Back to Journals » Cancer Management and Research » Volume 15
Fecal Carriage of Extended-Spectrum β-Lactamase and Carbapenemase-Producing Enterobacteriaceae Among Oncology and Non-Oncology Patients at Jimma Medical Center in Ethiopia: A Comparative Cross-Sectional Study
Authors Temsegen W, Gorems K , Mekonnen M, Fufa D , Kassa T
Received 11 June 2023
Accepted for publication 11 October 2023
Published 1 November 2023 Volume 2023:15 Pages 1217—1231
DOI https://doi.org/10.2147/CMAR.S422376
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Wubalech Temsegen,1,2 Kasahun Gorems,2,3 Mekidim Mekonnen,1 Diriba Fufa,4 Tesfaye Kassa1
1School of Medical Laboratory Science, Faculty of Health Sciences, Jimma University, Jimma, Ethiopia; 2Microbiology Diagnostic Laboratory Unit of Jimma Medical Center, Jimma, Ethiopia; 3Department of Microbiology, Immunology and Parsitology, St Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 4Department of Pediatrics & Child Health, School of Medicine, Jimma University, Jimma, Ethiopia
Correspondence: Kasahun Gorems, Department of Microbiology, Immunology and Parsitology, St Paul’s Hospital Millennium Medical College, P. O. Box, 1271, Addis Ababa, Ethiopia, Tel +251925494531, Email [email protected]; [email protected] Tesfaye Kassa, School of Medical Laboratory Sciences, Jimma University, P. O. Box 788, Jimma, Ethiopia, Tel +251931057195, Email [email protected]; [email protected]
Purpose: Extended-spectrum β-lactamase (ESBL) and carbapenemase-producing Enterobacteriaceae (CPE) are among the major threats to global health because of their encoded protection against key antibiotics.
Methods: A comparative cross-sectional study was conducted among oncology and non-oncology patient groups (1:1; n = 214) on a consecutive sampling approach. Stool or rectal swab was collected from June 2021 to November 2021 and screened for ESBL-PE and CPE using ChromID-ESBL media. Confirmation for the enzymes was made by using combination disc and modified carbapenem inactivation methods, respectively. Disk diffusion method was used to determine antimicrobial susceptibility testing following the recommendations of CLSI 2022. SPSS software version 23 was used for data analysis.
Results: Fecal carriage prevalence of ESBL-PE was found in 90 (84.1%) of oncology participants and in 77 (71.9%) of non-oncology patients (p = 0.032). Escherichia coli was the most common ESBL-PE isolate in 82 (62.5%) and 68 (88.3%) of oncology and non-oncology patients, followed by Klebsiella oxytoca [15 (11.5%) versus 6 (7.8%)], respectively. Out of the total ESBL-PE isolates from both oncology and non-oncology patient groups, the maximum level of resistance was observed against ciprofloxacin 177 (86.3%), trimethoprim-sulfamethoxazole 103 (80.3%), tetracycline 97 (75.8%), whereas enhanced susceptibility was appreciated to tigecycline 200 (97.6%), meropenem 162 (79.0%), and ertapenem 145 (70.7%) with no significant difference between oncology and non-oncology group. Carbapenemase-producing isolates from oncology patients were 12 (11.2%), whereas it was 4 (3.7%) (p = 0.611) from non-oncology group. Bacterial isolates from oncology in this study showed a trend of multiple drug resistance of 113 (88.3%).
Conclusion: The results revealed alarmingly high carriage rates of ESBL and CPE among all study participants. Moreover, the isolates showed increased resistance rates to alternative drugs and had multiple antibiotic-resistant patterns. Hence, it is important to emphasize strict adherence to antimicrobial stewardship program as well as infection prevention and control practices.
Keywords: carriage rate, oncology, extended-spectrum beta-lactamase, carbapenemase, Enterobacteriaceae, Ethiopia
Introduction
Extended-spectrum-β-lactamase- (ESBL-PE) and carbapenemase- producing-Enterobacteriaceae (CPE) are serious and critical pathogens in healthcare systems. Major clinical problems are widespread due to these pathogens and constitute a great challenge for physicians in their management of patients.1 For instance, bacteremia caused by ESBL-PE has been associated with higher mortality rate than the non-ESBL-PE isolates.2
The Global Cancer Observatory reported 19,292,789 new cases of cancer and 9,958,133 deaths in 2020 globally.3 In Ethiopia, cancer accounts for approximately 5.8% of the total national mortality. Although population-based data do not exist in the country with the exception for Addis Ababa, it is estimated that the annual incidence of cancer is approximately 60,960 cases, and the annual mortality is expected to be over 44,000.4 Broad-spectrum β-lactam agents are the cornerstone in the treatment of cancer patients with suspected infection due to Enterobacteriaceae. Therefore, the increasing prevalence of multidrug resistance due to ESBL and CPE poses an urgent threat to vulnerable patients.5
During the past few decades, the ever-increasing use of some of the non-carbapenem b-lactams as first-line treatment in the community and cancer patients has raised doubts about their efficacy. This is because, their use has led to selective pressure in favor of emergence of bacteria that have acquired resistance enzymes.6 ESBLs are one of them currently spreading rapidly among Enterobacteriaceae, largely due to genes located on plasmids that can also disseminate across species barriers.7,8 Such propagation of these bacterial strains among cancer patients is an emerging public health concern.9
Various studies have shown that there is a strong relationship between malignancy and microbial infections. Those studies also indicated that infectious diseases are one of the salient noncancerous causes of death among cancer cases. In developing countries, infectious diseases cause the death of one in five cancer patients.10 Since bacterial infections are the most common problems among cancer cases, they are likely to result in immunodeficiency disorders owing to surgery or invasive procedures, lack of proper nutrition, repeated hospitalizations, chemotherapy, radiation therapy, and aggressive immunosuppressive cancer treatment interferences.11
