Back to Journals » Infection and Drug Resistance » Volume 17
Fatal Case of Pneumocystis Jirovecii Pneumonia (PJP) During Treatment for Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) Syndrome
Authors Zhou C, Li J, Zhou F, Huang L, Liu X, Li H
Received 1 November 2023
Accepted for publication 10 January 2024
Published 16 January 2024 Volume 2024:17 Pages 153—159
DOI https://doi.org/10.2147/IDR.S447694
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chaoe Zhou,1,* Jun Li,2,* Fude Zhou,3 Lei Huang,4 Xinmin Liu,1 Haichao Li5
1Department of Geriatrics, Peking University First Hospital, Beijing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Beijing Tsinghua Changgung Hospital, Beijing, People’s Republic of China; 3Renal Division, Department of Medicine, Peking University First Hospital, Beijing, People’s Republic of China; 4Department of Clinical Laboratory, Peking University First Hospital, Beijing, People’s Republic of China; 5Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinmin Liu, Department of Geriatrics, Peking University First Hospital, Tel +86-10-8357-2128, Beijing, 100034, People’s Republic of China, Email [email protected] Haichao Li, Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Tel +86-10-8357-2128, Beijing, 100034, People’s Republic of China, Email [email protected]
Abstract: Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is an acute, rare and potentially fatal drug reaction. To date, limited studies have reported secondary Pneumocystis jirovecii pneumonia (PJP) infection during the treatment of DRESS syndrome. A 53-year-old man was admitted to the hospital due to a persistent fever lasting for 5 days. He had a medical history of hypertension, psoriasis, urticaria, and had recently been treated with carbamazepine for nerve spasm two weeks ago. After admission, the patient presented with a high fever accompanied by chills, abdominal pain, bilateral upper limb muscle pain, and generalized lymph nodes enlargement. Laboratory tests revealed elevated eosinophils and atypical lymphocytes. Subsequently, the patient developed multiple internal organ complications, including oliguria, elevated serum creatinine, liver enzymes, and cardiac troponin I. Based on diagnostic criteria, the patient was diagnosed with DRESS syndrome. To manage the DRESS syndrome, the patient was successively or simultaneously prescribed methylprednisolone, cyclosporin and intravenous immunoglobulin, resulting in an improvement of the condition. However, during the treatment, the patient was infected with Pneumocystis jirovecii. Despite targeted therapy with trimethoprim/sulfamethoxazole, primaquine and clindamycin successively, no remission was observed. Chest CT scans exhibited multiple exudations in both lungs, indicative of interstitial pneumonia. Unfortunately, the patient’s oxygenation progressively declined, leading to his untimely demise. This rare case further highlights the need for clinicians to be aware of the risk of Pneumocystis jirovecii infection in DRESS syndrome patients treated with long-term and high-dose glucocorticoid therapy.
Keywords: DRESS syndrome, Pneumocystis jirovecii pneumonia, carbamazepine, glucocorticoid therapy, case report
Introduction
The Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare, complex, and potentially life-threatening hypersensitivity reaction caused by certain drugs. It typically manifests with a skin rash, fever, facial edema, hematologic abnormalities (elevated eosinophils and/or presence of atypical lymphocytes), lymphadenopathy, and multiple organ dysfunction in severe cases. Its incidence ranges from 1:1000 to 1:10,000 people, with a mortality rate as high as 10%.1 In DRESS syndrome, visceral organs are involved in nearly 90% of patients, and in about half of patients’ multiple organs may be involved, most commonly affecting the liver, kidneys, and lungs. The involvement of visceral organs is directly associated with mortality.2 The liver is the most frequently affected visceral organ in DRESS syndrome (75% of cases), hepatitis of varying severity is often seen, often with elevated levels of liver transaminases, alkaline phosphatase, and creatinine. The kidneys are also commonly affected in DRESS syndrome (10 to 30% of cases).3 Acute kidney injury was observed in 96% patients, 4% patients had isolated proteinuria and hematuria without acute kidney injury, oliguria and anuria were reported in 18% and 6% patients, respectively.4 Although lung involvement in DRESS syndrome is less common (5 to 25% of cases), it is associated with a more severe clinical course and potentially worse outcomes. DRESS involvement in the lungs can manifest in a variety of manifestations, ranging from mild cough or dyspnea with chest imaging nonspecific interstitial changes to acute respiratory distress syndrome with life-threatening hypoxic respiratory failure. Symptoms of cough and shortness of breath were present in 72% of patients at the time of presentation, shortness of breath was more common (81%) than cough (19%).2 Furthermore, the heart can also be affected in DRESS syndrome, often leading to myocarditis. Cardiac enzymes including creatinine kinase and troponin-I may be elevated.5
The precise pathogenesis of DRESS remains unclear but virus reactivation and a drug-specific immune response are considered key factors.6 The most common virus reported to be reactivated is human herpesvirus 6 (HHV-6),7 other herpes virus (HHV-7), Epstein Barr virus (EBV), or Cytomegalovirus (CMV) reactivations have been reported to be associated with the onset of DRESS.8 So far, there is limited research on DRESS syndrome complicated by pneumocystis jirovecii (PJ) infection. In this study, we presented a case of carbamazepine-induced DRESS associated with secondary PJ infection during the treatment of DRESS syndrome. It is crucial for clinicians to be aware of the risk of DRESS syndrome during carbamazepine treatment and remain vigilant for the development of PJ infection in patients with DRESS syndrome who are receiving long-term and high-dose glucocorticoid therapy in the future.
