Back to Journals » Drug Design, Development and Therapy » Volume 10
Feasibility of sequential adjuvant chemotherapy with a 3-month oxaliplatin-based regimen followed by 3 months of capecitabine in patients with stage III and high-risk stage II colorectal cancer: JSWOG-C2 study
Authors Tsuruta A, Yamashita K, Tanioka H, Tsuji A, Inukai M, Yamakawa T, Yamatsuji T , Yoshimitsu M, Toyota K, Yamano T, Nagasaka T, Okajima M
Received 8 May 2016
Accepted for publication 5 July 2016
Published 23 November 2016 Volume 2016:10 Pages 3827—3835
DOI https://doi.org/10.2147/DDDT.S112322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Prof. Dr. Wei Duan
Atsushi Tsuruta,1,* Kazuki Yamashita,2,* Hiroaki Tanioka,3 Akihito Tsuji,4,5 Michio Inukai,6 Toshiki Yamakawa,7 Tomoki Yamatsuji,8 Masanori Yoshimitsu,9 Kazuhiro Toyota,10 Taketoshi Yamano,11 Takeshi Nagasaka,12 Masazumi Okajima13
On behalf of the Japan Southwest Oncology Group (JSWOG)
1Department of Digestive Surgery, Kawasaki Medical School Hospital, 2Department of Surgery, 3Department of Medical Oncology, Okayama Rosai Hospital, Okayama, 4Department of Medical Oncology, Kobe City Medical Center General Hospital, Kobe, 5Department of Clinical Oncology, Faculty of Medicine, Kagawa University Hospital, Kagawa, 6Department of Medicine, Okayama Saiseikai General Hospital, Okayama, 7Department of Surgery, Kagawa Prefectural Central Hospital, Takamatsu, 8Department of General Surgery, Kawasaki Medical School, Okayama, 9Department of Surgery, Hiroshima City Asa Hospital, Hiroshima, 10Department of Surgery, National Hospital Organization Higashihirosima Medical Center, Higashihiroshima, 11Department of Surgery, Kurashiki Medical Center, 12Department of Gastroenterological Surgery, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, 13Department of Surgery, Hiroshima City Hospital, Hiroshima, Japan
*These authors contributed equally to this work
Background: Six months of oxaliplatin-based chemotherapy is the standard adjuvant chemotherapy for completely resected stage III colorectal cancer (CRC). Also, patients with stage II CRC who are considered to be at high risk of disease recurrence often receive the same adjuvant chemotherapy treatment. We prospectively investigated the extent and degree of neuropathy suffered by stage III and high-risk stage II resectable CRC patients who underwent sequential approach involving 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine.
Patients and methods: Patients with completely resected stage III and high-risk stage II CRC aged ≥20 years were eligible. Patients were treated with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CAPOX) for 3 months followed by capecitabine (2,500 mg/m2 on days 1–14 every 3 weeks) for 3 months. Primary end points were frequency and the grade of oxaliplatin-induced neurotoxicity as evaluated using the physician-based Common Terminology Criteria for Adverse Events version 4.0 (CTCAE) grading and the patient-based scale, self-reported Patient Neurotoxicity Questionnaire.
Results: Ninety-one patients were enrolled and 86 patients assessed. Eighty-four percent of patients completed the planned oxaliplatin-based therapy for 3 months, and 63% of patients completed all treatments for the full 6 months. Overall incidences of grade 3 or 4 peripheral sensory or motor neuropathy according to the CTCAE were 3.5% and 1.2%, respectively. Regarding the peripheral sensory neuropathy, the proportion of Patient Neurotoxicity Questionnaire (grade C–E) and CTCAE (grade 2–4) at months 1.5/3/6 were 11.3/22.1/29.4% and 5.3/4.4/11.3%, respectively (Spearman correlation coefficient: 0.47).
Conclusion: A sequential approach to adjuvant chemotherapy with 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine was tolerated by patients and associated with a low incidence of neuropathy.
