Back to Journals » Clinical Epidemiology » Volume 12
Feasibility Study of Advanced Cardiovascular Screening in Middle-Aged Patients with Diabetes
Authors Lindholt JS , Frystyk J, Hallas J , Rasmussen LM, Diederichsen ACP
Received 19 January 2020
Accepted for publication 1 April 2020
Published 6 May 2020 Volume 2020:12 Pages 447—455
DOI https://doi.org/10.2147/CLEP.S246636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Jes Sanddal Lindholt,1 Jan Frystyk,2 Jesper Hallas,3 Lars Melholt Rasmussen,4 Axel Cosmus Pyndt Diederichsen5
1Elitary Research Centre of Individualised Medicine in Arterial Disease (CIMA), Department of Cardiothoracic and Vascular Surgery, Odense University Hospital, Odense, Denmark; 2Department of Endocrinology, Odense University Hospital, Odense, Denmark; 3Institute of Pharmacology, University of Southern Denmark, Odense, Denmark; 4Elitary Research Centre of Individualised Medicine in Arterial Disease (CIMA), Department of Clinical Biochemistry and Pharmacology, University Hospital Odense, Odense, Denmark; 5Elitary Research Centre of Individualised Medicine in Arterial Disease (CIMA), Department of Cardiology, University Hospital Odense, Odense, Denmark
Correspondence: Axel Cosmus Pyndt Diederichsen
Department of Cardiology, University Hospital Odense, J. B. Winsløws Vej 4, Odense 5000, Denmark
Tel +45 40191227
Email [email protected]
Purpose: Cardiovascular mortality remains high among patients with diabetes compared with the general population. The primary aim was to evaluate the interest in and demand for advanced cardiovascular screening in patients with diabetes; the secondary aim was to explore its efficiency in detecting unprotected subclinical cardiovascular disease (CVD).
Patients and Methods: In a cross-sectional design, randomly selected 40– 60-year-old men and women with diabetes were invited to the screening trial. Screening encompassed (1) a comprehensive medical interview; (2) non-contrast computed tomography scanning to quantify coronary artery and aortic valve calcification, to measure left atrial size, to assess heart rhythm and to detect aortic and iliac dilatations; (3) ankle and brachial blood pressure measurements; and (4) blood and urine samples for measurements of HbA1c, lipid profile, renal function, NT-pro B-type natriuretic peptide (pro-BNP) and albuminuria. Primary outcome was participation rate; secondary outcome was rate of unprotected subclinical CVD.
Results: Of 465 invited patients, 191 (41.1%) attended screening. The participation rate was 40% (95% CI:33– 47) for males and 42% (95% CI:36– 48) for females. Twenty-four patients were excluded due to previous CVD. The remaining patients’ mean age was 52 years; 58% were males. Subclinical CVD was found in 64%, with a male preponderance (males 75% (95% CI:66– 83; females 49% (95% CI:37– 60)). Presence of severe coronary artery calcification (score ≥ 400) showed a male preponderance (males 19% (95% CI:12– 27); females 7% (95% CI:3– 16)). Aortic valve calcification, enlarged left atrial volume, atrial fibrillation, aortic dilatations, peripheral artery disease or increased pro-BNP were uncommon, and without any sex differences. Unprotected subclinical CVD was very common, and medical treatment was intensified in 60% (95% CI:53– 68) of patients.
Conclusion: We propose a feasible cardiovascular screening examination from which middle-aged patients with diabetes may benefit. However, the participation rate may be too low to warrant screening.
Keywords: diabetes, cardiovascular disease, screening, risk factors, coronary artery calcification, CT scanning
Introduction
Large-scale studies have reported a 3-fold higher mortality and a considerably shorter life expectancy in persons with type 2 diabetes (T2D) than in healthy persons.1 This is primarily due to an increased incidence of cardiovascular disease (CVD).2,3 Several attempts have been made to target CVD risk factors; however, clinical outcomes have only rarely been successful.4 Even though all persons with T2D are recommended a healthy lifestyle, a recent clinical trial with 8.5 years of lifestyle intervention found no decrease in CVD incidence despite a significant reduction in several CVD risk factors.5,6 The same disappointing observation applies to treatment of hyperglycemia. Hence, the use of intensive therapy to target a near-normal HbA1c level does not decrease CVD risk.7 Statin therapy is recommended in most cases, but the optimal antithrombotic treatment has not been established; nor are optimal blood pressure targets unanimously defined.8 Thus, we see an unmet need for other approaches to identify and treat persons with T2D at risk of CVD.
