 From the family level, Erysipelotrichaceae and Ruminococcaceae was higher, and Prevotellaceae, Actinomycetaceae, Coriobacteriaceae, Oscillospira, Streptococcaceae, Bifidobacteriaceae were lower in children with ASD.24,25 From the genus level (Figure 1), Clostridium, Alkaliflexus, Desulfovibrio, Acetanaerobacterium, Parabacteroides, Lactobacillus, Sutterella, Odoribacter, Butyricimonas, Prevotella, Dorea, Collinsella, Lachnoclostridium, Bifidobacterium, Coprobacillus elevated, and Weissella, Helcococcus, Alkaliphilus, Anaerofilum, Pseudoramibacter, Streptococcus, Anaerovorax, Lactococcus, Leuconostoc, Ethanoligenens, Veillonella, Flavonifractor, Haemophilus, Eisenbergiella, Akkermansia, Faecalibacterium, Parasutterella, Paraprevotella reduced in children with ASD.26–28 Clostridium bolteae, Bacteroides vulgatus and Faecalibacterium prausnitzii were higher from the species level in autistic children than neurotypical children.29 Elevated amounts of GABA, serotonin, lipopolysaccharides and p-cresol were also found in the blood of children with ASD,30–33 while the amount of homocysteine, methionine, glutathione, S-adenosylmethionine,33 arachidonic acid, docosahexaenoic acid and eicosapentaenoic acid34 decreased. More evidence show that microbiological changes in autistic children with gastrointestinal symptoms are associated with digestive enzyme deficiency,35 poor carbohydrate absorption,36 selective diet,37 increased bacterial toxins,38 and neuro-inflammatory signaling alteration.39 Microbiota related metabolic disorder may be involved in the pathogenesis of gastrointestinal symptoms in patients with ASD. Differential metabolites are involved in the metabolism of several neurotransmitters, such as serotonin, dopamine, histidine and gamma-aminobutyric acid.29 Overproduction of pathogenic bacteria or reduction of probiotics can lead to gut microbiota disturbance, mediating a variety of neurological and psychological diseases.40 On the whole, normal gut microbiota plays an important role in maintaining stable gut-brain axis function.
 |
Table 1 Elevated and Decreased Gut Microbiota and Metabolites in the Blood of Children with ASD |
Microbiota-Gut-Brain Axis
The microbiota-gut-brain axis consists of complex network and multiple pathways that allow signals to be sent between the microbiota and the brain.41 The microbiota also regulates sensory processing, social behavior and stress responses directly or indirectly through a variety of neural activities and signaling molecules.42 Conversely, the brain can directly regulate the composition and function of gut microbiota by releasing neuroactive compounds that act on certain gut microbial receptors, or indirectly by regulating the motility and secretory activity of the gut.43
Gut microbiota ferment dietary fiber to produce short-chain fatty acids (SCFAs), and transform tryptophan (TRP) in protein foods into metabolites with different functions in the host body. SCFAs such as acetate, butyrate and propionate can increase the expression of claudin and occludin, thereby reducing the blood-brain barrier permeability. SCFAs and TRP metabolites block the activation of transcription factors in astrocytes and microglias by blocking proinflammatory factors, leading to cerebral homeostasis.44 Recent studies have suggested that gut microbiota metabolites, such as propionic acid and butyrate can have effects on mitochondrial activity in lymphoblastoid cell line from children with ASD.45,46 4-cresol produced by clostridium difficile can affect GABAergic and glutamate transport, causing neurological and physiological changes in patients with ASD.47,48
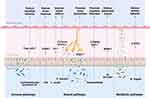
Gut microbiota affects brain development and neurobehavior through immune, neural and metabolic pathways.18 Key findings of the pathways involved in the pathophysiology of several neurological and psychological diseases are summarized (Figure 2). In the animal studies of ASD, the relative abundance of Lachnospiraceae bacterium A2 was associated with reduced repetitive behavior and increased Treg cells in mesenteric lymph nodes.49 Lactobacillus reuteri (L. reuteri) acts on the vagus nerve and restores social-induced synaptic plasticity in the ventral tegmental region of the midbrain. L. reuteri could increase the level of oxytocin (OT) and affect oxytocin-dopamine system to improve the social defects in mice. L. reuteri was ineffective in mice lacking oxytocin receptors.50 Enterobacter faecalis (E. faecalis) reduced corticosterone levels in stressed mice by inhibiting activation of the HPA axis, influencing social behavior in mice through neuronal circuits that mediate the stress response in the brain.51