Keywords: oxaliplatin, peripheral neurotoxicity, adjuvant chemotherapy, Patient Neurotoxicity Questionnaire
Introduction
Colorectal cancer (CRC) is the third leading cause of cancer death in both male and female patients worldwide.1,2 Stage IV cancer has a poor prognosis,3 but patients with stage III colon cancer who undergo radical surgery and standard adjuvant chemotherapy demonstrate a much better prognosis than those with stage IV cancer. Standard adjuvant chemotherapy for stage III colon cancer involves 6 months of folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CAPOX) in National Comprehensive Cancer Network guidelines.4 On the other hand, the 2014 Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines for the treatment of CRC recommend the following adjuvant regimens: 6 months 5-fluorouracil (5FU) and leucovorin (LV), tegafur/uracil + LV, capecitabine, FOLFOX, and CAPOX.5 The Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer study (MOSAIC) demonstrated that 6 months of adjuvant FOLFOX treatment was associated with a significant increase in 6-year overall survival in patients with stage III colon cancer compared with LV5FU2 treatment.6,7 The National Surgical Adjuvant Breast and Bowel Project C-07 trials (NSABP C-07) also showed that adding oxaliplatin to 5FU significantly increased disease-free survival (DFS) in stage II and III colon cancer.8 The NO16968 (XELOX in Adjuvant Colon Cancer Treatment) study (XELOXA) demonstrated a statistically significant difference in 3-year DFS using CAPOX in the adjuvant treatment of stage III colon cancer.9 The established administration period of adjuvant chemotherapy is 6 months based on the publication of two Phase III studies.10,11 In National Comprehensive Cancer Network guidelines, 6 months of oxaliplatin-based chemotherapy is the standard adjuvant chemotherapy for completely resected stage III CRC.4 However, Chau et al12 reported that the probability of 12 weeks of protracted venous infusion of 5FU being inferior to 6 months of bolus 5FU/LV was extremely low. Protracted venous infusion of 5FU produced significantly less neutropenia. The advantage of a shorter duration of adjuvant chemotherapy was suggested. Dose-dependent oxaliplatin-induced peripheral neurotoxicity (PN) often develops and may become irreversible. Therefore, optimal treatment duration of adjuvant therapy remains controversial. In MOSAIC, grade 3 peripheral sensory neuropathy (PSN) during treatment was reported in 12.5% in the FOLFOX group and 0.2% in the 5FU+LV (LV5FU2) group.6,7 In NSABP C-07, grade 3/4 PSN occurred in 8.4% in the (5FU+LV+Oxaliplatin) FLOX group compared with 0.7% in the 5FU+LV (FULV) group.8 In XELOXA, grade 3/4 PSN occurred in 11% of patients in the CAPOX group versus 1% in the 5FU/folic acid (FA) group.9 In MOSAIC, XELOXA, and NSABP C-07, 3-year DFS (%) was 78.2, 70.9, and 76.1, respectively, while the median dosage of oxaliplatin (mg/m2) was 810, 905, and 677, respectively.6,8,9 Although the median dose of oxaliplatin in NSABP C-07 was lower than that in MOSAIC or XELOXA, there was similarity in 3-year DFS among MOSAIC, NSABP C-07, and XELOXA. The median dosage of oxaliplatin in NSABP C-07 was equivalent to eight courses of FOLFOX. Five trials in the International Duration Evaluation of Adjuvant Chemotherapy have enrolled patients to test whether 3 months of oxaliplatin-based adjuvant therapy was noninferior for DFS to 6 months of the therapy in patients with stage III colon cancer. According to the JSCCR guidelines, 6 months of oxaliplatin-based adjuvant chemotherapy in stage III colon cancer has not always been recommended.5 de Gramont et al13 reported that the estimated incidence of grade 3 neuropathy calculated for patients exposed to FOLFOX did not increase before five cycles. Therefore, we set the duration of oxaliplatin treatment in this study to 3 months.
In this study, we prospectively investigated the extent and degree of peripheral neuropathy in patients with stage III and high-risk stage II CRC who underwent a sequential approach involving 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine, and verified the feasibility of the sequential regimen.
Patients and methods
Study design
This prospective Phase II study was performed at multiple centers in Japan. The institutional review boards of all participating institutions in the Japan Southwest Oncology Group (JSWOG) approved the study protocol. We evaluated the frequency and grade of PSN and peripheral motor neuropathy (PMN) in patients with R0 resected high-risk stage II and stage III CRC.