Case Presentation
A 53-year-old man was admitted to the hospital due to a fever lasting for 5 days. He had a medical history of hypertension, psoriasis, urticaria, and had recently been treated with carbamazepine for nerve spasm two weeks ago. Five days before his hospitalization, the patient presented with high fever (39°C), accompanied by chills, abdominal pain, bilateral upper limb muscle pain, and enlargement of retro-occipital lymph nodes. One day before his admission, the patient experienced trunk pain, facial edema, and diffuse erythema on the extremities and trunk. A detailed timeline of diagnosis and treatment is provided in Figure 1.
 |
Figure 1 The main timeline for diagnosis and treatment characteristics associated with DRESS syndrome. |
Upon admission, laboratory tests revealed that the white blood count (WBC) was 9.03×10^9/L (reference interval 3.5–9.5×10^9/L), eosinophils (EOS) were 1.01×10^9/L (reference interval 0.02–0.52×10^9/L), alanine transferase (ALT) was 251 IU/L (reference interval 9–50 IU/L), aspartate aminotransferase (AST) was 144 IU/L (reference interval 15–40 IU/L), and lactate dehydrogenase (LDH) was 366 IU/L (reference interval 100–240 IU/L). Abdominal ultrasonic examination revealed enhanced hepatic parenchymal echo and changes in the cholecystectomy. The electrocardiogram (ECG) showed normal results. Ultrasonography of the lymph nodes revealed multiple enlarged lymph nodes in the bilateral neck, axillary, and inguinal regions. CMV IgG/M, EBV IgG/M, epidemic hemorrhagic fever antibody, anti-nuclear antibody, and nine respiratory virus antibodies were negative.
After admission, the patient developed oliguria (<400mL/24h) and showed elevated serum creatinine levels, liver enzymes, and cardiac troponin I. The patient was diagnosed with DRESS syndrome based on diagnostic criteria (Table 1). Methylprednisolone (80mg, qd) and intravenous immunoglobulin (IVIG) (5g, qd) were prescribed, resulting in significant relief of skin rash and edema. However, the patient’s symptoms worsened after IVIG was discontinued. Laboratory indicators such as eosinophils and transaminases fluctuated throughout the course of treatment (Figure 2), and cyclosporine (50mg, bid) was prescribed. Subsequently, serum creatinine and aminotransferase levels decreased, indicating effective treatment (Figure 2).
 |
Table 1 RegiSCAR DRESS Validation Score 2007 [9] |
 |
Figure 2 Changes in important laboratory indicators during hospitalization. |
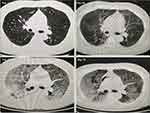
However, 63 days after admission, the patient developed fever (38.5°C) accompanied by chills and dyspnea. Laboratory examination revealed an increase in hypersensitive C-reactive protein levels (Figure 2). Blood gas analysis (conducted in room air) indicated a pH of 7.495, pCO2 of 35.5 mmHg, and pO2 of 55 mmHg. Chest computed tomography (CT) revealed diffuse interstitial infiltrations with ground-glass shadow in both lungs, suggestive of a PJ infection (Figure 3). Empirical therapy with trimethoprim/sulfamethoxazole (TMP-SMX) (4 tablets, q8h) was initiated.