Coronary artery calcification (CAC) is easily detectable by cardiac non-contrast CT scanning. This method is highly specific of an underlying subclinical atherosclerotic coronary artery disease. In the general population as well as in patients with diabetes, the extent of CAC is significantly associated with an increased risk of CVD.9 Interestingly, one-third of persons with T2D have no CAC and harbour a risk of CVD close that of the general population.10,11 Conversely, a large number of persons with T2D suffer from silent, undiagnosed significant coronary artery disease (CAC score above 400). They have five times higher mortality rates than the general population.12 Therefore, CAC score measurements may be useful for optimizing CVD risk stratification by identifying persons with undiagnosed disease, who should receive intensified preventive treatment, and to identify low-risk persons in whom a healthy lifestyle should be sufficient.
The dual aim of this pilot study was, firstly to evaluate the interest in and demand for advanced cardiovascular screening in patients with diabetes; secondly to explore its efficiency in detecting unprotected subclinical CVD.
Patients and Methods
This is a cross-sectional observational study of randomly included 40–60-year-old females and males with diabetes and living in the municipalities of Middelfart, Assens, Kerteminde, Odense and Bogense in Denmark. The patients were identified through the Odense University Pharmacoepidemiological Database (OPED).13 Diabetes was defined as collecting antidiabetic treatment (ATC code A10) at a pharmacy during the period from 1 November 2016 to 31 October 2017. In Demark, all citizens have a unique civil registration (CPR) number. This number is used when dispensed medication is collected at a pharmacy. OPED contains data on reimbursed prescriptions from the above municipalities and the CPR number of the person to whom the medicine is prescribed. Invited to participate in the study were all patients with T2D born from the 1st to the 9th day in all months of the year. No exclusion criteria were applied. Non-responders were re-invited after three months. The screening examination was performed at Odense University Hospital from October 2018 to February 2019. All participants signed informed consent forms on the day of screening. The project was approved by the Southern Denmark Region Committee on Health Research Ethics (S-20180066) and the Danish Data Protection Agency (18/40178).
Invitation and Booking
Patients were invited as previously described in the Danish Cardiovascular Screening Trial (DANCAVAS).14 In brief, patients received an electronic letter of invitation with information about the screening program. If interested, an appointment was booked online or by phone or e-mail. The interest in and demand for the screening programme was evaluated in terms of participation rate.
Screening Sessions
Screening examinations were arranged at 10-minute intervals and encompassed:
A Medical Interview
The interview was based on a thorough review of a questionnaire sent to prospective participants along with the screening invitation. The questionnaire addressed the following issues: 1) Duration of diabetes, diabetes type, diabetic complications and medical treatment (classified as metformin, insulin, SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors and others). 2) Quality of life (Eur-QoL 5D). 3) Family history of diabetes and CVD. 4) Prior CVD (stroke, myocardial infarction, coronary revascularisation, heart valve surgery, aneurysm or peripheral artery disease). 5) Smoking and alcohol use. 6) Symptoms from chest and lower extremity (dyspnoea, palpitations or pain). 7) Use of other medication (classified as thrombocyte aggregation inhibitors, anticoagulant, lipid-lowering drugs, thiazides, β-blockers, angiotensin-converting-enzyme inhibitor, angiotensin II receptor blockers, calcium blockers, potassium-saving diuretics, loop diuretics and others).
A Whole-Body Non-Contrast CT Scanning
The scanning was performed to quantify coronary artery and aortic valve calcification, to measure left atrial size, to assess heart rhythm and to detect aortic and iliac dilatations or aneurysms. After training and evaluation, the radiographers assessed CAC, heart rhythm and aneurysms, while a research assistant assessed aortic valve calcification and left atrial size. All measurements were performed blinded to patient characteristics. The CT scan protocol has been published previously.15 Radiation dose during whole-body CT scanning was on average 4.7 mSv and without any sex difference.