Some patients with ASD also have similar symptoms such as anxiety, depression and motor dysfunction. In the studies of depression and anxiety, mice fed with Lactobacillus rhamnosus (L. rhamnosus) showed less emotional stress and anxiety, with elevated level of gamma-aminobutyric acid (GABA) mRNA expression in the hippocampus.52 The metabolite 4-EPS produced by the gut microbiota of mice was absorbed into the blood and circulated through the whole body into the brain, influencing the complex behavior of mice by affecting the formation of myelin sheath in the brain. 4-EPS promoted the anxiety behavior of mice and Bacteroides fragilis (B. fragilis) probably alleviate the process.53 In a pilot study conducted in patients with irritable bowel syndrome, Bifidobacterium longum NCC3001 (BL NCC3001) played a role in decreasing depression scores and improving the quality of life by upregulating the level of brain derived neurotrophic factor (BDNF) in the hippocampus probably.54
In the studies of neurodegenerative disorder, the α-synuclein (αSyn) accumulates in the intestinal nervous system and travels to the brain via the vagus nerve, causing neurodegeneration and motor dysfunction. Gut microbiota from patients with Parkinson’s disease enhanced motor dysfunction in mice, indicating that changes in the gut microbiota could influence motor deficits.55 The Bacteroidetes raised the metabolism of the pro-inflammatory polyunsaturated fatty acid by activating the C/EBPβ/AEP pathway, which enhanced microglial cell activation and neuroinflammation, and promotes pathological and cognitive disorders of Alzheimer’s disease.56 These classic pathways in the microbiota-gut-brain axis can be further explored in the study of ASD.
In the studies of ASD-related genes, animal studies have shown that EPHB6 deficiency mediates autism-like behavior in mice by inducing gut microbiota dysregulation, which leads to vitamin B6 and dopamine deficiency, and thereby induces excitatory or inhibitory imbalance of pyramidal neurons in the prefrontal cortex through the dopamine D1 receptor pathway.57 In drosophila melanogaster, loss of KDM5 function resulted in impaired intestinal barrier function, dysbiosis with intestinal microecology, and abnormal social behavior. KDM5 protein is a histone demethylase that regulates innate immune signaling and bacterial disturbances. The social behavior, longevity and cellular phenotype of drosophila with KDM5 protein deficiency were partially saved by antibiotic treatment or feeding lactobacillus plantarum.58
FMT Studies in ASD Animal Models
FMT studies in ASD animal models were summarized (Table 2). Transplanting gut microbiota from ASD or typically-developing donors into germ-free mice showed that colonization with gut microbiota of ASD patients was effective to induce signature ASD behaviors.59 It has been reported that the ASD mouse models can be established from the offspring by repeatedly transplanting fecal extract of children with ASD into pregnant mice.60
 |
Table 2 FMT Studies in ASD Animal Models |
In an animal experiment, fecal samples from ASD and control children were transplanted into germ-free mice. ASD and control transplanted mice showed different behavioral characteristics of ASD and significant differences in gut microbiota. Metabolomics analysis showed that the changes of amino acid and 5-hydroxytryptamine (5-HT) metabolic pathways were significant in serum metabolomics of both children and mice with ASD after transplantation. ASD mice showed lower expression of 5-HT reabsorption transporter and serotonin 1A receptor proteins, and higher expression of tryptophan hydroxylase 1 protein in the colon.61