As for oxaliplatin-based chemotherapy, the rates of grade 3 PSN at sixth and twelfth courses (%) were 4.16 and 12.4 in MOSAIC. NSABP C-07 demonstrated that the rate of grade 3 PSN was 8.4% at the end of treatment. Therefore, the expected incidence rate of grade 3 PSN was assumed to be 4.16% at the end of this sequential approach involving 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine. In order to detect at least one patient of grade 3 PSN with a probability of 95%, 73 patients would be required for the study to achieve the expected incidence rate of grade 3 PSN. Therefore, we decided that a total of at least 80 patients should be enrolled to account for exclusions from the analysis.
This study was conducted in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Research revised in 2004 and was approved by the institutional review board of each participating hospital (Supplementary material). Written informed consent was obtained from all patients who participated in the study. This study was registered in the UMIN Clinical Trials Registry as UMIN000004934.
Eligibility
All patients who had no history of chemotherapy or radiotherapy, had histologically confirmed high-risk stage II or stage III according to the JSCCR guidelines, and had an Eastern Cooperative Oncology Group performance status of 0 or 1 were eligible. High-risk features for stage II were a high histological grade, lymphovascular or perineural invasion, a mucinous component, T4 stage, extramural vein invasion, symptomatic bowel obstruction or perforation at diagnosis, and removal of <12 lymph nodes. Surgical resection was performed with the assurance of no residual disease, and postoperative adjuvant chemotherapy could start within 8 weeks after surgery. Organ function was assessed as follows: white blood cell count, 3,000–12,000/mm3; neutrophil count, ≥1,500/mm3; platelet count, ≥100,000/mm3; total bilirubin, <2.0 mg/dL; aspartate aminotransferase and alanine aminotransferase, <100 IU/L; and serum creatinine, ≤1.5 mg/dL. All patients provided signed informed consent to participate. Patients with any of the following conditions were excluded: a history of severe drug allergy, clinically active multiple primary neoplasms, pregnancy, a history of CRC, severe PSN or PMN, uncontrolled hypertension, clinically significant cardiovascular disease, severe mental and neurological disorders, uncontrolled active infection, severe diarrhea, dementia, or uncontrolled diabetes mellitus.
Chemotherapy
All patients enrolled in the study received an oxaliplatin-based regimen for 3 months: modified FOLFOX (mFOLFOX6) for six cycles or CAPOX for four cycles, followed by capecitabine for four cycles (Figure 1). The decision of whether to use mFOLFOX or CAPOX was the choice of the physician.
The study treatment was delayed if any of the following criteria were not met on the scheduled day of administration or the previous day: neutrophil count, ≥1,500/mm3; platelet count, ≥75,000/mm3; aspartate aminotransferase and alanine aminotransferase, ≤150 IU/L; serum creatinine, ≤1.5 mg/dL; total bilirubin, ≤2.0 mg/dL; no diarrhea within 24 hours; grade 0–1 stomatitis; or grade 0–2 PSN or PMN. If grade 3 or higher hematologic toxicity or nonhematologic toxicity occurred, the dose of oxaliplatin was reduced by two steps: 75 mg/m2 in the first step and 65 mg/m2 in the second step in the mFOLFOX regimen, and 100 mg/m2 in the first step and 85 mg/m2 in the second step in the CAPOX regimen. For dose adjustment of 5FU in patients with grade 3 or higher hematologic toxicity or nonhematologic toxicity, the infusion dose was reduced by two steps: 2,000 mg/m2 in the first step and 1,600 mg/m2 in the second step. The bolus dose was also reduced in two steps in these patients: 300 mg/m2 in the first step and 200 mg/m2 in the second step. In the CAPOX, capecitabine was reduced to 1,500 mg/m2 from the initial 2,000 mg/m2 and then to 1,000 mg/m2. When the patient required a more than two-step dose reduction to recover from any adverse effect, adjuvant chemotherapy was terminated.