Colonization with ESBL-PE, typically in the gastrointestinal tract, has been linked to subsequent infection due to multidrug-resistant (MDR) pathogens. Patients colonized unknowingly in the community may contribute for the subsequently transmission of the bacteria to other patients.12 This is of particular concern in cancer patients where oncological treatment frequently requires the use of surgery and chemotherapy or radiotherapy in health care facilities. This increases the risk of hospital-acquired infections mainly due to MDR bacteria.7 Patients with hematologic malignancies remain delicately vulnerable to infection with gram-negative bacteria which are carbapenem and expanded-spectrum β-lactam resistant ones such as Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia.13
Since cancer patients are frequently exposed to cytotoxic treatments that contribute to the weakening of their immune system and the destruction of the mucosal barrier, such situation facilitates gut permeability and inflammation. These promote microbial translocation from the digestive tract to the bloodstream.14 Recently, a significant increase in the prevalence of ESBL-PE fecal carriage has been reported in a variety of settings, including in hospitalized patients, newborns, infants, and healthy children, as well as in pediatric emergencies.12 Some of the publications indicated that one of the many independent risk factors for developing infections caused by ESBL-PE is prior colonization of the gastrointestinal tract with those bacteria.15
A recent cohort study performed by Cornejo-Juarez et al16 among patients with hematologic malignancies revealed that patients with fecal ESBL-PE colonization were 3.5 times more likely to develop ESBL-PE bacteremia than those without. This finding is in contrast to a similar study performed by Arnan et al17 in which no correlation was identified between ESBL-E fecal colonization and infection in a neutropenic population. Although the mortality rate was similar among ESBL-PE colonized and noncolonized individuals, the presence of fecal ESBL-E was associated with longer hospitalizations, a reduced time to death, and higher hospital costs.16
Carriage of resistant Enterobacteriaceae strains as a normal gut flora may serve as a reservoir of resistance genes. These genes may be acquired subsequently by intra or inter bacterial species to cause infection.18 The study planned to rule out any potential carriage of ESBL-PE and/or CPE which may results in subsequent infections of which may pose fatal consequences especially among cancer patients (oncology population) as this population is immunocompromised through the use of different kind of cancer treatment such as chemotherapy. According to oncology health expert reports from Jimma Medical Center (JMC), cancer patients who have appeared repeatedly were due to febrile illnesses that may be due to bacterial infections. Yet, data on the carriage rate of ESBL-PE and CPE among oncologic patients was not known in the center or in Ethiopia at large. Hence, this study was planned for the first time to assess the fecal carriage rate of ESBL-PE and CPE among oncology and non-oncology cases of JMC for comparison. Besides, the bacterial susceptibility pattern was also performed for other antibiotics.
Materials and Methods
Study Design, Period and Area
A comparative cross-sectional study was conducted at JMC, the largest medical center in southwestern Ethiopia. This study was conducted at JMC from June 2021 to November 2021. JMC have two oncology treatment centers (pediatric and adult oncology unit). The pediatric oncology unit (age ranged upto18 years) has a capacity of 15 rooms and 19 beds. Trimethoprim-sulfamethoxazole is used among the patients as antimicrobial prophylaxis. Patients with fever and severe neutropenia receive ceftriaxone plus gentamicin as initial treatment in the unit unless severe sepsis or septic shock is suspected in whom ceftazidime plus vancomycin is initiated.19 Currently, as much as 120 new cases are coming to the pediatric oncology unit per annum among which around 80 cases are under follow-up (personal communication). On the other hand, JMC Adult oncology unit has a capacity of 12 beds in 6 rooms with a patient flow of around 350 per year among which about 100 of them are under follow-up (personal report).
The sample size was determined by a double population proportion formula using Epi-info Version 7. Since there was no similar study conducted on the area so far, by considering chemotherapy for cancer patient’s use as an exposure variable, it was decided to take 50% of exposure in both groups, and 50% of prevalence was given to “non-oncology patients”. This was dependent on the assumption that patients attending the oncology ward have a 20% higher ESBL-PE carriage rate than “non-oncology patients”. By using a power of 80% and a confidence level of 95% for the two sample sizes with a 1:1 proportion, the initial sample size was 206. By considering 4% nonresponse rate, the final sample size was 214 with an equal size assigned for patients attending oncology wards (107), and another 107 for non-oncology patients.
Study Participants and Data Collection
Clinical and demographic information were collected using pretested questionnaire and patient records, which included age, sex, residential address, duration of illness, previous history of hospital admissions, antibiotic treatment use in the last three months, and presence of underlying diseases (hematological malignancies, nephroblastoma, neuroblastoma, different sarcomas, breast cancer, esophageal cancer, lung cancer, etc.). A trained nurse was assigned as data collector. The study enrolled all cancer patients attending the oncology unit during the study period after obtaining informed consent from oncology patients or parents/caregivers.
Recruitment of Comparator Groups: The Non-Oncology Patients
Comparative volunteer non-oncologic patient group was included from the pediatric and medical outpatient departments in JMC. All the selected patients have not been in oncology units for suspected diagnosis of malignancies, or admitted in the center and who did not take any antibiotics in two weeks period were enrolled as comparator. The study participants were recruited and selected after their informed written consent to participate in the study was signed. Families or caregivers were consented after the objective of the study was explained clearly to them.