A study showed that FMT from healthy human gut microbiota significantly improved anxiety-like and repetitive behaviors and increased serum chemokine levels in ASD mice, including GRO-α (CXCL1), MRIP-1 α (CCL3), MCP-3 (CCL7), RANTES (CCL5) and Eotaxin (CCL11), which are involved in neural development and synaptic transmission of the central nervous system.62 Another study found that FMT from naive wild-type mice improved memory deficits and social withdrawal of the Fmr1 knock out mice, with normalizing the content of A. muciniphila to wild-type level and decreasing the levels of TNFα and Iba1 in their brains.63 Moreover, studies have shown that FMT therapy repair the social interaction of the ASD mouse models induced by propionic acid. FMT treatments significantly increased the abundance of Clostridium, Bifidobacterium, Marvinbryantia and Butyricicoccus, and were negatively correlated with succinic acid synthesis, pyruvate metabolism, nitrogen metabolism, β-lactam resistance and peptidoglycan synthesis. Propionic acid significantly increased the abundance of fecal C. perfringens, decreased the content of Clostridium cluster IV and increased the expression level of BDNF in the hippocampus. FMT treatment could restore fecal Clostridium spp. balance, normalize BDNF expression and increase GST levels.64,65
It was reported that the addition of p-cresol to drinking water induced social deficits and repetitive behaviors in ASD mice. P-cresol-treated mice exhibited more frequent head shakes and circling events, but similar hyperactivity and anxiety compared with normal mice, indicating that p-cresol selectively induced ASD core symptoms. Transplanting the fecal microbiota of p-cresol treated mice to normal mice induces typical ASD behavior and increases p-cresol production. However, transplantation of fecal microbiota from normal mice to p-cresol treated mice could restore their social behavior, VTA dopamine neuronal excitability and fecal p-cresol levels.66 On the whole, social behavioral abnormalities in ASD mice can be corrected by the administration of FMT.
Effects of FMT on ASD
Effect of FMT on Gastrointestinal and Neurobehavioral Outcomes
FMT studies in ASD patients were summarized (Table 3). A small open-label clinical trial was conducted in 18 children diagnosed with ASD. FMT was performed after 2 weeks of antibiotic treatment and intestinal clearance. The Gastrointestinal Symptom Rating Scale (GSRS) showed about 80% improvement in gastrointestinal symptoms. Behavioral symptoms of ASD also improved significantly, and continued to improve after 8 weeks of treatment. Sequencing analysis showed that part of the donor microbiota was successfully transplanted, the intestinal environment was improved, the total bacterial diversity and the abundance of Bifidobacteria, Prevotella and Dephosphococcus were increased, and remained unchanged after 8 weeks.67 8 autistic children showed improvement in gastrointestinal symptoms and a greater degree of improvement in ASD symptoms after 2 years of FMT treatment. Significant changes in the gut microbiota of children who received FMT persisted 2 years after treatment, including a significant increase in microbial diversity and an increase in the relative abundance of Bifidobacteria and Prevotella, suggesting the long-term safety and efficacy of FMT in improving ASD symptoms.68
 |
Table 3 FMT Studies in ASD Patients |
In China, 16 children with typical development (TD) and 40 children with ASD were enrolled for 12 weeks. Children with ASD were treated with FMT for 4 weeks and followed up for 8 weeks. At the first week, there were significant differences in behavior, gastrointestinal symptoms and microbiota between children with ASD and TD. FMT could improve gastrointestinal and ASD symptoms and serum neurotransmitter levels. Both oral and rectal FMT improved gastrointestinal symptoms, and there was no significant difference between the two approaches. FMT can promote the colonization of donor microbiota and transfer the bacterial community of children with ASD to TD or donor children. The abundance of Eubacterium coprostanoligenes was positively correlated with high GSRS score, while FMT reduced its abundance and might be associated with the improvement of ASD symptoms.69 In addition, a multicentre, double-blind, randomised, placebo-controlled trial of FMT for ASD is ongoing in China currently.70
The fecal and plasma metabolites of 18 children with ASD and 20 healthy children were compared. Among the 619 plasma metabolites detected in children with ASD, the levels of niacinamide nucleoside and other metabolites decreased, while the levels of octanoate and heptanoate increased. Among the 699 fecal metabolites detected, there was no significant difference between the two groups, but the level of p-cresol sulfate was not significantly increased in the children with ASD. After FMT, the plasma metabolic profile of children with ASD changed significantly, including nicotinate/nicotinate and purine-related metabolites, and the reduction of fecal sulfate level of p-cresol was similar to that of healthy children.71
