Back to Journals » Infection and Drug Resistance » Volume 12
Fatal Liver Infection Caused By Clostridium perfringens After Common Bile Duct Stenting Due To Pancreatic Cancer: A Case Report
Authors Xu J , Wang Y, Cui H, Chen J
Received 14 June 2019
Accepted for publication 13 October 2019
Published 24 October 2019 Volume 2019:12 Pages 3343—3347
DOI https://doi.org/10.2147/IDR.S219472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Jingyong Xu,1 Yanbin Wang,2 Hongyuan Cui,1 Jian Chen1
1Department of General Surgery, Beijing Hospital, National Centre of Gerontology, Beijing 100730, People’s Republic of China; 2Department of General Surgery, Beijing Aero Space General Hospital, Beijing 100076, People’s Republic of China
Correspondence: Jian Chen
Department of General Surgery, Beijing Hospital, Beijing 100730, People’s Republic of China
Email [email protected]
Background: Intra-abdominal Clostridium perfringens, especially liver infection, is rare and fatal. It often occurs in patients with immunodeficiency due to various factors, such as cancer, diabetes mellitus, and organ transplantation. The identification of gram-positive bacilli in septicemia, the presence of gas-forming liver damage and intravascular hemolysis are manifestations of Clostridium perfringens infection. The episode deteriorates rapidly and has a high mortality rate.
Case presentation: This case involved a 60-year-old man with infection onset 2 weeks after common bile duct stenting for obstructive jaundice caused by unresectable pancreatic cancer. Abdominal computed tomography (CT) revealed gas-containing lesions in the liver. Blood culture showed Clostridium perfringens. Though aggressively rescued, he died within 24 hrs after admission.
Conclusion: Clostridium perfringens liver infection is rare but leads to a severe prognosis rapidly. High awareness of this condition is key for early diagnosis and effective treatment.
Keywords: liver infection, Clostridium perfringens, stenting, pancreatic cancer
Background
Liver infection caused by Clostridium perfringens is rare but fatal. It may induce a wide variety of clinical manifestations, ranging from asymptomatic to systemic infection and death.1 Massive hemolysis and gas-forming liver damage are classical features that may prompt early recognition and treatment. However, the episode deteriorates rapidly and leads to high mortality.2 This case report involved a 60-year-old man with an onset of infection 2 weeks after common bile duct stenting for obstructive jaundice caused by pancreatic cancer. Written informed consent was provided by the patient’s wife to allow the case details to be published, and the Ethics Committee of Beijing Hospital approved our publication of this case.
Case Presentation
A 60-year-old man was admitted to the emergency room of Beijing Hospital in the afternoon of March 8, 2016 with severe epigastric dilation, stomachache and fever. He had localized epigastric pain for 2 days before being admitted without fever. In the morning before admission, he began vomiting, and the abdominal pain spread to the entire stomach along with a high fever of 39.5°C. Two weeks earlier, he received an endoscopic biliary stenting operation due to obstructive jaundice caused by unresectable pancreatic cancer. The serum total bilirubin (TBIL) decreased from 160.7 μmol/L before the procedure to 46.2 μmol/L at discharge, and the direct bilirubin (DBIL) decreased from 124 μmol/L to 37.9 μmol/L. Nothing uncomfortable happened until the onset of abdominal pain. In physical examination, his blood pressure was 95/65 mmHg, his pulse 110 bpm. The abdominal examination showed abdominal distention, hepatomegaly with a liver span of 2 cm below the right costal margin, epigastric tenderness with mild rebounding pain, and almost no gurgling sound.
According to the history of pancreatic cancer and endoscopic intervention, we first excluded several common conditions, such as pancreatitis, cholangiolitis, especially retrograde type after stenting, and some stent-related problems, such as stent dislocation and stent reobstruction. Therefore, relevant blood tests and cultures and abdominal CT scans were administered immediately.