Isolation and Identification of Bacteria
Stool or Rectal Swab Sample Collection and Transportation
Stool specimens or rectal swabs were collected from oncology and non-oncologic patients. Each patient or guardian was instructed on how to collect stool specimens. Fecal specimen was collected in a sterile wide-mouth screw-capped container and labeled with a unique ID number, date, and time of collection. All the samples were delivered for culture within 30 minutes of collection to the center diagnostic microbiology laboratory. Samples were kept in the refrigerator for 1–2 hours if unprocessed immediately. A rectal swab was collected from infants and weak patients unable to give stool specimens. Each swab was immersed immediately into a Cary-Blair transport medium and transported to JMC diagnostic Microbiology Laboratory.20
Screening and Identification of ESBL and C-P Enterobacteriaceae
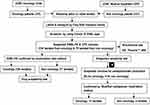
All samples were screened for ESBLs and CPE organisms using ChromID ESBL (bioMe´rieux, Marcy l’Etoile, France), a commercial ESBL-selective chromogenic agar medium (Figure 1). Plates were inoculated and incubated at 37°C for 18–24 h. If there was no growth, the plates were reincubated for an additional 24 h. Each morphologically suspected colony of Enterobacteriaceae was selected for identification by their pigment production on ChromID ESBL agar.
Species identification was performed with the conventional biochemical methods including colony morphology, oxidase test, triple sugar iron agar, citrate utilization test, urease test, motility, lysine decarboxylation tests, sulfur and indole production (all biochemical media from Liofilchem® s.r.l., Italy). Confirmation of ambiguous isolates were made by using BD Phoenix™ M50 system instrument at Ethiopian Public Health Institute, Addis Ababa.
Phenotypic Confirmation of ESBLs with a Combination Disk Test
Potential ESBL-PE producers were isolated from ChromID ESBL agar media. Final confirmation test were performed directly from the plates on suspected colonies by combination disk method. Cefepime (30 μg) and cefotaxime (30 μg) alone as well as its combination with clavulanic acid (30 μg g/10 μg), were placed at a distance of 25 mm, center to center, on Mueller Hinton agar (MHA) (Liofilchem® s.r.l., Italy) plates previously inoculated with a bacterial suspension of 0.5 McFarland turbidity standard. The plates were incubated overnight (18–24 hrs) at 37°C aerobically. Bacterial isolates that showed an increase in the inhibition zone diameter of ≥5 mm for combination disks versus cefepime or cefotaxime disk alone were confirmed as ESBL producers.21
Phenotypic Confirmation of CPE Production
Carbapenemase-producing isolates of Enterobacteriaceae were screened based on the non-susceptibility of isolates to ertapenem (≤18 mm). It is often the most sensitive indicator of carbapenemase production. It was confirmed by using the modified carbapenem inactivation method (mCIM).22 Briefly, 1 μLloopful of the Enterobacteriaceae isolate from blood agar plates was emulsified in 2 mL trypticase soy broth (TSB). A meropenem (10 μg) disk was then immersed in the suspension and incubated for a minimum of 4 h at 35°C. A 0.5 McFarland suspension of freshly grown E. coli ATCC 25922 was prepared and lawn of this bacterium was made on MHA plate. The meropenem disk was removed from the TSB suspension and placed on a MHA plate previously inoculated with the indicator strain. Plates were incubated at 35°C in ambient air for 18−24 h. An inhibition zone diameter of 6–15 mm or colonies within a 16–18 mm zone was considered to be a positive result, and a zone of inhibition ≥19 mm was considered to be a negative result.22
Antimicrobial Susceptibility Testing
Antibiotic susceptibility testing was performed by using the Kirby Bauer disc diffusion method on MHA.23 Briefly, pure culture isolates were taken to make suspensions equivalent to a 0.5 McFarland standard. A sterile cotton swab was dipped into the standardized suspension and used to evenly inoculate MHA plates and allowed to stand for about 15 minutes before applying disks. Different types of antibiotic discs (all from Liofilchem® s.r.l., Italy) were placed including: piperacillin/tazobactam (100/10µg), cefotaxime (30µg), ceftazidime (30µg), tetracycline (30µg), cefoxitin (30µg), cefepime (30µg), aztreonam (30µg), meropenem (10µg), tigecycline (15µg), ertapenem (10µg), gentamicin (10µg), ciprofloxacin (5µg), and trimethoprim-sulfamethoxazole (1.25/23.75µg). The plates were read and interpreted according to CLSI guidelines by measuring the zone of inhibition after overnight incubation at 37°C for 16–18 hours.24
Statistical Analysis
Data were handled for statistical analysis by SPSS version 23 software. Descriptive and frequency statistics for percentage and mean were calculated. Odds ratios and Chi-square tests were employed to compare the bacterial profile and antibiotic resistance of gut bacteria between the two groups: oncologic and non-oncologic study participants. Any variables that showed a p value ≤0.05 were considered statistically significant.
Results
Sociodemographic Characteristics of the Study Participants
A total of 214 study participants (107 oncology cases with various types of cancer and 107 non-oncology patients) were enrolled in this study at JMC and they were screened for fecal ESBL-PE and CPE carriage. The mean age of oncology patients was 28.01 ± 17.23 years and ranged from 1 to 84 years; for non-oncology patients, the mean age was 20.40 ± 17.65 years and ranged from 2 months to 80 years. Nearly 47% of oncology and 60% of non-oncology patients were males. The male to female ratio of the study participants was 1.14:1. Almost 60.7% of the oncology and 54.2% of the non-oncology patients lived in rural settings (Table 1).
 |
Table 1 Sociodemographic Characteristics of Oncology and Non-Oncology Study Participants from June 2021 to November 2021 at Jimma Medical Center, Jimma, Southwest Ethiopia |
Clinical Profile of Oncology Patients Participants
The most predominant cancer types among the oncology patient groups were carcinoma, leukemia, lymphoma, and different forms of sarcoma, with frequency rates of 49 (45.8%), 24 (22.4%), 22 (20.7%) and 10 (9.5%) cases, respectively. From the oncology participant group, 21 (≈20%) of them had been hospitalized, and another 51 (47.7%) cases had taken antimicrobial therapy in the past 3 months at the study time mainly ceftriaxone alone or in conjunction with other antibiotics [47/56 (83.9%)] followed by trimethoprim-sulfamethoxazole [38/56 (67.9%)]. Most oncology participants (88.8%) had been on different cycles of cancer chemotherapy treatments (Table 2).