FMT is a novel method in the treatment of children with ASD, which can relieve not only gastrointestinal symptoms, but also neurobehavioral symptoms.72 However, as data on the long-term effects of this treatment is still limited, further research is needed, but it can be expected, based on contemporary scientific evidence and experimental studies, that FMT will be formally applied in the treatment of ASD in the future. Unresolved questions include which microbiome in the gut is beneficial and which is harmful, how to safely and effectively implant beneficial bacteria into the body, and how to extract and eliminate harmful microbes before transplantation.73 In future studies, large-sample randomized controlled clinical studies are needed to determine the mechanism of FMT in the treatment of ASD and the optimal method of FMT.
Risk of Adverse Events
All adverse events of FMT were classified as short-term (48 hours after FMT) and long-term (3 months after FMT).74 FMT is highly effective for treating recurrent clostridioides difficile infection (RCDI). In a large single-center prospective study conducted in 609 adult patients with RCDI, more than 60% of the patients had diarrhea during short-term follow-up after FMT, but it lasted less than a week, and the long-term risk of adverse events and complications of infection is low.75 A single-center retrospective study was performed to investigate the safety of FMT in children, the incidence of short-term adverse events was 26.32% (30/114), and the most common short-term adverse events were abdominal pain, diarrhea, fever and vomiting, all of which were self-limited and asymptomatic within 48 hours. In the study, no relevant long-term adverse events happened during 3-month follow-up, only one child with primary immunodeficiency died of sepsis and liver failure 4 weeks later after treatment with FMT for chronic intractable diarrhea.76 An open-label study and two-year follow-up showed that FMT is relatively safe in significantly alleviating gastrointestinal and neurobehavioral symptoms in children with ASD, without any adverse negative side effects. In general, FMT appears safe both in the short-term and long-term.
Conclusion
The microbiota-gut-brain axis plays an important role in the gastrointestinal symptoms and neurodevelopmental dysfunctions in ASD patients. FMT can alter the gut microbiota and improve the gastrointestinal as well as neurobehavioral symptoms in ASD patients, which is a promising treatment strategy. However, not only well designed human studies are needed to confirm the effect, but also elucidated research to explore the mechanism. Specific microbiota interventions need to be identified to further optimize treatment options.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Young S, Hollingdale J, Absoud M, et al. Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus. BMC Med. 2020;18:146. doi:10.1186/s12916-020-01585-y
2. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392:508–520. doi:10.1016/S0140-6736(18)31129-2
3. Zeidan J, Fombonne E, Scorah J, et al. Global prevalence of autism: a systematic review update. Autism Res. 2022;15:778–790. doi:10.1002/aur.2696
4. Svoboda E. Could the gut microbiome be linked to autism? Nature. 2020;577:S14–S5. doi:10.1038/d41586-020-00198-y
5. Leader G, Abberton C, Cunningham S, et al. Gastrointestinal symptoms in autism spectrum disorder: a systematic review. Nutrients. 2022;14:1471. doi:10.3390/nu14071471
6. Sanlier N, Kocabas Ş. The effect of probiotic, prebiotic and gut microbiota on ASD: a review and future perspectives. Crit Rev Food Sci Nutr. 2021;1–12. doi:10.1080/10408398.2021.1973957
7. Sorboni SG, Moghaddam HS, Jafarzadeh-Esfehani R, Soleimanpour S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev. 2022;35:e0033820. doi:10.1128/CMR.00338-20
8. Saurman V, Margolis KG, Luna RA. Autism spectrum disorder as a brain-gut-microbiome axis disorder. Dig Dis Sci. 2020;65:818–828. doi:10.1007/s10620-020-06133-5
9. Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37:984–995. doi:10.1016/j.clinthera.2015.04.002
10. Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29:79–84. doi:10.1097/MOG.0b013e32835a4b3e
11. Chen CC, Chiu CH. Current and future applications of fecal microbiota transplantation for children. Biomed J. 2021;45:11–18.