Evaluation of frequency and grade of PSN and PMN
Hausheer et al14 have advocated the patient self-reported Patient Neurotoxicity Questionnaire (PNQ) as a new measurement to quantify symptoms and severity of chemotherapy-induced PN. PNQ-oxaliplatin was used for measuring and evaluating neurotoxicity associated with patients receiving oxaliplatin. This questionnaire was filled out at baseline and at months 1.5, 3, and 6 after beginning adjuvant chemotherapy (Figure 2). The grade of oxaliplatin-induced neurotoxicity was evaluated using the physician-based Common Terminology Criteria for Adverse Events (CTCAE) at every cycle of chemotherapy. Regarding PSN, grade 1 was asymptomatic: loss of deep tendon reflexes or paresthesia; grade 2 was moderate symptoms: limiting instrumental activities of daily life (ADL); grade 3 was severe symptoms: limiting self-care ADL; and grade 4 was life-threatening consequences: urgent intervention indicated in the CTCAE. Regarding PMN, grade 1 was asymptomatic: clinical or diagnostic observations only, intervention not indicated; grade 3 was severe symptoms: limiting self-care ADL, assistive device indicated in the CTCAE; and grade 2 and 4 were the same as PSN.
  | Figure 2 PNQ – oxaliplatin. |
The PNQ was distributed to the patient in the hospital while receiving chemotherapy and sent in an envelope to the JSWOG office within 1 week of completion. The attending physician could not inspect the questionnaire.
Statistical analysis
The primary objective of this study was to assess the frequency and degree of PSN or PMN. The secondary end points were the proportion of patients who completed oxaliplatin-based therapy, the proportion who completed adjuvant chemotherapy, the proportion who underwent each treatment selection, DFS, adverse events, and comparison of efficacy and adverse events between FOLFOX and CAPOX. All statistical analyses were carried out using the Statistical Package for the Social Sciences software version 22.0 (IBM Corporation, Armonk, NY, USA). P-values of <0.05 were considered to indicate statistical significance.
Results
Patients
In total, 91 patients from eleven hospitals were enrolled from March 2011 to December 2012. Two patients were excluded for not fulfilling the eligibility criteria, and three were excluded for refusing to continue treatment (Figure 3). Table 1 shows the characteristics of all eligible 86 patients.
  | Table 1 Patient characteristics (N=86) |
Treatment
In the first half of the 6-month adjuvant chemotherapy regimen, 30 patients received mFOLFOX (median dose of oxaliplatin, 479 mg/m2) and 56 patients received CAPOX (median dose of oxaliplatin, 490 mg/m2). Overall, 83.7% of patients completed the oxaliplatin-based therapy and 65.1% of patients completed all treatments. There were no significant differences in the proportion of patients who completed mFOLFOX and those who completed CAPOX (Table 2).
Toxicity
Adverse events are summarized in Table 3. The most common grade ≥3 adverse events were neutropenia (9.3% of all patients, 3.3% of the mFOLFOX group, and 12.5% of the CAPOX group) and diarrhea (8.1% of all patients, 10.0% of the mFOLFOX group, and 7.1% of the CAPOX group). Other severe toxicities were increasing alanine aminotransferase concentration (5.8% of all patients) and anorexia (4.7% of all patients). There was no significant difference in grade ≥3 hand-and-foot syndrome between the mFOLFOX group (3.3%) and the CAPOX group (3.6%). With respect to PN, grade ≥3 PSN occurred in 3.5% of all patients, 3.3% of the mFOLFOX group, and 3.6% of the CAPOX group. Grade ≥3 PMN occurred in 1.2% of all patients, 0.0% of the mFOLFOX group, and 1.8% of the CAPOX group showing a lower tendency than PSN. In all-grade adverse events, the mFOLFOX group suffered from anemia or oral mucositis more than the CAPOX group with significant difference.
Evaluation of neurotoxicity
The CTCAE and PNQ were used to compare the degree of neurotoxicity among the patients. Figure 4 shows the frequency of neurotoxicity at four points: baseline and months 1.5, 3, and 6 after induction of adjuvant chemotherapy. The numbers of completed PNQs collected were 86, 71, 68, and 53 at the four points from baseline to month 6, while the numbers of completed CTCAE evaluated by physicians were 86, 75, 68, and 53, respectively. There was no significant difference between the numbers of surveys collected. The incidence of grade C or higher sensory neurotoxicity according to the PNQ (PNQ-sensory) gradually increased from 2.3% to 11.3% at month 1.5, to 22.1% at month 3, and to 28.3% at month 6. Conversely, the incidence of grade ≥2 sensory neurotoxicity according to the CTCAE (CTCAE-sensory) was 1.2%, 5.3%, 4.4%, and 11.3% from baseline to month 6. The CTCAE-sensory showed that the incidence remained low until month 3 and was markedly increased at month 6. There was significant difference between the change of PNQ-sensory and that of CTCAE-sensory (P=0.042). The incidence of motor neurotoxicity according to the PNQ (PNQ-motor) and CTCAE (CTCAE-motor) remained low from baseline to month 6.