 |
Table 2 Frequency of Clinical Profile of the Study Participants from June 2021 to November 2021 at Jimma Medical Center, Jimma, Southwest Ethiopia |
Magnitude of ESBL-Producing Enterobacteriaceae Among Oncology and Non-Oncology Patients
A total of 138 suspected ESBL producing Enterobacteriaceae strains were isolated from oncology patients. ESBL production was confirmed in 128 (92.8%) using a combination disk test (Table 3, Figure 1). On the other hand, a total of 79 suspected ESBL producing Enterobacteriaceae strains were isolated from non-oncology cases of which 77 (97.5%) were confirmed using a combination disk test (Table 3).
 |
Table 3 Bacterial Profile of ESBL- and CPE Carriage Rate Among Oncologic and Non-Oncologic Cases from June 2021 to November 2021 at Jimma Medical Center, Jimma, Southwest Ethiopia |
Out of the 128 ESBL producing Enterobacteriaceae isolates, 9 different bacterial species were identified with the predominant bacterial isolate being E. coli (n = 82, 64.0%) followed by K. oxytoca. (n = 15, 11.7%) and K. pneumonia (n = 13, 10.2%). On the other hand, out of 77 ESBL isolated from non-oncology patients, only 3 types of Enterobacteriaceae species were identified with E. coli (n = 68, 88.3%) followed by K. oxytoca (n = 6, 7.8%) and K. pneumonia (n = 3, 3.9%). The proportions of the first three bacterial isolates distribution did not show difference being oncologic or non-oncologic cases, χ2 (3, N = 208)=6.59, p > 0.05 (p = 0.0862) (Table 3).
Among the culture-positive samples from oncology patients (90 cases), 51 (56.7%) of them revealed single bacterial organisms, while the remaining 39 patients (43.3%) were positive for two or more species. From non-oncology culture-positive samples (77 patients), 73 (97.4%) of them were only single organisms, while the remaining 2 (2.6%) were positive for two bacteria (Table 3). The prevalence of ESBL and C-PE isolated among oncology cases was 84.1% (95% CI [76.6–90.7]) and 12.1% (95% CI [6.5–19.6]), respectively. There was statistically significant difference in the isolation frequency of ESBL-PE among oncology and non-oncology cases;p = 0.032 (Table 4).
 |
Table 4 Prevalence of ESBL and C-P Enterobacteriaceae Isolated from Oncology and Non-Oncology Patients from June 2021 to November 2021 at Jimma Medical Center, Southwest Ethiopia |
According to this study, the colonization rate of ESBL-PE among pediatrics and adult oncology was 87.7% and 78.6%, respectively. However, it was 68.9% and 76.1% among patients with non-oncologic symptoms (Figure 2). In both cases, there was no significant difference in the rate of colonization, p >0.05.
 |
Figure 2 Isolation of ESBL-PE against age of patients with oncology and non-oncology group from June 2021 to November 2021 at Jimma Medical Center, Jimma, Southwest Ethiopia. |
Magnitude of Carbapenemase-Producing Enterobacteriaceae Among Oncology and Non-Oncology Patients
Among the total 138 ESBL-PE suspected strains isolated from oncology patients, 54 of them were screened for carbapenemase elaboration (Figure 1). Further confirmation test identified 12 (12/54, 22.2%) of the isolates as carbapenemase producers (Table 4). From non-oncology group, out of the 77 ESBL-PE isolates, 9 of them were screened for carbapenemase elaboration. Four of the 9 were confirmed as carbapenemase elaborators. The overall magnitudes of carbapenemase-producing Enterobacteriaceae among oncology and non-oncology were 11.2% (n = 12/107) and 3.7 (n = 4/107), P = 0.46 respectively. The frequent isolates with carbapenemase producers among oncology patients was observed in E. coli, 7.5% (n = 8/107); followed by K. oxytoca, 1.9% (n = 2/107) and C. diversus, 1.9% (n = 2/107).
Antimicrobial Resistance Pattern of ESBL-Producing Enterobacteriaceae Oncology and Non-Oncology Patients
Antimicrobial susceptibility tests were performed on 128 isolates from oncology and 77 from non-oncology study participants. Out of 13 antimicrobial agents tested, the antimicrobial susceptibility pattern (as shown in Table 5) among oncology patients showed high resistance rates to non-β-lactam antibiotics, including ciprofloxacin, trimethoprim-sulfamethoxazole, and tetracycline were with 105 (80.2%), 103 (80.5%), and 97 (75.8%) frequency, respectively. Around forty percent of the isolates from oncology patients were resistant to ertapenem. On the other hand, susceptibility of isolates was seen to tigecycline 123 (96.1%), and meropenem 92 (71.9%). Isolates from non-oncology showed resistance to ciprofloxacin, 72 (93.5%); however, they were susceptible to tigecycline 77 (100%), meropenem 70 (90.9%) and ertapenem 68 (88.3%).
 |
Table 5 Antimicrobial Resistance Patterns of ESBL-E Isolates from Oncologic and Non-Oncologic Cases from June 2021 to November 2021 at Jimma Medical Center, Jimma, Southwest Ethiopia |
Generally, out of the total ESBL-PE isolates from oncology and non-oncology patients, the maximum level of resistance was seen towards ciprofloxacin 177 (86.3%), trimethoprim-sulfamethoxazole 103 (80.3%), tetracycline 97 (75.8%), whereas better susceptibility was seen to tigecycline 200 (97.6%), meropenem 162 (79.0%) and ertapenem 145 (70.7%) (Table 5).