12. Liptak R, Gromova B, Gardlik R. Fecal microbiota transplantation as a tool for therapeutic modulation of non-gastrointestinal disorders. Front Med. 2021;8:665520. doi:10.3389/fmed.2021.665520
13. Borsom EM, Lee K, Cope EK. Do the bugs in your gut eat your memories? Relationship between gut microbiota and alzheimer’s disease. Brain Sci. 2020;10:814. doi:10.3390/brainsci10110814
14. Vendrik KEW, Ooijevaar RE, de Jong PRC, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. doi:10.3389/fcimb.2020.00098
15. Chinna Meyyappan A, Forth E, Wallace CJK, Milev R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry. 2020;20:299. doi:10.1186/s12888-020-02654-5
16. Rosenfeld CS. Microbiome disturbances and autism spectrum disorders. Drug Metab Dispos. 2015;43:1557–1571. doi:10.1124/dmd.115.063826
17. Tan Q, Orsso CE, Deehan EC, et al. Probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in the treatment of behavioral symptoms of autism spectrum disorder: a systematic review. Autism Res. 2021;14:1820–1836. doi:10.1002/aur.2560
18. Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120. doi:10.3389/fncel.2017.00120
19. Xie X, Li L, Wu X, et al. Alteration of the fecal microbiota in Chinese children with autism spectrum disorder. Autism Res. 2022;15:996–1007. doi:10.1002/aur.2718
20. Lou M, Cao A, Jin C, et al. Deviated and early unsustainable stunted development of gut microbiota in children with autism spectrum disorder. Gut. 2021;gutjnl-2021–325115. doi:10.1136/gutjnl-2021-325115
21. Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–S16. doi:10.1086/341914
22. Zou R, Xu F, Wang Y, et al. Changes in the gut microbiota of children with autism spectrum disorder. Autism Res. 2020;13:1614–1625. doi:10.1002/aur.2358
23. Wan Y, Zuo T, Xu Z, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut. 2022;71:910–918. doi:10.1136/gutjnl-2020-324015
24. Coretti L, Paparo L, Riccio MP, et al. Corrigendum: gut microbiota features in young children with autism spectrum disorders. Front Microbiol. 2019;10:920. doi:10.3389/fmicb.2019.00920
25. Sun H, You Z, Jia L, Wang F. Autism spectrum disorder is associated with gut microbiota disorder in children. BMC Pediatr. 2019;19:516. doi:10.1186/s12887-019-1896-6
26. Finegold SM, Dowd SE, Gontcharova V, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi:10.1016/j.anaerobe.2010.06.008
27. Zhang M, Ma W, Zhang J, He Y, Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep. 2018;8:13981. doi:10.1038/s41598-018-32219-2
28. Ding X, Xu Y, Zhang X, et al. Gut microbiota changes in patients with autism spectrum disorders. J Psychiatr Res. 2020;129:149–159. doi:10.1016/j.jpsychires.2020.06.032
29. Dan Z, Mao X, Liu Q, et al. Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut Microbes. 2020;11:1246–1267. doi:10.1080/19490976.2020.1747329
30. Grimaldi R, Cela D, Swann JR, et al. In vitro fermentation of B-GOS: impact on faecal bacterial populations and metabolic activity in autistic and non-autistic children. FEMS Microbiol Ecol. 2017;93:fiw233. doi:10.1093/femsec/fiw233
31. Marler S, Ferguson BJ, Lee EB, et al. Brief report: whole blood serotonin levels and gastrointestinal symptoms in autism spectrum disorder. J Autism Dev Disord. 2016;46:1124–1130. doi:10.1007/s10803-015-2646-8
32. Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22:361–368. doi:10.3748/wjg.v22.i1.361
33. Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010;9:2996–3004. doi:10.1021/pr901188e
34. Jory J. Abnormal fatty acids in Canadian children with autism. Nutrition. 2016;32:474–477. doi:10.1016/j.nut.2015.10.019