We also studied the strength of the relationship between the PNQ and CTCAE using Spearman’s rank correlation coefficient. The correlation coefficient of the obtained data between the PNQ-sensory and CTCAE-sensory was 0.468 (P<0.01), while that between the PNQ-motor and CTCAE-motor was 0.127 (P=0.038) (Table 4). In Spearman’s rank correlation coefficient, positive rs indicate positive linear association. Moreover in graded interpretation, the rs ranged from 0.4 to 0.7 mean moderate correlation, while the rs ranged from 0.1 to 0.3 mean weak correlation. Therefore, a positive correlation between the PNQ-sensory and CTCAE-sensory was suggested.
The incidence of grade 1 or 2 PSN during treatment was 77.9% and decreased to 25.3% at month 6. Conversely, the incidence of grade 3 PSN during treatment was 3.5%, but decreased to 0.0% at month 6 (Figure 5). Moreover, we compared the transition of the PNQ and CTCAE at months 1.5 and 3 between patients in whom PSN still remained at month 6 (PSN+) and those in whom PSN was not present at that time (PSN−) and analyzed the data (Figure 6). There was no significant difference between these two groups of patients at month 1.5, but there was a significant difference at month 3 in both PNQ and CTCAE. In the PSN+ group, the PNQ had already reached nearly 50% at month 3 despite a much lower incidence in the PSN− group at the same time point. The CTCAE at month 3 also showed a significant difference between the PSN+ group and PSN− group.
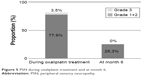  | Figure 5 PSN during oxaliplatin treatment and at month 6. |
Disease-free survival
DFS was one of secondary end points of this study. However, follow-up of this study is not complete yet. As shown in Figure 7, the results that we can make reference to at this stage are that 1-year DFS was 91.6% and that there was no difference between mFOLFOX and CAPOX in 1-year DFS.
  | Figure 7 DFS. |
Discussion
The development of PN is the characteristic adverse event of oxaliplatin therapy. The incidence of neurotoxicity associated with oxaliplatin, including cold-related transient paresthesia or dysesthesia, is reportedly very high.15 In one study, the incidence of PN in oxaliplatin-based treatment was 18.2% (grade ≥3).13 Another randomized controlled trial reported PN rate of 9% (grade >3).16 The incidence of grade ≥3 PN (%) was 12.4 in MOSAIC and 8.4 in NSABP C-07.7,8 MOSAIC showed that the median dose of oxaliplatin was 810 mg/m2.6 In NSABP C-07, the median dose of oxaliplatin was 677 mg/m2, with interquartile range from 320 to 763 mg/m2.8 Namely, the median dosage of oxaliplatin in this study (479 mg/m2) was less than that of both studies.7,8
In the initial stage after induction, oxaliplatin-induced PN is reversible in some cases and subsides until the next administration of oxaliplatin. However, long-term oxaliplatin chemotherapy is associated with a possibility of marked worsening of PN. In MOSAIC, grade 1, 2, and 3 PN was observed in 11.9%, 2.8%, and 0.7% of the patients examined, respectively, even at month 48.7 Prevention of PN by calcium and magnesium infusions or Goshajinkigan, a traditional Japanese herbal medicine, was ineffective in two Phase III studies.17,18
The rates of grade 3 PSN at the sixth and twelfth courses of chemotherapy were 4.16% and 12.4%, respectively, in MOSAIC. NSABP C-07 showed that the rate of grade 3 PSN was 8.4% at the end of treatment.6,8 In this study, the proportion of grade 3 PSN (3.3%) and PMN (1.2%) during treatment was lower than that previously reported at 6 months of adjuvant treatment. Moreover, no patients had grade 3 PSN at month 6, while the incidence of PSN was 1.3% in MOSAIC at that time.5 Therefore, a sequential approach involving 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine can be a safe treatment.