Ankle and Brachial Blood Pressure
Blood pressure was measured to detect peripheral artery disease and hypertension. The method has been published previously.15
Blood and Urine Samples
Samples were obtained to measure HbA1c, lipid profile, renal function, NT-Pro B-type natriuretic peptide (pro-BNP) and microalbuminuria.
Subclinical Cardiovascular Disease
Subclinical CVD was defined as described below.
Coronary Artery Calcifications
The Agatston method was used to calculate the CAC score. A CAC score above the age and sex-specific median was regarded as abnormal. The CAC score was categorised based on commonly used cut-points indicating atherosclerotic plaque burden: 0 (none), 1–99 (mild), 100–399 (moderate) and ≥400 AU (severe).9,12
Atrial Fibrillation
If the P waves were missing or the rhythm was irregular during CT scanning, atrial fibrillation was suspected; and confirmed or rejected by a subsequent ECG or occasionally by seven days of Holter monitoring.
Heart Failure
Pro-BNP is a sensitive marker of congestive heart failure.16 In the present study, patients with pro-BNP values above 125 pg/mL were referred to echocardiography to confirm or deny heart failure.
Aneurysms
Aortic/iliac dilatations or aneurysms were defined as ascending aorta ≥50 mm, arcus aorta ≥45mm, descending aorta ≥35mm, abdominal aorta ≥30mm or a common iliac artery ≥20mm.14
Peripheral Artery Disease
Peripheral artery disease was defined as an ankle-brachial index of 0.9 or less, or at least 1.4.14
Aortic Valve Calcification
Aortic valve calcification measured by CT scan has been documented to be an early marker of aortic valve stenosis and has recently been approved by the European Society of Cardiology for assessment of aortic stenosis.17 Reference values are so far not applicable, but in the present study we considered values ≥300 AU of significance.18
Left Atrial Size
Left atrial size measured by echocardiography is an important marker of congestive heart failure.19 We have recently evaluated a CT-based method for measuring left atrial size,20 but so far reference values are not applicable. In the present study, we, therefore, considered a left atrial size outside the 95% confidence interval (CI) as enlarged. Left atrial size was adjusted by body surface area.
Unprotected Subclinical Cardiovascular Disease
The possible benefit of the screening programme was evaluated in terms of detecting unprotected subclinical CVD. Patients with increased CAC score, aneurysm or peripheral arterial disease were invited to a consultation conducted by a study nurse; and acetylsalicylic acid (75 mg daily) and atorvastatin (80 mg daily) were prescribed if not already initiated. Patients with HbA1c above individual target (Appendix, Table A) and a CAC score ≥100, peripheral artery disease, atrial fibrillation or high LDL (≥3.0 mmol/L despite maximal lipid-lowering therapy) were invited to a consultation conducted by a cardiologist and an endocrinologist; and Empagliflozin (10 mg daily), Semaglutid (initially 0.25 mg weekly), Rivaroxaban (20mg daily) and/or a PCSK9 inhibitor were initiated if necessary. Echocardiography was performed in case of prior unknown atrial fibrillation or unexplained, increased pro-BNP. Patients with an aneurysm were referred to surgery or routine follow-up scanning according to guidelines. Patients and their general physician received a written report with selected test results; and hereafter, future medical treatment was planned at the discretion of the patient’s family physician.
Statistical Analysis
Demographic data are reported as numbers and corresponding percentages (95% CI), mean ± standard deviation (SD) or median with Q1 and Q3. Patients were stratified and analysed according to sex and risk group allocation. Risk group allocation was based on the number of risk factors outside the target range: smoking (being a current smoker at study entry), systolic and diastolic blood pressure (cut-off value ≥130 mm Hg for systolic blood pressure or ≥80 mmHg for diastolic blood pressure), HbA1c (cut-off value ≥ individual HbA1c target), LDL (cut-off value ≥2.6 mmol/l) and albuminuria (cut-off value ≥30 mg/L).21 The association between subclinical CVD and baseline variables was investigated by univariate and multivariate multiple logistic regression analysis. The independent variables included in the model were number of risk factors, age, sex, diabetes duration, diabetes type, Body Mass Index (BMI), family history of CVD and eGFR. In the multiple regression model, age or diabetes duration were included as their interaction is obvious. All the remaining variables were forced into the model, as they are known to be associated with CVD. A negative binomial regression model was performed using the count of subclinical CVD findings as the dependent variable.