Multidrug Resistance Pattern of ESBL-Producing Enterobacteriaceae
Among the total ESBL-producing Enterobacteriaceae isolates (n = 131), multidrug resistance (resistance to at least 3 antibiotics in a different class) was observed in 87.5% (n=112/128). Surprisingly, complete (100%) MDR was noted for K. ozaenae, Citrobacter spp., K. rhinoscleromatis, and Providence spp. (Table 6).
 |
Table 6 Multidrug Resistance Patterns of ESBL-Producing Enterobacteriaceae Isolates Among Oncology Patients from June 2021 to November 2021 at Jimma Medical Center, Jimma, Southwest Ethiopia |
Discussion
Gastrointestinal colonization with ESBL-PE and CPE is a growing public health threat to humans worldwide and worrisome in patients. It has been linked to subsequent infection due to these pathogens and may contribute to patient-to-patient transmission.12 Gastrointestinal tract (GIT) colonization has also been of particular concern among oncology patients due to the effect of cytotoxic chemotherapy and extensive uses of broad-spectrum antibiotics that alter the gut microbiome. Carriage of ESBL and C-P Enterobacteriaceae strains in the normal gut flora may serve as a good reservoir of resistance genes transferring to strains that able to cause infection.10
Over the last decade, the prevalence of ESBL-PE and CPE has dramatically increased worldwide. In Ethiopia, especially in cancer patients, there was no data available with regard to fecal carriage of ESBL-PE and CPE. We consider, this is the first study showing the alarmingly high prevalence of ESBL-PE and CPE colonization of the GIT among oncology patients. In this study, the majority of ESBL-PE was isolated from patients with hematological malignancies (89.5%) compared with solid tumors. This fact was the same as the results from patients with hematological malignancies observed in a study from Algeria (73%)12 and India (60%).25 The reason for this situation might be related to the immunocompromised state, especially during periods of neutropenia and frequent use of antibiotics in this population.25
In our study, the prevalence of ESBL-PE fecal carriage was 84.1% (95% CI 76.6–90.7). The current finding was higher than the studies done in Africa (16–65%)12,14,26–33 and elsewhere (7.69–63%)29–32,34–36 in different group of patients. This result was also higher than a study conducted among cancer patients in eastern Iran (50%).37 This variation might be due to the difference in the study population, inappropriate use of antibiotics, variation in antibiotic resistance control and prevention measures, and variation in the method of ESBL detection.
In this study, the prevalence of ESBL-PE among pediatric oncology patients was 87.7%. This was also higher than the study conducted on oncology pediatric units in Algeria (54%),12 India (58%),25 and the Czech Republic (19%).38 It was also higher than those of other categories of children: 21% in children without cancer admitted to a pediatric unit in Madagascar,29 31% in children with severe acute malnutrition in Niger,39 and 25% in healthy children in Lebanon.40 This vast variation in the prevalence rate of ESBL-PE fecal carriage is possibly related to the overuse of β-lactam antibiotic drugs. ESBL colonization further amplifies after repeated antibiotic usage as well as due to cross infections in the oncology unit. This was demonstrated in a study where 80% of sensitive organisms acquired resistance and 40% of non-ESBL and non-CPE organisms converted to ESBL and CPE, respectively.25
In our study, the majority of bacterial ESBL-PE isolates were E. coli, followed by K. oxytoca. This finding is consistent with the results of other recent studies in Spain,41 Mexico,42 and Lebanon,43 which have reported alarmingly high levels of fecal colonization by ESBL-producing E. coli strains in neutropenic patients with hematological malignancies.17 This result may be inconsistent with a study conducted in Algeria that reported K. pneumonia isolates, followed by E. coli.12 Similar results were reported by Zachariah and his team,44 and Thacker et al25 showed that K. pneumonia isolates were the most frequent in children with cancer. The possible reasons for such variation in the bacterial profile might be attributed to the difference in climatic and geographic location of the study sites.
In this study, antimicrobial sensitivity testing showed high resistance to non-β-lactam antibiotics in ESBL-PE strains, as was previously reported by other authors.12,38 This is observed to ciprofloxacin 177 (86.3%), trimethoprim-sulfamethoxazole 103 (80.3%), and tetracycline 97 (75.8%). This finding is in contrary to with the previous study conducted by Montazeri et al that showed ciprofloxacin (28.6%).45 Our study showed better susceptibility to tigecycline (96.2%), and meropenem (71%) although the study carried out by Medboua-Benbalahfound approximately100%susceptibility against meropenem.12 The major reasons for this discrepancy between results might be the different types of antibiotic usage in the prophylaxis and treatment of cancer cases in various geographical areas and the difference in antibiotic usage policy.
In this study, ESBL-producing Enterobacteriaceae exhibited increased resistance to carbapenem drugs, such as meropenem (29%) and ertapenem (41.2%). This finding is alarming compared with the studies conducted in Addis Ababa, Ethiopia, Zimbabwe, as well as in India28,34,46 where all reported complete susceptibility to carbapenem drugs. This increased resistance rate to carbapenems might be linked with the enlarged use of carbapenem drugs in private and public health facilities in this geographic location. The overuse and misuse of such antibiotics during the COVID-19 pandemics might also contribute to the increased rates of resistance.
In the present study, ESBL-producing Enterobacteriaceae were also resistant to multiple non-β-lactam antibiotics, including ciprofloxacin (80.2%), trimethoprim and sulfamethoxazole (80.2%), and tetracycline (75.6%). ESBL producers showing significant resistance to ciprofloxacin and trimethoprim –sulfamethoxazole were reported from a similar studies in Chad,30 Burkina Faso,47 Arba Minch, Ethiopia,33 Morocco,27 Egypt,14 and Tanzania26 in the ranges between 58–68.5% and 60–100%, respectively. This may be due to the fact that resistance gene coding for ESBL production is usually found on the same mobile genetic elements, which may also carry resistance genes for non-beta-lactam antibiotics to dispense them coresistance. Further selection of resistant strains may be facilitated by the irrational overuse of antimicrobial agents as well as prescribing drugs without antimicrobial susceptibility testing in patients.