Correlation between PNQ and CTCAE by Spearman’s correlation coefficient demonstrated stronger correlation between PNQ and CTCAE in sensory neurotoxicity than that in motor neurotoxicity (Table 4). The reason might be that oxaliplatin-induced PN appears as sensory neurotoxicity rather than motor neurotoxicity. The proportion of patients with more than grade C PN on the PNQ-sensory began to increase at month 1.5 of treatment and continued to increase gradually until month 6. However, the proportion of grade ≥2 on the CTCAE-sensory hardly showed any difference until month 3 of treatment. A significant increase appeared for the first time at the 6-month inquiry using the CTCAE, which was the final assessment point. Therefore, the PNQ appears to detect oxaliplatin-induced neurotoxicity earlier than the CTCAE.
The PNQ is an evaluation system to which the patient can voluntarily respond, while the CTCAE is a different evaluation system to which the patient responds to the medical questions through the attending physician. This may mean that the PNQ outcome is the result of an active response of the patient and the CTCAE outcome is the result of a passive response.
The proportion of patients with PN on the CTCAE-sensory at month 6 was 11.3%, while that on the PNQ-sensory had already reached the same level at month 1.5. The PNQ could recognize severe PN more than 4 months earlier than the CTCAE. On the other hand, Figure 6 demonstrates that both the PNQ and the CTCAE showed significant differences between the PSN+ and PSN− groups at month 3. Therefore, both the PNQ and CTCAE at month 3 can assess whether continuation of oxaliplatin treatment is possible. In other words, consideration at the 3-month point in oxaliplatin-based adjuvant chemotherapy should be crucial. Moreover, the proportion in CTCAE at 1.5 months in PSN+ group was found to be almost zero, whereas in PSN− group, the CTCAE at 1.5 months was close to 10%. That would be the reason why patients tend not to report their mild degree of PSN to their physician.
The results concerning DFS, one of the secondary study end points in this study, is still immature. As for 1-year DFS, there is similarity among the results of MOSAIC, NSABP C-07, and this study.5,7
Conclusion
Although the number of patients in this study was small, the present study results met the primary end point. A sequential approach involving 3 months of an oxaliplatin-based regimen followed by 3 months of capecitabine is a safe adjuvant treatment for CRC. In addition, assessment of PSN at month 3 with PNQ and CTCAE should provide useful information to prevent persistent PSN after oxaliplatin-based adjuvant treatment.
Acknowledgments
We thank Frederick H Hausheer, MD, FACP, BioNumerik Pharmacoceutical, Inc. for his granting permission to use PNQ-oxaliplatin. We also thank Dr Hideo Okumura, Dr Yasuo Oka, and Dr Yuji Yamamoto for collecting data and for their great support. We thank the patients who participated in this study and their families and physicians. This study was presented at the ESMO 17th World Congress on Gastrointestinal Cancer 2015 (July 1–4, Barcelona, Spain) as a poster presentation (P-246). The poster’s abstract was published in “Poster Abstracts” in Annals of Oncology 26 (Supplement 4), iv1–iv100, 2015. This paper is the first to publish as an original article.
Author contribution
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North Am. 2002;82(5):1075–1090. | ||
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology, Colon Cancer version 2. 2016. | ||
Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2014 for the treatment of colorectal cancer. Int J Clin Oncol. 2015;20(2):207–239. | ||
Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. | ||
Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC Trial. J Clin Oncol. 2009;27(19):3109–3116. | ||
Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. | ||
Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–1471. | ||
O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16(1):295–300. | ||
Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23(34):8671–8678. | ||
Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16(4):549–557. | ||
de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. | ||
Hausheer FH, Schilsky RL, Bain S, et al. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15–49. | ||
Boku N, Ohtsu A, Hyodo I, et al. Phase II study of oxaliplatin in japanese patients with metastatic colorectal cancer refractory to fluoropyrimidines. Jpn J Clin Oncol. 2007;37(6):440–445. | ||
Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. | ||
Loprinzi CL, Qin R, Dakhil SR, et al. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol. 2014;32(10):997–1005. | ||
Oki E, Emi Y, Kojima H, et al. Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol. 2015;20(4):767–775. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