Analyses were performed using Stata (StataCorp. 2017. Stata Statistical Software: Release 15.1 College Station, TX: StataCorp LLC).
Results
A total of 465 patients were invited; 41% (95% CI:37–46) attended screening. The participation rate was 40% (95% CI:33–47) for females, 42% (95% CI:36–48) for males. Twenty-four patients were excluded from further analysis due to previous CVD, leaving a study group of 167 patients with diabetes. Baseline characteristics of the participants are presented in Table 1. Their mean age was 52 years, and 97 (58%) were males. Apart from family history of CVD and HDL values, no gender differences were observed. Median duration of diabetes was 9 years (Q1-Q3: 4–15 years), and 137 (82%) had T2D. Mean HbA1c was 55 mmol/mol; mean BMI was 32.9 kg/m2. Statins and antihypertensive drugs were used by approx. 60%; anti-thrombotics by 10%. Metformin was used by 72%; insulin by 31%; and SLGT-2i, GPL-1a or DPP-4i by 11–13%.
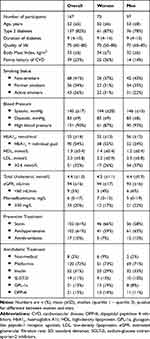 |
Table 1 Baseline Characteristics |
Among participants, 60 (36%) patients had no subclinical CVD, while 107 (64%) had subclinical CVD which showed a male preponderance (males 75% (95% CI:66–83); females 49% (95% CI:37–60)) (Table 2). Sixty-eight (41%) patients had no CAC (27% (95% CI:19–36) of all males; 61% (95% CI:49–72) of all females). The presence of heavily calcified coronaries (CAC score ≥ 400) was more frequent among males (19% (95% CI:12–27)) than among females (7% (95% CI:3–16)). Aortic valve calcification, enlarged left atrial volume, aortic aneurysms, atrial fibrillation, peripheral artery disease or increased pro-BNP were quite uncommon, and without any sex differences.
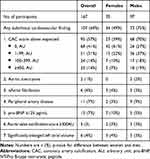 |
Table 2 Subclinical Cardiovascular Disease Among Females and Males |
Regarding the number of risk factors outside the target range, 26% were current smokers, while 34% were former smokers; 90% had increased blood pressure; 54% had HbA1c above the individual target; and 32% had increased LDL, while 20% had micro- or macro-albuminuria. Only six patients had no cardiovascular risk factors besides diabetes, and therefore we collapsed the patients with zero or one risk factor into one group. No patients had all five risk factors outside the target range. Accordingly, patients were allocated to one of four risk groups. When stratifying patients for analyses according to these four risk groups, we saw a slight variation; but, generally, presence of subclinical CVD within a risk group was distributed in the same range as for the overall analysis (Table 3). This was also the case for each of the individual findings. The CAC score was above the expected level in 57% (95% CI:41–71) of patients with zero or one risk factor, and in 39% (95% CI: 20–61) of patients with four risk factors. Opposite this, 41% (95%:27–57) of patients with zero or one risk factor had no CAC, while this was the case in 56% (95% CI:34–75) of patients with four risk factors. In the multivariate regression analyses, only diabetes duration (OR=1.11 per year; 95% CI:1.04–1.18), T2D as opposed to T1D (OR=14.9; 95% CI:2.9–76.5) and male sex (OR=4.41; 95% CI:2.06–9.46) were associated with subclinical CVD, while BMI, family history of CVD, eGFR and number of risk factors were not (Table 4). In a supplementary negative binomial regression model including number of subclinical CVD as dependent variable, we found no differences.
