Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Factors Associated with the Non-Exacerbator Phenotype of Chronic Obstructive Pulmonary Disease
Authors Bouhuis D, Giezeman M, Hasselgren M, Janson C , Kisiel MA, Lisspers K , Montgomery S, Nager A, Sandelowsky H , Ställberg B , Sundh J
Received 9 October 2022
Accepted for publication 6 March 2023
Published 6 April 2023 Volume 2023:18 Pages 483—492
DOI https://doi.org/10.2147/COPD.S392070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Dennis Bouhuis,1 Maaike Giezeman,1,2 Mikael Hasselgren,1,2 Christer Janson,3 Marta A Kisiel,4 Karin Lisspers,5 Scott Montgomery,6– 8 Anna Nager,9 Hanna Sandelowsky,7,9,10 Björn Ställberg,5 Josefin Sundh11
1School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden; 2Centre for Clinical Research and Education, Karlstad, Sweden; 3Department of Medical Sciences, Respiratory, Allergy and Sleep Research, Uppsala University, Uppsala, Sweden; 4Department of Medical Sciences, Occupational and Environmental Medicine, Uppsala University, Uppsala, Sweden; 5Department of Public Health and Caring Sciences, Family Medicine and Preventive Medicine, Uppsala University, Uppsala, Sweden; 6Clinical Epidemiology and Biostatistics, School of Medical Sciences, Faculty of Medicine and Health, Örebro, Sweden; 7Clinical Epidemiology Division, Department of Medicine, Solna, Karolinska Institutet, Stockholm, Sweden; 8Department of Epidemiology and Public Health, University College, London, UK; 9Department of Neurobiology, Care Sciences and Society, Division of Family Medicine and Primary Care, Karolinska Institutet, Stockholm, Sweden; 10Academic Primary Health Care Centre, Stockholm, Sweden; 11Department of Respiratory Medicine, Faculty of Medicine and Health, Örebro University, Örebro, Sweden
Correspondence: Josefin Sundh, Department of Respiratory Medicine, Faculty of Medicine and Health, Örebro University, Örebro, Sweden, Tel +46702349517, Email [email protected]
Background: Patients with chronic obstructive pulmonary disease (COPD) and no exacerbations may need less maintenance treatment and follow-up. The aim was to identify factors associated with a non-exacerbator COPD phenotype.
Methods: Cross-sectional analysis of 1354 patients from primary and secondary care, with a doctor’s diagnosis of COPD. In 2014, data on demographics, exacerbation frequency and symptoms using COPD Assessment Test (CAT) were collected using questionnaires and on spirometry and comorbid conditions by record review. The non-exacerbator phenotype was defined as having reported no exacerbations the previous six months. Multivariable logistic regression with the non-exacerbator phenotype as dependent variable was performed, including stratification and interaction analyses by sex.
Results: The non-exacerbator phenotype was found in 891 (66%) patients and was independently associated with COPD stage 1 (OR [95% CI] 5.72 [3.30– 9.92]), stage 2 (3.42 [2.13– 5.51]) and stage 3 (2.38 [1.46– 3.88]) compared with stage 4, and with CAT score < 10 (3.35 [2.34– 4.80]). Chronic bronchitis and underweight were inversely associated with the non-exacerbator phenotype (0.47 [0.28– 0.79]) and (0.68 [0.48– 0.97]), respectively. The proportion of non-exacerbators was higher among patients with no maintenance treatment or a single bronchodilator. The association of COPD stage 1 compared with stage 4 with the non-exacerbator phenotype was stronger in men (p for interaction 0.048). In women, underweight and obesity were both inversely associated with the non-exacerbator phenotype (p for interaction 0.033 and 0.046 respectively), and in men heart failure was inversely associated with the non-exacerbator phenotype (p for interaction 0.030).
Conclusion: The non-exacerbator phenotype is common, especially in patients with no maintenance treatment or a single bronchodilator, and is characterized by preserved lung function, low symptom burden, and by absence of chronic bronchitis, underweight and obesity and heart failure. We suggest these patients may need less treatment and follow-up, but that management of comorbid conditions is important to avoid exacerbations.