35. Saad K, Eltayeb AA, Mohamad IL, et al. A randomized, placebo-controlled trial of digestive enzymes in children with autism spectrum disorders. Clin Psychopharmacol Neurosci. 2015;13:188–193. doi:10.9758/cpn.2015.13.2.188
36. Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi:10.1371/journal.pone.0024585
37. Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124:680–686. doi:10.1542/peds.2008-2933
38. Finegold SM, Summanen PH, Downes J, Corbett K, Komoriya T. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe. 2017;45:133–137. doi:10.1016/j.anaerobe.2017.02.008
39. Settanni CR, Bibbò S, Ianiro G, et al. Gastrointestinal involvement of autism spectrum disorder: focus on gut microbiota. Expert Rev Gastroenterol Hepatol. 2021;15:599–622. doi:10.1080/17474124.2021.1869938
40. Xu HM, Huang HL, Zhou YL, et al. Fecal microbiota transplantation: a new therapeutic attempt from the gut to the brain. Gastroenterol Res Pract. 2021;2021:6699268. doi:10.1155/2021/6699268
41. Rutsch A, Kantsjö JB, Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11:604179. doi:10.3389/fimmu.2020.604179
42. Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. doi:10.3389/fnint.2013.00070
43. Chernikova MA, Flores GD, Kilroy E, Labus JS, Mayer EA, Aziz-Zadeh L. The brain-gut-microbiome system: pathways and implications for autism spectrum disorder. Nutrients. 2021;13:4497. doi:10.3390/nu13124497
44. Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J Psychiatry. 2021;43:293–305. doi:10.1590/1516-4446-2020-0987
45. Rose S, Bennuri SC, Davis JE, et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry. 2018;8:42. doi:10.1038/s41398-017-0089-z
46. Frye RE, Rose S, Chacko J, et al. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl Psychiatry. 2016;6:e927. doi:10.1038/tp.2016.189
47. Roussin L, Prince N, Perez-Pardo P, Kraneveld AD, Rabot S, Naudon L. Role of the gut microbiota in the pathophysiology of autism spectrum disorder: clinical and preclinical evidence. Microorganisms. 2020;8:1369. doi:10.3390/microorganisms8091369
48. Gevi F, Belardo A, Zolla L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165859. doi:10.1016/j.bbadis.2020.165859
49. van de Wouw M, Walsh CJ, Vigano GMD, et al. Kefir ameliorates specific microbiota-gut-brain axis impairments in a mouse model relevant to autism spectrum disorder. Brain Behav Immun. 2021;97:119–134. doi:10.1016/j.bbi.2021.07.004
50. Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101:246–59 e6. doi:10.1016/j.neuron.2018.11.018
51. Wu WL, Adame MD, Liou CW, et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature. 2021;595:409–414. doi:10.1038/s41586-021-03669-y
52. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi:10.1073/pnas.1102999108
53. Needham BD, Funabashi M, Adame MD, et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022;602:647–653. doi:10.1038/s41586-022-04396-8
54. Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153:448–59 e8. doi:10.1053/j.gastro.2017.05.003
55. Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–80 e12. doi:10.1016/j.cell.2016.11.018
56. Chen C, Liao J, Xia Y, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. 2022;71:2233–2252. doi:10.1136/gutjnl-2021-326269
57. Li Y, Luo ZY, Hu YY, et al. The gut microbiota regulates autism-like behavior by mediating vitamin B(6) homeostasis in EphB6-deficient mice. Microbiome. 2020;8:120. doi:10.1186/s40168-020-00884-z