In this study, high fecal carriage rate of ESBL-PE was identified, with isolates highly resistant to most antibiotics. Such organisms have high risk for nosocomial infections as well as dissemination of resistant strains in health care system and community, presenting subsequent infection with high morbidity and mortality outcomes. The overall carriage rate of CPE was 12 (11.2%) (95% CI; 6.5–19.6) using the modified carbapenem inactivation method. This finding is in line with the study conducted in India (14.8%)34 and Pakistan (18.5%)48 among hospitalized patients. This was higher than that of non-oncology patients, 4 (3.7%) isolated in this study (p.value=0.069). This result was also higher than those of a study performed in Addis Ababa, Ethiopia (2%),28 Arba Minch, Ethiopia (1.43%),33 Morocco (1.8%),27 Egypt (5%),14 China (6.6%)49 and China (8.5%).50 The possible reason might be the difference in carbapenemase detection methodological difference, sample size, and study participants and due to the widespread use of carbapenems for treating ESBL-associated infections. In the present study, the highest carbapenemase production was recorded in E. coli 7.5%. Opposed to our finding, the study conducted in different areas showed that K. pneumoniae was reported in Arba Minch 1.43%,33 in Egypt at 5%,14 and in China at 11.6%.50 This might be attributed to the difference in climatic and geographic variation of the study sites.
The limitations of this study include the fact that cancer patients confirmed to have ESBL-PE and CPE colonization were not given immediate decontamination management by discussing with the responsible body because there was no effective strategy or guideline for such patient population, and it is not clear what agents may be used to avoid the selection and dissemination of superbugs in the gut microbiome as well as in the environment.
Conclusion and Recommendation
The overall prevalence of ESBL-PE carriage from the gastrointestinal tract was higher. E. coli followed by K. oxytoca were the predominant bacterial isolates among cancer patients. The ESBL-PE pathogens have shown a high level of antibiotic resistance in the study area. The majority of the bacterial isolates had multiple drug-resistant features. Almost all the isolated bacteria showed a considerable level of resistance, particularly to ciprofloxacin, trimethoprim-sulfamethoxazole, and tetracycline. The isolated bacteria exhibited a high level of ciprofloxacin resistance among both groups (105 (82.0%) and 72 (93.5%)), respectively. Tigecycline, and meropenem have better action against the isolates. In general, the results of this study revealed ESBL-PE and CPE recovered from cancer patients are alarmingly shocking in Jimma area and can becoming a major public health problem in the management of patients with oncology cases.
It is highly recommended future research target to answer the questions of the true impact of colonization due to ESBL-PE and CPEin the development of infections. Culture and antimicrobial susceptibility tests should be considered mandatory before prescribing any antibiotics to minimize empirical treatment and emergence of drug-resistant strains in the community of both oncology and non-oncology patients. It is strongly recommended to emphasize strengthening infection prevention and control plan against ESBL-PE and CPE to avoid patient to patient transmission among oncology and non-oncology patients in healthcare settings.
Data Sharing Statement
The data sets generated and/or analyzed for this study are available from the corresponding author on reasonable request.
Ethics Approval
Ethical clearance and approval were obtained from the Institutional Review Board (IRB) of Jimma University Institute of Health with reference number JHRPGN/166 before conducting the research. The study was done as per the Helsinki declaration. Permission was also obtained from the health facilities team where the study was conducted. Informed voluntary assent was obtained from children, and written consent was obtained from all adult patients and parents/guardians of the children involved before commencing the study. Patients with a positive result were communicated about their result to their respective physician for proper management.
Consent for Publication
Not Applicable – This manuscript does not contain any individual person’s data.
Acknowledgments
The authors are grateful to all the study participants for their willingness to participate in this study. Additionally, we would like to thank the staff of Jimma University Medical Center. We are also grateful to data collector Mrs. Sara Kedir and Mr.Teketele for constructive comments suggestions and support during data collection and result communication.
Author Contributions
All authors made a significant contribution to the work reported, from the conception, study design, execution, acquisition of data, analysis to interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; agreed on the journal to which the article should be submitted; reviewed and agreed on the article in its current version and agree to take responsibility and be accountable for the contents of the article.
Funding
The research was funded by Institute of Health, Jimma University, Ethiopia.
Disclosure
The authors declare that the study was carried out in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Catho G, Huttner BD. Strategies for the eradication of extended-spectrum beta-lactamase or carbapenemase-producing Enterobacteriaceae intestinal carriage. Expert Rev Anti Infect Ther. 2019;17(8):557–569. doi:10.1080/14787210.2019.1645007
2. Rottier WC, Ammerlaan HS, Bonten MJ. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67(6):1311–1320. doi:10.1093/jac/dks065
3. The global cancer observatory; 2018. Available from: https://gco.iarc.fr/today.
4. National cancer control plan 2016–2020. Available from: https://www.iccpportal.org/sites/default/files/plans/NCCP%20Ethiopia%20Final%20261015.pdf.