Keywords: COPD, exacerbations, lung function, CAT, body mass index, chronic bronchitis, sex, heart failure
Introduction
Several chronic obstructive pulmonary disease (COPD) phenotypes have been identified,1 including the emphysema phenotype,2 COPD with systemic inflammation3 and the frequent exacerbator phenotype. The frequent exacerbator phenotype, characterized by more than two exacerbations per year, has received considerable attention.4,5
An exacerbation is characterized by increased dyspnea and/or cough and sputum that worsens in < 14 days.6 Exacerbations in COPD are known to be associated with disease progression, worse health-related quality of life (HRQL), and higher risk for re-exacerbations and mortality.7–9 Risk factors associated with exacerbations are well studied and include previous exacerbations, worse lung function and presence of chronic bronchitis.9,10
Recent studies have reported less exacerbations and lower mortality risk with inhaled corticosteroids (ICS) in addition to long-acting muscarinic antagonists (LAMA) and long-acting beta-2-agonists (LABA) in this patient group.11,12 However, due to increased risk of ICS side-effects, such as pneumonia,13,14 selection of patients with low exacerbation risk may also be of clinical importance. Identifying a potential non-exacerbator phenotype could uncover a patient group requiring less medication and less monitoring from the health care providers, but such studies are lacking.
Thus, the aim of this study was to explore the prevalence of a non-exacerbator phenotype, to identify factors associated with this phenotype and to investigate if these factors differ by sex.
Materials and Methods
Data Collection
This was a cross-sectional study with data from the second cohort of the PRAXIS study, a real world observational study of COPD in central Sweden.15 Data were collected in 2014 using patient questionnaires and medical record review. In total, 2920 patients with a doctor’s diagnosis of COPD (International Classification of Diseases, tenth revision, ICD-10-code J44) were randomly selected from 14 secondary and 76 primary health care centres. The inclusion criterion was a recorded diagnosis of COPD between 2007–01-01 and 2010–12-31, and the exclusion criterion was inability (cognitive or linguistic) or unwillingness to complete the patient questionnaire. The number of participating centres was chosen to reflect the Swedish health care system, where the majority of patients with COPD are managed in primary care. The data collection process is shown in Figure 1.
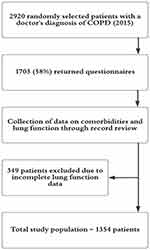 |
Figure 1 Flow chart describing the selection and data collection within the study. Abbreviation: COPD, chronic obstructive lung disease. |
The study was approved by the Ethical Board of Uppsala, Sweden (D-Nr 2010/090). Written consent was received by all participants. The data accessed complied with relevant data protection and privacy regulations.
Variables
The questionnaires provided information on sex, age, height, weight, current smoking status, chronic bronchitis, level of education, symptom burden, number of exacerbations during the previous six months and present maintenance inhaled treatment. Chronic bronchitis is defined as productive cough at least three months the previous two years,16 and in this study chronic bronchitis denoted self-reported doctor's diagnosis of chronic bronchitis.
Age was categorized in approximate tertiles: <66, 66–69 and ≥70 years. Body mass index (BMI) was calculated and categorized based on previous knowledge of prognostic values as <22, 22–30 and ≥30 kg/m2.17–19 Smoking status was categorized as never smoking, ex smoking, occasional smoking and current daily smoking, and was dichotomized as current daily smoking or not in subsequent regression analyses. Level of education was divided into high and low, where high was defined as two or more years beyond Swedish compulsory school of nine years. Symptom burden was assessed by the COPD Assessment Test (CAT),20 where CAT ≥10 indicates a high symptom burden.21 An exacerbation was defined as an emergency visit or prescription of a course of oral glucocorticoids or antibiotics due to worsening respiratory symptoms during the previous six months and dichotomized as 0 or ≥1. Data collection in the first PRAXIS cohort was started in 2005, before the GOLD definition of frequent exacerbations previous 12 months was introduced. At this time, a shorter period of six months for exacerbation history was chosen to minimize recall bias. Level of inhaled treatment was categorized as no maintenance treatment single bronchodilator (long-acting muscarinic antagonists or long-acting beta-2-agonists), double bronchodilator (long-acting muscarinic antagonists and long-acting beta-2-agonists) or inhaled corticosteroids in any combination.