66 days after admission, the patient was transferred to the respiratory intensive care unit for further treatment. Etiological results revealed a large number of PJ cysts in the sputum. Targeted therapy with TMP-SMX (4 tablets, q8h) was prescribed. In light of persistent fever and inadequate improvement in oxygenation, the administration of TMP-SMX was adjusted to primaquine (30mg, qd) in conjunction with clindamycin (600mg, q8h). However, due to the development of methemoglobinemia likely caused by primaquine’s side effects, primaquine and clindamycin were discontinued, and TMP-SMX (4 tablets, q8h) was resumed. No PJ cysts were detected in subsequent sputum tests.
Given the patient extended treatment with methylprednisolone and cyclosporine, a positive (1,3)-β-D-glucan and galactomannan test was observed. The presence of spores and mycelium in the sputum suggested possible candida or aspergillus infections, prompting antifungal treatments with caspofungin (50mg, qd) and voriconazole (200mg, qth). Tests for HHV-6, CMV, EBV, and other respiratory viruses were negative. Blood cultures detected Staphylococcus epidermidis, which was considered a contaminant microorganism. Empirical therapy with meropenem (1g, bid) and fosfomycin (4g, q8h) was initiated to prevent bacterial infections.
Following the patient transferred to the intensive care unit, there was an escalation in the medications administered. The patient developed an intensified skin rash marked by redness and desquamation. Blood tests revealed a surge in lgE levels to 4208 kU/L, indicative of a recurrent or persistent of DRESS syndrome. Chest CT exhibited multiple exudations in both lungs, suggestive of interstitial pneumonia (Figure 3). Blood gas analysis indicated decreased oxygenation. Beyond potential infections, the manifestations also raised concerns of immune-related lung damage. In response, high doses of methylprednisolone (500mg, 2 days) were administered. Regrettably, the patient’s oxygenation progressively declined, blood gas analyses reflected a worsening condition. Unfortunately, the patient passed away.
Discussion
Different diagnostic criteria scores have been developed to help clinicians confirm or exclude the diagnosis of DRESS. RegiSCAR has devised a widely accepted scoring system for DRESS, as shown in Table 1.9 According to the RegiSCAR criteria, the diagnosis of DRESS syndrome in this patient was definitive, with a score of more than 5 points. The patient presented with symptoms including fever, eosinophilia, atypical lymphocytosis, enlarged lymph nodes, extensive skin rash, and involvement of the lungs, kidneys, and liver damage. Previous study had indicated that about 50 different drugs had the potential to cause DRESS syndrome, with carbamazepine being identified as the main culprit.10 In this case, carbamazepine was identified as the causative drug for causing DRESS syndrome, as the symptoms manifested two weeks after the initiation of carbamazepine treatment and in the absence of any other drugs.
Prior to the diagnosis of DRESS syndrome, the administration of carbamazepine was ceased. Following the diagnosis of DRESS syndrome, the patient was promptly administered glucocorticoid and intravenous immunoglobulin. Subsequently, immunoglobulin was discontinued and cyclophosphamide was prescribed. It is widely acknowledged that identification and prompt withdrawal of the culprit drug is the mainstay of the treatment for patients with DRESS. Systemic corticosteroids have been recognized as the gold standard treatment for ameliorating clinical symptoms of DRESS at the acute phase. The skin rash and fever resolve rapidly within a few days after initiation of systemic corticosteroid therapy. The usual dosage is prednisolone 40–50 mg/day. Systemic corticosteroids need to be tapered over 6–8 weeks to prevent the various symptoms of the syndrome from relapsing.8
However, it should be noted that prolonged and high-dose glucocorticoids therapy may reactivate viruses such as HHV-6 or CMV and worsen the condition in a minority of patients. Furthermore, immunosuppression is commonly observed in patients with DRESS syndrome,11 rendering them susceptible to opportunistic infections. In this case, the patient developed a PJ infection while received glucocorticoids and immunosuppressant cyclophosphamide for DRESS syndrome. Thus, patients receiving high-dose corticosteroid treatment (equivalent to ≥20 mg of prednisone daily for ≥1month) who have an additional cause of immunodeficiency (such as additional immunosuppressive medication) should receive PJP prophylaxis. This finding was consistent with previous published study.12
As we all known PJ is an opportunistic fungal pathogen that can cause severe pneumonia in immunocompromised individuals.13 Reactivation of latent infection or person-to-person transmission can lead to PJP. In DRESS syndrome, herpesvirus reactivation may be considered as immune reconstitution inflammatory syndrome (IRIS), which occurs during immune recovery from an immunocompromised state. Therefore, the appearance of pneumonia in this patient may also be interpreted as IRIS, which is due to recovery from an immunocompromised state caused by treatment for DRESS syndrome. It is difficult to determine whether the pneumonia in this patient was a simple opportunistic infection due to the immunocompromised state or a manifestation of pneumonia as IRIS, this still needs further research.