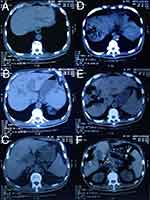
Laboratory data and changes are shown in Tables 1–3. The emergency abdominal CT scan shown in Figure 1 with comparison with past radiologic materials revealed irregular gas-containing lesions in the liver, containing liver parenchymal moth-eaten destruction and a pneumatized bile duct without obvious inflammation around, and the stent was right in the common bile duct without obstruction. It could be deduced that the gas came retrogradely from the duodenum because of the damage to Voter’s ampulla or was generated by several kinds of special bacterial infection. The infection originated from the intrahepatic duct and deteriorated with adjacent parenchymal damage, and all the aerated lumens communicated with the bile ducts.
 |
Table 1 Complete Blood Count |
 |
Table 2 Coagulation Studies And Myocardial Markers |
 |
Table 3 Serum Biochemistry Levels |
According to the parameters, we diagnosed the patient with severe intrahepatic infection caused by enterogenous aerogenes, multiorgan deficiency involving hepatic, renal, and cardiac injuries and coagulopathy. We treated him with a board-spectrum antibiotic (biapenem 0.3 g Q12H and ornidazole 100 mg Q12H intravenously) and vigorous fluid resuscitation under intensive care. Ten hours after hospitalization, his blood pressure began falling, and inotropic support was started with intravenous infusion of norepinephrine. Nevertheless, his clinical condition deteriorated with multiorgan failure. Finally, he succumbed at the 20th hour of hospitalization to sudden cardiac arrest.
The method of blood specimen culture was as follows: the blood sample was taken aseptically, injected into culture bottles (BacT/ALERT® SA and SN, BioMérieux Company, Lyon, France) immediately, and put into the BacT/ALERT automated blood culture system. Twenty-four hours later, the BacT/ALERT SN bottle was positive for alarm, and gram-positive bacilli were seen on direct smears. The bacilli were transferred to a Colombian medium blood plate and cultivated in both aerobic and anaerobic environments at 37°C. Twenty-four hours later, gray-white, round, serrated colonies with double-hemolytic rings were seen on the plate in the anaerobic environment. Finally, the blood culture results revealed a Clostridium perfringens infection according to an automatic bacterial identification system (Vitek®2 COMPACT, BioMérieux Company, Lyon, France).
Discussion
Clostridium perfringens is a ubiquitous, gram-positive, anaerobic bacillus, which is a normal inhabitant of the human gastrointestinal tract. Clostridium perfringens grows fast, with a doubling time of approximately 7 min, and its virulence is due to its toxin production, which contributes to the pathogenesis of the infection.3 The main toxin is phospholipase C lecithinase (α-toxin), which damages the structural integrity of the cell membrane and then leads to spherocytosis and subsequent hemolysis. α-Toxin is also a key pathogenic factor in gas gangrene. Other toxic factors (such as β- and ε- toxins) may act on the vascular endothelium, leading to capillary leakage.
Though the incidence of Clostridium perfringens bacteremia is low (0.97/100,000 in patients who are hospitalized and 0.7/100,000 in nonselected populations), Clostridium perfringens liver abscess is even rarer.4–6 There are various risk factors for clostridium infection, including any condition with insufficient immune function, such as elderly age, poorly controlled diabetes, cirrhosis and malignancy, especially gastrointestinal and genitourinary malignancies.3 Intra-abdominal is the major site of Clostridium perfringens infection. In the case reported here, the patient was suffering from pancreatic head cancer and jaundice caused by obstruction of the distal common bile duct. Pneumocholangiosis after ERCP is a common clinical phenomenon. Most of the gas is distributed along the bile duct, and the wall of the bile duct is smooth. However, the pneumoconiosis in this case manifested as extensive dilatation and rigidity of bile ducts at all levels and concomitant destruction of the liver. Therefore, we postulate that after common bile duct stenting, the clostridium organisms migrated to the liver via the damaged Vater’s ampulla and caused local infection in the liver parenchyma, which was later demonstrated by the bacterial culture.