 |
Table 3 Subclinical Cardiovascular Disease According to the Number of Risk Factors Outside Target Range |
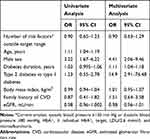 |
Table 4 Univariate and Multivariate Logistic Regression. Associations Between Presence of Subclinical Cardiovascular Disease and Various Cardiovascular Risk Factors |
As shown in Table 5, prescriptions of medical treatment were similar between patients with and without subclinical CDV; this was also the case for CAC score groups (Appendix, Table B). At the follow-up consultations, medical treatment was intensified in 60% (95% CI:53–68) of patients. Empagliflozin or Semaglutide were prescribed to 15 patients (9%), aspirin to 86 (52%), Rivaroxaban to 4 (2%), atorvastatin to 93 (56%) and PCSK9 inhibitor to 1 (<1%). Thirteen (8%) patients were referred for echocardiography, and 2 (<1%) were offered surveillance due to aortic dilatations.
 |
Table 5 Baseline Treatment and Subclinical Cardiovascular Disease |
Discussion
In this study, patients with T2D seem not to demand or recognise the need for a cardiovascular screening examination as their participation rate was very low. According to the WHO, for a screening program to be effective, its participation rate should exceed 70%. Thus, the poor participation rate in the present study reflects the presence of potential barriers that must be overcome to ensure high screening efficiency. We have recently completed patient inclusion in the large-scale randomized screening trial, DANCAVAS, which included men aged 65–74 years from the general population.14 The screening examination was similar to the one performed in the present study, but the participation rate was significantly higher (16,768 were invited and 10,471 attended; participation rate 62.4%). The poor participation rate in the present study may be explained by sub-optimal self-care among patients with diabetes albeit self-care is essential as it has been found to correlate positively with fewer complications and better quality of life.22 Poor self-care may be due to social deprivation including low socio-economic status. Unfortunately, in the present study, we have no data from non-participants allowing us to determine if participants differ from the non-participants in this respect. However, we do believe that a number of the participants in the present study may have reduced self-care, as evidenced by the fact that a significant proportion had an HbA1c level above their individual target as well as high blood pressure and elevated LDL. Of notice, almost half of the patients were not in antihypertensive and lipid-lowering treatment. Additionally, the majority were obese, and one-fourth were smokers; factors that have also generally associated with low socio-economic status. Finally, we also found a relatively poor quality of life. This is a pilot study, and before conducting a large-scale trial, we may need to evaluate how to improve attendance rate. One strategy for improving enrolment may be to strengthen patients’ involvement, which has been shown to impact participation rate in screening trials.23
In the clinic, physicians evaluate traditional risk factors before prescribing preventive medical treatment. In our study, we found a significant number of unprotected patients despite subclinical CVD. Moreover, we found no difference in preventive treatment between those with and those without subclinical CVD. This is very unfortunate, but may be explained by the lack of association between traditional risk factors and subclinical CVD. We, therefore, intensified preventive medical treatment in the majority of our patients. These findings suggest that some patients might benefit from a CVD screening examination. A more comprehensive screening examination may, therefore, help optimize risk stratification by distinguishing those with subclinical CVD who should receive intensified treatment from low-risk persons in whom a healthy lifestyle should be sufficient to counteract subclinical CVD. However, our study is not a randomised intervention trial; thus, the efficacy of the screening cannot be demonstrated.
A number of small randomised CVD screening and intervention trials including patients with diabetes have presented conflicting findings.24–28 Two systematic reviews including approx. 3300 patients reported a quite similar relative risk reduction from screening of 0.73 (95% CI 0.55–0.97) and 0.72 (95% CI 0.49–1.06), respectively.29,30 Numerous reasons may explain the disappointing results of these screening trials. First, the wrong screening tool may have been used. Hence, three studies used exercise electrocardiogram for screening which is now considered unsuitable. Second, the wrong intervention may have been used. The five trials intended to detect asymptomatic diabetics with coronary stenosis and to revascularize patients. It has since been established that revascularization is not indicated in asymptomatic patients.31 Third, event rates may have been low and the studies accordingly underpowered. In our pilot study, we aimed to address the above limitations. As screening tool, we used non-contrast CT to measure CAC score, among others. CAC score has a high sensitivity, also in asymptomatic patients with diabetes, and it is a strong and independent predictor of future CVD. Another advantage of CAC score measurement is that it is cost-effective compared with other non-invasive examinations. However, the radiation dose may give rise to some concerns. We prescribed preventive medical treatment as the intervention of choice, which has been demonstrated to cause clinically relevant reductions in cardiovascular risk, with hazard ratios between 0.72 and 0.88.32–36 However, before a large-scale randomised clinical trial is launched we need to address the problem of low attendance consider the low attendance rate.