58. Chen K, Luan X, Liu Q, et al. Drosophila histone demethylase KDM5 regulates social behavior through immune control and gut microbiota maintenance. Cell Host Microbe. 2019;25:537–52 e8. doi:10.1016/j.chom.2019.02.003
59. Sharon G, Cruz NJ, Kang DW, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177:1600–18 e17. doi:10.1016/j.cell.2019.05.004
60. Qi Z, Lyu M, Yang L, et al. A novel and reliable rat model of autism. Front Psychiatry. 2021;12:549810. doi:10.3389/fpsyt.2021.549810
61. Xiao L, Yan J, Yang T, et al. Fecal microbiome transplantation from children with autism spectrum disorder modulates tryptophan and serotonergic synapse metabolism and induces altered behaviors in germ-free mice. mSystems. 2021;6. doi:10.1128/mSystems.01343-20
62. Chen K, Fu Y, Wang Y, et al. Therapeutic effects of the in vitro cultured human gut microbiota as transplants on altering gut microbiota and improving symptoms associated with autism spectrum disorder. Microb Ecol. 2020;80:475–486. doi:10.1007/s00248-020-01494-w
63. Goo N, Bae HJ, Park K, et al. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020;262:118497. doi:10.1016/j.lfs.2020.118497
64. Abuaish S, Al-Otaibi NM, Abujamel TS, et al. Fecal transplant and bifidobacterium treatments modulate gut clostridium bacteria and rescue social impairment and hippocampal BDNF expression in a rodent model of autism. Brain Sci. 2021;11:1038. doi:10.3390/brainsci11081038
65. Abuaish S, Al-Otaibi NM, Aabed K, et al. The efficacy of fecal transplantation and bifidobacterium supplementation in ameliorating propionic acid-induced behavioral and biochemical autistic features in juvenile male rats. J Mol Neurosci. 2022;72:372–381. doi:10.1007/s12031-021-01959-8
66. Bermudez-Martin P, Becker JAJ, Caramello N, et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 2021;9:157. doi:10.1186/s40168-021-01103-z
67. Kang DW, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. doi:10.1186/s40168-016-0225-7
68. Kang DW, Adams JB, Coleman DM, et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821. doi:10.1038/s41598-019-42183-0
69. Li N, Chen H, Cheng Y, et al. Fecal microbiota transplantation relieves gastrointestinal and autism symptoms by improving the gut microbiota in an open-label study. Front Cell Infect Microbiol. 2021;11:759435. doi:10.3389/fcimb.2021.759435
70. Chen Y, Xueying Z, Jiaqu C, et al. FTACMT study protocol: a multicentre, double-blind, randomised, placebo-controlled trial of faecal microbiota transplantation for autism spectrum disorder. BMJ Open. 2022;12:e051613. doi:10.1136/bmjopen-2021-051613
71. Kang DW, Adams JB, Vargason T, Santiago M, Hahn J, Krajmalnik-Brown R. Distinct fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. mSphere. 2020;5. doi:10.1128/mSphere.00314-20
72. Żebrowska P, Łaczmańska I, Łaczmański Ł. Future directions in reducing gastrointestinal disorders in children with ASD using fecal microbiota transplantation. Front Cell Infect Microbiol. 2021;11:630052. doi:10.3389/fcimb.2021.630052
73. Tu T, Zhao C. Treating autism spectrum disorder by intervening with gut microbiota. J Med Microbiol. 2021;70. doi:10.1099/jmm.0.001469
74. Li SS, Zhu A, Benes V, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586–589. doi:10.1126/science.aad8852
75. Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology. 2021;160:1961–9 e3. doi:10.1053/j.gastro.2021.01.010
76. Zhang XY, Wang YZ, Li XL, et al. Safety of fecal microbiota transplantation in Chinese children: a single-center retrospective study. World J Clin Cases. 2018;6:1121–1127. doi:10.12998/wjcc.v6.i161.1121
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.