The clinical episode of this special infection usually deteriorates rapidly, with a high mortality rate ranging from 70% to 100%.7 Intravenously administered high-dose penicillin and surgical debridement of all involved tissue were thought to be the first choice of treatment.3 When surgical debridement is difficult, hyperbaric oxygen therapy is another choice that can decrease the toxin production rate and make the environment less anaerobic for the bacteria to grow.8,9 In our patient, the condition deteriorated rapidly with multiorgan insufficiency, and there was no chance for surgical intervention. Thus, the patient had a dreadful prognosis.
Law et al reviewed 20 cases of Clostridium perfringens liver abscess between 1990 and 2011, and only 6 patients survived, and 5 had the primary focus of infection removed.9 Of these 20 cases, four were advanced malignancies (20%), including two pancreatic, one hepatocellular and one rectal cancer.10–12 After that, a few more cases were published. Among all the cases reported, most of the latest cases were of infection caused by therapeutic intervention, such as transcatheter arterial chemoembolization (TACE).13,14 The case we reported above is the first published case caused by common bile duct stenting. Since palliative therapy for advanced malignancies has developed well in recent years, some interventional procedures have been widely used, and longer-term survival can be achieved. New complications constantly emerge, including some severe conditions, such as Clostridium perfringens infection, which we should keep in mind.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi:10.1128/CMR.3.1.66
2. van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68:343–346.
3. Ohtani S, Watanabe N, Kawata M, Harada K, Himei M, Murakami K. Massive intravascular hemolysis in a patient infected by a Clostridium perfringens. Acta Med Okayama. 2006;60:357–360. doi:10.18926/AMO/30725
4. Yang CC, Hsu PC, Chang HJ, Cheng CW, Lee MH. Clinical significance and outcomes of Clostridium perfringens bacteremia–a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17:e955–e960. doi:10.1016/j.ijid.2013.03.001
5. Ngo JT, Parkins MD, Gregson DB, et al. Population-based assessment of the incidence, risk factors, and outcomes of anaerobic bloodstream infections. Infection. 2013;41:41–48. doi:10.1007/s15010-012-0389-4
6. Law ST, Lee MK. A middle-aged lady with a pyogenic liver abscess caused by Clostridium perfringens. World J Hepatol. 2012;4:252–255. doi:10.4254/wjh.v4.i8.252
7. Ng H, Lam SM, Shum HP, Yan WW. Clostridium perfringens liver abscess with massive haemolysis. Hong Kong Med J. 2012;16:310–312.
8. Wilkinson D, Doolette D. Hyperbaric oxygen treatment and survival from necrotizing soft tissue infection. Arch Surg. 2004;139:1339–1345. doi:10.1001/archsurg.139.12.1339
9. Rajendran G, Bothma P, Brodbeck A. Intravascular haemolysis and septicaemia due to Clostridium perfringens liver abscess. Anaesth Intensive Care. 2010;38:942–945. doi:10.1177/0310057X1003800522
10. Bätge B, Filejski W, Kurowski V, Klüter H, Djonlagic H. Clostridial sepsis with massive intravascular hemolysis: rapid diagnosis and successful treatment. Intensive Care Med. 1992;18:488–490. doi:10.1007/BF01708587
11. Fondran J, Williams GB. Liver metastasis presenting as pneumoperitoneum. South Med J. 2005;98:248–249. doi:10.1097/01.SMJ.0000153196.84534.9A
12. Eckel F, Lersch C, Huber W, Weiss W, Berger H, Schulte-Frohlinde E. Multimicrobial sepsis including Clostridium perfringens after chemoembolization of a single liver metastasis from common bile duct cancer. Digestion. 2000;62:208–212. doi:10.1159/000007815
13. Oshima S, Takaishi K, Tani N, et al. Two cases of liver abscess caused by Clostridium perfringens after transcatheter arterial chemoembolization. Gan To Kagaku Ryoho. 2013;40:1795–1797.
14. Li JH, Yao RR, Shen HJ, et al. Clostridium perfringens infection after transarterial chemoembolization for large hepatocellular carcinoma. World J Gastroenterol. 2015;21:4397–4401. doi:10.3748/wjg.v21.i14.4397
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.