Strengths and Limitations
A number of important limitations to this study must be acknowledged. 1) We aimed to invite random patients with diabetes to the screening trial. To ensure a high external validity, the patients were therefore identified in the general population. However, the participation rate was very low, which introduces a strong selection bias. This bias must be overcome in the future to ensure continued use of cardiovascular screening examinations among diabetics. 2) A prescription database was used to identify patients redeeming antidiabetic medical treatment (defined as ATC code A10) within the past year. This entailed some limitations. First, as some non-diabetics are also treated with ATC A10 (like Metformin, which is used to treat insulin resistance seen in polycystic ovary syndrome), the invitation may have reached a number of patients who did not belong to the target group, thereby contributing to a low participation rate. Second, patients only treated with lifestyle intervention, like diet and exercise, did not receive an invitation, as they were not included in the prescription database. 3) The low number of patients entails a low precision of several of our estimates. Even so, we found that a screening examination may be useful for targeting otherwise unprotected high-risk patients who would benefit from preventive medications. 4) Patient groups with high morbidity and mortality, like patients with high CAC, may be subject to more systematic underrepresentation because of the cross-sectional design of the present study than would have been the case if a cohort design had been used. This could explain the low predictive value of well-established cardiovascular risk factors in our study. On the other hand, since we aimed to describe the expected characteristics of enrolees in a screening endeavour rather than to identify cardiovascular risk factors, our use of a cross-sectional design could be viewed as a strength as well. 5) Blood pressure was measured as part of the ankle-brachial index measurement. This is stressful and may have caused a falsely increased blood pressure in a number of patients. Accordingly, some patients may have been misclassified as having a risk factor.
Conclusion
In this pilot study, we propose a feasible screening trial. A large proportion of patients had subclinical CVD, but remained unprotected. Although this is not an outcome trial, these patients may benefit from screening as evidenced by the post-intervention intensified medical preventive treatment. These findings suggest the merits of imaging-based screening and a more individualised approach in treatment of patients with diabetes. However, the low participation rate represents a problem, and further studies should evaluate how to improve attendance before a large-scale randomised screening trial is conducted.
Acknowledgments
The Elitary Research Centre of Individualised Medicine in Arterial Disease, Odense University Hospital, supported the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi:10.1056/NEJMoa1504347
2. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi:10.1136/bmj.38678.389583.7C
3. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841.
4. Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi:10.1016/S0140-6736(11)60698-3
5. Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951–964. doi:10.1016/S2213-8587(17)30327-3
6. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145–154.
7. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
8. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
9. Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the multi-ethnic study of atherosclerosis. JAMA Cardiol. 2017;2(12):1332–1340. doi:10.1001/jamacardio.2017.4191
10. Malik S, Budoff MJ, Katz R, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the multi-ethnic study of atherosclerosis. Diabetes Care. 2011;34(10):2285–2290. doi:10.2337/dc11-0816
11. Shaikh K, Li D, Nakanishi R, et al. Low short-term and long-term cardiovascular and all-cause mortality in absence of coronary artery calcium: a 22-year follow-up observational study from large cohort. J Diabetes Complications. 2019;33(9):616–622. doi:10.1016/j.jdiacomp.2019.05.015
12. Valenti V, Hartaigh BÓ, Cho I, et al. Absence of coronary artery calcium identifies asymptomatic diabetic individuals at low near-term but not long-term risk of mortality: a 15-year follow-up study of 9715 patients. Circ Cardiovasc Imaging. 2016;9(2):e003528. doi:10.1161/CIRCIMAGING.115.003528
13. Hallas J, Hellfritzsch M, Rix M, Olesen M, Reilev M, Pottegård A. Odense pharmacoepidemiological database: a review of use and content. Basic Clin Pharmacol Toxicol. 2017;120(5):419–425. doi:10.1111/bcpt.12764
14. Lindholt JS, Rasmussen LM, Søgaard R, et al. Baseline findings of the population-based, randomized, multifaceted Danish cardiovascular screening trial (DANCAVAS) of men aged 65–74 years. Br J Surg. 2019;106(7):862–871. doi:10.1002/bjs.11135
15. Kvist TV, Lindholt JS, Rasmussen LM, et al. The dancavas pilot study of multifaceted screening for subclinical cardiovascular disease in men and women aged 65–74 years. Eur J Vasc Endovasc Surg. 2017;53(1):123–131. doi:10.1016/j.ejvs.2016.10.010
16. Ibrahim NE, Januzzi JL
17. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791. doi:10.1093/eurheartj/ehx391
18. Paulsen NH, Carlsen BB, Dahl JS, et al. Association between aortic valve calcification measured on non-contrast computed tomography and aortic valve stenosis in the general population. J Cardiovasc Comput Tomogr. 2016;10(4):309–315. doi:10.1016/j.jcct.2016.05.001
19. Bombelli M, Facchetti R, Cuspidi C, et al. Prognostic significance of left atrial enlargement in a general population: results of the PAMELA study. Hypertension. 2014;64(6):1205–1211. doi:10.1161/HYPERTENSIONAHA.114.03975
20. Fredgart MH, Carter-Storch R, Møller JE, et al. Measurement of left atrial volume by 2D and 3D non-contrast computed tomography compared with cardiac magnetic resonance imaging. J Cardiovasc Comput Tomogr. 2018;12(4):316–319. doi:10.1016/j.jcct.2018.04.001
21. Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi:10.1056/NEJMoa1800256
22. Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013;12(1):14. doi:10.1186/2251-6581-12-14
23. Crocker JC, Ricci-Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. 2018;363:k4738. doi:10.1136/bmj.k4738
24. Turrini F, Scarlini S, Mannucci C, et al. Does coronary atherosclerosis deserve to be diagnosed early in diabetic patients? The DADDY-D trial. screening diabetic patients for unknown coronary disease. Eur J Intern Med. 2015;26(6):407–413. doi:10.1016/j.ejim.2015.05.006
25. Lièvre MM, Moulin P, Thivolet C, et al. Detection of silent myocardial ischemia in asymptomatic patients with diabetes: results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials. 2011;12(1):23. doi:10.1186/1745-6215-12-23
26. Faglia E, Manuela M, Antonella Q, et al. Risk reduction of cardiac events by screening of unknown asymptomatic coronary artery disease in subjects with type 2 diabetes mellitus at high cardiovascular risk: an open-label randomized pilot study. Am Heart J. 2005;149(2):e1–6. doi:10.1016/j.ahj.2004.07.027
27. Young LH, Wackers FJ, Chyun DA, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301(15):1547–1555. doi:10.1001/jama.2009.476
28. Muhlestein JB, Lappe DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312(21):2234–2243. doi:10.1001/jama.2014.15825
29. Clerc OF, Fuchs TA, Stehli J, et al. Non-invasive screening for coronary artery disease in asymptomatic diabetic patients: a systematic review and meta-analysis of randomised controlled trials. Eur Heart J Cardiovasc Imaging. 2018;19(8):838–846. doi:10.1093/ehjci/jey014
30. Rados DV, Pinto LC, Leitão CB, Gross JL. Screening for coronary artery disease in patients with type 2 diabetes: a meta-analysis and trial sequential analysis. BMJ Open. 2017;7(5):e015089. doi:10.1136/bmjopen-2016-015089
31. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477.
32. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860.
33. Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med. 2010;170(12):1024–1031. doi:10.1001/archinternmed.2010.182
34. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi:10.1056/NEJMoa1615664
35. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827
36. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