Information on spirometry data and comorbid conditions were obtained from medical records covering the period of 2005 to 2014. Ischemic heart disease (IHD), heart failure, atrial fibrillation, depression or anxiety were identified using International Classification Codes (ICD)-10. IHD was defined as any occurrence of stable angina, acute coronary syndrome, percutaneous coronary intervention or presence of a coronary artery bypass graft at baseline.
COPD staging was based on the forced expiratory volume in one second in percentage of predicted value (FEV1%pred) according to the Global Lung Function Initiative (GLI),22 and categorized as GOLD stage 1 (≥80%pred), stage 2 (50–79%pred), stage 3 (30–49%pred) and stage 4 (<30%pred).21 If spirometry was repeated during the period, the highest value of FEV1%pred was used.
Statistics
Statistics were performed using IBM SPPS version 28.0 (IBM Corporation, Armonk, NY, USA). Differences in characteristics between COPD-patients with and without exacerbations were analysed using cross-tabulations and χ2-test and presented as counts and percentages.
The proportion of patients with no exacerbations was calculated within spirometric stages and within treatment categories. Logistic regression was used to analyse associations of patient characteristics with the status of having no exacerbations the previous six months. Factors investigated, listed in Table 1 and Table 2 were chosen a-priori based on a theoretical basis and confirmed in both univariable and multivariable analysis. The multivariable model was repeated stratified by sex, and with interaction analysis using multiplicative interaction terms of sex and investigated factors. A p-value of <0.05 was considered statistically significant.
 |
Table 1 Patient Characteristics |
 |
Table 2 Associations with the Non-Exacerbator COPD Phenotype |
Results
In total, 1703 (58%) out of 2920 patients with a doctor's diagnosis of COPD returned the questionnaire and left written consent, whereof 1354 had available spirometry data and were included in the study. Of these, 891 (66%) reported having no exacerbations the previous six months. The proportion of patients without exacerbations decreased with severity of COPD but the non-exacerbator phenotype was present in all stages (Table 1 and Figure 2).
 |
Figure 2 The non-exacerbator phenotype by severity of COPD proportions of patients with no exacerbations in every COPD stage. Abbreviation: COPD, chronic obstructive lung disease. |
Overall, 47% of the population had inhaled corticosteroids in some form. The proportion of non-exacerbators was 84% in patients with no maintenance treatment, 70% with one single bronchodilator, 52% with double bronchodilators and 54% in patients with any combination including inhaled corticosteroids. In addition, the non-exacerbator phenotype more often had CAT <10 and were current daily smokers, while BMI <22 kg/m2, chronic bronchitis, heart failure, ischemic heart disease, atrial fibrillation and depression/anxiety were significantly more common in patients with exacerbations (Table 1). In multivariable logistic regression, COPD stages 1–3 and CAT <10 were independently and statistically significantly positively associated, and chronic bronchitis and underweight inversely associated, with the non-exacerbator phenotype (Table 2 and Figure 3). The results remained substantially unchanged after further adjustment for level of inhaled treatment (data not shown).
Stratification and interaction analyses by sex showed that BMI <22 and >30 kg/m2 were statistically significantly inversely associated with the non-exacerbator phenotype in women but not in men, and heart failure with the non-exacerbator phenotype in men but not in women (Table 3 and Figure 3). In addition, the association of COPD stage 1 with the non-exacerbator phenotype was statistically significantly of higher magnitude in men than in women (Table 3 and Figure 3).
 |
Table 3 Differences by Sex |
Discussion
The primary findings of this real-world study were that a majority of patients with COPD had a non-exacerbator phenotype, and that higher lung function, lower symptom burden, and absence of chronic bronchitis and underweight were significantly associated with the non-exacerbator phenotype. Secondary findings were that factors associated with the non-exacerbator phenotype differed by sex, as absence of underweight and obesity were associated with the non-exacerbator phenotype in women but not in men, absence of heart failure was associated with the non-exacerbator phenotype in men but not in women, and the association of preserved lung function with the non-exacerbator phenotype was of higher magnitude in men compared with women.