5. Woodworth KR, Walters MS, Weiner LM, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006–2017. Morbid Mortal Weekly Rep. 2018;67(13):396. doi:10.15585/mmwr.mm6713e1
6. Montes CV, Vilar-Compte D, Velazquez C, Golzarri MF, Cornejo-Juarez P, Larson EL. Risk factors for extended spectrum β-lactamase-producing Escherichia coli versus susceptible E. coli in surgical site infections among cancer patients in Mexico. Surg Infect. 2014;15(5):627–634. doi:10.1089/sur.2013.189
7. Kariuki S, Hart CA. Global aspects of antimicrobial-resistant enteric bacteria. Curr Opin Infect Dis. 2001;14(5):579–586. doi:10.1097/00001432-200110000-00012
8. Falagas M, Karageorgopoulos DE. Extended-spectrum β-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–354. doi:10.1016/j.jhin.2009.02.021
9. Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi:10.1016/S1473-3099(08)70041-0
10. Lin L, Jia L, Fu Y, et al. A comparative analysis of infection in patients with malignant cancer: a clinical pharmacist consultation study. J Infect Public Health. 2019;12(6):789–793. doi:10.1016/j.jiph.2019.03.021
11. Jiang A-M, Shi X, Liu N, et al. Nosocomial infections due to multidrug-resistant bacteria in cancer patients: a six-year retrospective study of an oncology Center in Western China. BMC Infect Dis. 2020;20(1):1–12. doi:10.1186/s12879-020-05181-6
12. Medboua-Benbalagh C, Touati A, Kermas R, et al. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae strains is associated with worse outcome in patients hospitalized in the pediatric oncology unit of Beni-Messous hospital in Algiers, Algeria. Microb Drug Resist. 2017;23(6):757–763. doi:10.1089/mdr.2016.0153
13. Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis. 2011;53(8):798–806. doi:10.1093/cid/cir492
14. Abdallah H, Alnaiemi N, Reuland E, et al. Fecal carriage of extended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae in Egyptian patients with community-onset gastrointestinal complaints: a hospital-based cross-sectional study. Antimicrob Resist Infect Control. 2017;6(1):1–7. doi:10.1186/s13756-017-0219-7
15. Reddy P, Malczynski M, Obias A, et al. Screening for extended-spectrum β-lactamase-producing Enterobacteriaceae among high-risk patients and rates of subsequent bacteremia. Clin Infect Dis. 2007;45(7):846–852. doi:10.1086/521260
16. Cornejo-Juárez P, Suárez-Cuenca JA, Volkow-Fernández P, et al. Fecal ESBL Escherichia coli carriage as a risk factor for bacteremia in patients with hematological malignancies. Support Care in Cancer. 2016;24(1):253–259. doi:10.1007/s00520-015-2772-z
17. Arnan M, Gudiol C, Calatayud L, et al. Risk factors for, and clinical relevance of, faecal extended-spectrum β-lactamase producing Escherichia coli (ESBL-EC) carriage in neutropenic patients with haematological malignancies. Eur j Clin Microbiol Infect Dis. 2011;30(3):355–360. doi:10.1007/s10096-010-1093-x
18. WHO publishes list of bacteria for which new antibiotics are urgently needed. Available from: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-newantibiotics-are-urgently-needed.
19. The pediatric cancer unit at Jimma university specialized hospital treated more than 100 children with cancer. Available from: https://semonegna.com/jush-pediatric-cancer-unit-treated-children-cancer/.
20. Nagata N, Tohya M, Takeuchi F, et al. Effects of storage temperature, storage time, and Cary-Blair transport medium on the stability of the gut microbiota. Drug Discov Ther. 2019;13(5):256–260. doi:10.5582/ddt.2019.01071
21. Disc tests for detection of ESBL-producing Enterobacteriaceae. Available from: https://www.liofilchem.com/images/prodottievidenza/antibiotic-disc/ESBL_DISC_TESTS.pdf.
22. Jing X, Zhou H, Min X, et al. The simplified carbapenem inactivation method (sCIM) for simple and accurate detection of carbapenemase-producing gram-negative bacilli. Front Microbiol. 2018;9:2391. doi:10.3389/fmicb.2018.02391
23. Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009;15:55–63.
24. M100: performance standards for antimicrobial susceptibility testing. Available from: https://www.nih.org.pk/wpcontent/uploads/2021/02/CLSI-2020.pdf.
25. Thacker N, Pereira N, Banavali S, et al. Alarming prevalence of community-acquired multidrug-resistant organisms colonization in children with cancer and implications for therapy: a prospective study. Indian J Cancer. 2014;51(4):442. doi:10.4103/0019-509X.175310
26. Kibwana UO, Majigo M, Kamori D, Manyahi J. High fecal carriage of extended Beta Lactamase producing Enterobacteriaceae among adult patients admitted in referral hospitals in Dar es Salaam, Tanzania. BMC Infect Dis. 2020;20(1):1–7. doi:10.1186/s12879-020-05272-4
27. Arhoune B, Oumokhtar B, Hmami F, et al. Rectal carriage of extended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J Global Antimicrob Resist. 2017;8:90–96. doi:10.1016/j.jgar.2016.11.004
28. Desta K, Woldeamanuel Y, Azazh A, et al. High gastrointestinal colonization rate with extended-spectrum β-lactamase-producing Enterobacteriaceae in hospitalized patients: emergence of carbapenemase-producing K. pneumoniae in Ethiopia. PLoS One. 2016;11(8):e0161685. doi:10.1371/journal.pone.0161685
29. Andriatahina T, Randrianirina F, Hariniana ER, et al. High prevalence of fecal carriage of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect Dis. 2010;10(1):1–8. doi:10.1186/1471-2334-10-204
30. Ouchar Mahamat O, Tidjani A, Lounnas M, et al. Fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in hospital and community settings in Chad. Antimicrob Resist Infect Control. 2019;8(1):1–7. doi:10.1186/s13756-019-0626-z