Conclusion
The prognosis of patients with DRESS syndrome complicated by PJP is extremely poor. This rare case further highlights the need for clinicians to be aware of the risk of PJ infection in DRESS syndrome patients treated with long-term and high-dose glucocorticoids. Pneumonia in this patient was a simple opportunistic infection due to the immunocompromised state or a manifestation of pneumonia as immune reconstitution inflammatory syndrome, this still needs further research.
Abbreviations
DRESS, Drug rash with eosinophilia and systemic symptoms; PJ, Pneumocystis jirovecii; HHV-6, Human herpesvirus 6; EBV, Epstein Barr virus; CMV, Cytomegalovirus; WBC, White blood count; EOS, Eosinophils; ALT, Alanine transferase; AST, Aspartate aminotransferase; LDH, Lactate dehydrogenase; ECG, Electrocardiogram; IVIG, Intravenous immunoglobulin; CT, Computed tomography; TMP-SMX, Trimethoprim/sulfamethoxazole; PJP, Pneumocystis jirovecii pneumonia; IRIS, immune reconstitution inflammatory syndrome.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee at Peking University First Hospital, Beijing, China, to publish the case details.
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report and of the accompanying images.
Funding
This work was supported by the Beijing Clinical Key Specialty (no. XKB2022B1002), Capital’s Funds for Health Improvement and Research (no. 2022-3-70212), and National High Level Hospital Clinical Research Funding (Interdepartmental Research Project of Peking University First Hospital) (no. 2023IR46). The funder had no role in the design, data collection, data analysis, and reporting of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. Clinical perspectives. J Am Acad Dermatol. 2013;68(5):
2. Taweesedt PT, Nordstrom CW, Stoeckel J, Dumic I. Pulmonary Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: a Systematic Review. Biomed Res Int. 2019;2019:7863815. doi:10.1155/2019/7863815
3. Cabañas R, Ramírez E, Sendagorta E, et al. Spanish Guidelines for Diagnosis, Management, Treatment, and Prevention of DRESS Syndrome. J Investig Allergol Clin Immunol. 2020;30(4):229–253. doi:10.18176/jiaci.0480
4. Dagnon da Silva M, Domingues SM, Oluic S, et al. Renal Manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome: a Systematic Review of 71 Cases. J Clin Med. 2023;12(14):4576. doi:10.3390/jcm12144576
5. Kano Y, Ishida T, Hirahara K, Shiohara T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med Clin North Am. 2010;94(4):743–759. doi:10.1016/j.mcna.2010.03.004
6. Pavlos R, Mallal S, Ostrov D, Pompeu Y, Phillips E. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. J Allergy Clin Immunol Pract. 2014;2(1):21–33. doi:10.1016/j.jaip.2013.11.005
7. Descamps V, Valance A, Edlinger C, et al. Association of human herpesvirus 6 infection with drug reaction with eosinophilia and systemic symptoms. Arch Dermatol. 2001;137(3):301–304.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. Management and therapeutics. J Am Acad Dermatol. 2013;68(5):
9. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156(3):609–611. doi:10.1111/j.1365-2133.2006.07704.x
10. Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–597. doi:10.1016/j.amjmed.2011.01.017
11. Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206(4):353–356. doi:10.1159/000069956
12. Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13. doi:10.4065/71.1.5
13. Weyant RB, Kabbani D, Doucette K, Lau C, Cervera C. Pneumocystis jirovecii: a review with a focus on prevention and treatment. Expert Opin Pharmacother. 2021;22(12):1579–1592. doi:10.1080/14656566.2021.1915989
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.