31. Chaudhary U, Agarwal S, Raghuraman K. Identification of extended spectrum beta lactamases, AmpC and carbapenemase production among isolates of Escherichia coli in North Indian tertiary care centre. Avicenna J Med. 2018;8(02):46–50. doi:10.4103/ajm.AJM_156_17
32. Hazirolan G, Mumcuoglu I, Altan G, Özmen B, Aksu N, Karahan Z. Fecal carriage of extended‑spectrum beta‑lactamase and AmpC Beta‑lactamase‑producing Enterobacteriaceae in a Turkish Community. Niger J Clin Pract. 2018;21(1):81–86. doi:10.4103/njcp.njcp_79_17
33. Aklilu A, Manilal A, Ameya G, Woldemariam M, Siraj M. Gastrointestinal tract colonization rate of extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae and associated factors among hospitalized patients in Arba Minch general hospital, Arba Minch, Ethiopia. Infect Drug Resist. 2020;13:1517–1526. doi:10.2147/IDR.S239092
34. Babu R, Kumar A, Karim S, et al. Faecal carriage rate of extended-spectrum β-lactamase-producing Enterobacteriaceae in hospitalised patients and healthy asymptomatic individuals coming for health check-up. J Global Antimicrob Resist. 2016;6:150–153. doi:10.1016/j.jgar.2016.05.007
35. Ruh E, Zakka J, Hoti K, et al. Extended-spectrum β-lactamase, plasmid-mediated AmpC β-lactamase, fluoroquinolone resistance, and decreased susceptibility to carbapenems in Enterobacteriaceae: fecal carriage rates and associated risk factors in the community of Northern Cyprus. Antimicrob Resist Infect Control. 2019;8(1):1–10. doi:10.1186/s13756-019-0548-9
36. Pérez CD-A, López-Fresneña N, Carlavilla ALR, et al. Local prevalence of extended-spectrum beta-lactamase (ESBL) producing Enterobacteriaceae intestinal carriers at admission and co-expression of ESBL and OXA-48 carbapenemase in Klebsiella pneumoniae: a prevalence survey in a Spanish University Hospital. BMJ open. 2019;9(3):e024879. doi:10.1136/bmjopen-2018-024879
37. Askari P, Hakimi F, Moghanni M, Sebzari AR, Namaei MH. Evaluation of fecal colonization with extended-spectrum beta-lactamase-producing Enterobacteriaceae and risk factors among cancer patients in Eastern Iran. Infect Epidemiol Microbiol. 2021;7(4):2. doi:10.52547/iem.7.4.277
38. Dolejska M, Brhelova E, Dobiasova H, et al. Dissemination of IncFIIK-type plasmids in multiresistant CTX-M-15-producing Enterobacteriaceae isolates from children in hospital paediatric oncology wards. Int J Antimicrob Agents. 2012;40(6):510–515. doi:10.1016/j.ijantimicag.2012.07.016
39. Woerther P-L, Angebault C, Jacquier H, et al. Massive increase, spread, and exchange of extended spectrum β-lactamase–encoding genes among intestinal Enterobacteriaceae in hospitalized children with severe acute malnutrition in Niger. Clin Infect Dis. 2011;53(7):677–685. doi:10.1093/cid/cir522
40. Hijazi S, Fawzi M, Ali F, Abd el galil K. Prevalence and characterization of extended-spectrum beta-lactamases producing Enterobacteriaceae in healthy children and associated risk factors. Ann Clin Microbiol Antimicrob. 2016;15(1):1–9. doi:10.1186/s12941-016-0121-9
41. Calatayud L, Arnan M, Liñares J, et al. Prospective study of fecal colonization by extended-spectrum-β-lactamase-producing Escherichia coli in neutropenic patients with cancer. Antimicrob Agents Chemother. 2008;52(11):4187–4190. doi:10.1128/AAC.00367-08
42. Golzarri MF, Silva-Sánchez J, Cornejo-Juárez P, et al. Colonization by fecal extended-spectrum β-lactamase-producing Enterobacteriaceae and surgical site infections in patients with cancer undergoing gastrointestinal and gynecologic surgery. Am J Infect Control. 2019;47(8):916–921. doi:10.1016/j.ajic.2019.01.020
43. Christophy R, Osman M, Mallat H, et al. Prevalence, antibiotic susceptibility and characterization of antibiotic resistant genes among carbapenem-resistant Gram-negative bacilli and yeast in intestinal flora of cancer patients in North Lebanon. J Infect Public Health. 2017;10(6):716–720. doi:10.1016/j.jiph.2016.10.009
44. Zachariah M, Al-Yazidi L, Bashir W, Al Rawas A, Wali Y, Pathare A. Spectrum of external catheter-related infections in children with acute leukemia—Single-center experience. J Infect Public Health. 2014;7(1):38–43. doi:10.1016/j.jiph.2013.06.005
45. Abbasi Montazeri E, Khosravi AD, Saki M, Sirous M, Keikhaei B, Seyed-Mohammadi S. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae causing bloodstream infections in cancer patients from southwest of Iran. Infect Drug Resist. 2020;Volume 13:1319–1326. doi:10.2147/IDR.S254357
46. Wilmore SS, Kranzer K, Williams A, et al. Carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae in HIV-infected children in Zimbabwe. J Med Microbiol. 2017;66(5):609. doi:10.1099/jmm.0.000474
47. Ouédraogo A-S, Sanou S, Kissou A, et al. Fecal carriage of Enterobacteriaceae producing extended-spectrum beta-lactamases in hospitalized patients and healthy community volunteers in Burkina Faso. Microb Drug Resist. 2017;23(1):63–70. doi:10.1089/mdr.2015.0356
48. Day KM, Ali S, Mirza IA, et al. Prevalence and molecular characterization of Enterobacteriaceae producing NDM-1 carbapenemase at a military hospital in Pakistan and evaluation of two chromogenic media. Diagn Microbiol Infect Dis. 2013;75(2):187–191. doi:10.1016/j.diagmicrobio.2012.11.006
49. Li B, Xu X-H, Zhao Z-C, Wang M-H, Cao Y-P. High prevalence of metallo-β-lactamase among carbapenem-resistant Klebsiella pneumoniae in a teaching hospital in China. Can J Microbiol. 2014;60(10):691–695. doi:10.1139/cjm-2014-0291
50. Liu B, Trout REL, Chu G-H, et al. Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine-and Metallo-Β-Lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. ACS Publications; 2019.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.