Our results are in agreement with previously reported data where the non-exacerbator phenotype seems to be stable over time.10 The finding that patients with low CAT scores and preserved lung function were more likely to have a non-exacerbator phenotype is consistent with previous studies.10,23,24 As exacerbations are defined as worsening of symptoms beyond normal day-to-day variation,9 it is logical that patients with a high symptom burden already in the stable phase are more likely to perceive their symptoms as exacerbations. In addition, frequent exacerbations cause an increased decline in lung function, emphasising the importance of preventing exacerbations to preserve lung function.25
The prevalence of comorbidity is high in COPD patients, as 86–98% have additional comorbid disease with an average of 1.2–4 comorbid conditions.26 Multimorbidity affects important clinical outcomes in COPD, such as HRQL, risk for exacerbations and mortality.26,27 Chronic bronchitis could exist as a single condition or as a clinical trait or phenotype of COPD but is not always present in COPD. Its pathophysiology includes hypersecretion of mucus by goblet cells due to exposure to smoking.21 Our finding that the absence of chronic bronchitis was independently associated with the non-exacerbator phenotype of COPD is consistent with previous research where chronic bronchitis has been associated with faster decline in lung function, decreased HRQL and frequent exacerbations. The combination of frequent exacerbations and chronic bronchitis is well known and pointed out as a COPD phenotype requiring specific treatment.28,29 The results of our study indirectly confirm the link between chronic bronchitis and exacerbations and highlight the importance of early smoking cessation in COPD-patients to avoid development of chronic bronchitis.
Differences by sex have been observed for many aspects of COPD, such as diagnostics30 management31 and clinical expression of COPD.32–34 In a study by Celli et al, women across all stages of COPD reported more dyspnoea and more exacerbations compared with men with similar lung function impairment, especially pronounced in COPD stages 1 and 2. The author suggested that women have a heightened perception of dyspnoea, which is reflected in worse HRQL.32 We speculate that our finding of a stronger association of mild lung function impairment with a non-exacerbator COPD in men may be due to sex-related differences in perception of dyspnoea.
Our finding that BMI <22 (mainly driven by a difference in women) is inversely associated with the non-exacerbator phenotype, is consistent with previous knowledge that low BMI is a negative prognostic factor for exacerbations and mortality in COPD.10,17,35 In a study by Lambert et al, a dose-dependent increase in dyspnoea and risk for severe exacerbations as well as a decrease in HRQL was found also with increasing BMI. Furthermore, stratification by sex showed HRQL to be more affected by obesity in women.36 However, to our knowledge, the inverse impact of obesity on the non-exacerbator phenotype in women shown in our study has not previously been described.
Our finding that the absence of heart failure was associated with the non-exacerbator phenotype in men is consistent with previous knowledge that heart failure increases the risk for exacerbations.37,38 Suggested reasons are the increased symptom burden with comorbid heart failure, and a possible underlying systemic inflammation.37,39 However, we have not found any previous studies reporting differences by sex in the association of heart failure with exacerbations. We speculate that this may partly be due to the prevalence of heart failure is higher in men with COPD, as well in this study (data not shown) as in other studies.40
The recent IMPACT11 and ETHOS12 studies of the benefit of adding ICS to LAMA/LABA therapy has raised the question whether all patients with COPD should be treated with ICS to prevent risk of exacerbations and increased mortality. Interestingly, we found that a majority of patients had no exacerbations, and that the non-exacerbator phenotype was more common in patients without ICS. This is constituent with previous real-word data where exacerbations per se is the main reason for stepping up treatment.41
In addition, a large Swedish primary care study reported that 74% of patients with COPD and no exacerbations before study entry remained exacerbation free for 12 months, and of these 77% remained exacerbation free for 24 months.10 Nevertheless, several studies have shown a high use of ICS treatment also in non-exacerbating patients with COPD.11,12
Subsequently, there is obviously a group of patients with no exacerbations where the absence of exacerbations cannot be explained by treatment with ICS. We believe that in times of high stress in the health care system, it is important to identify patients where less frequent treatment and follow-up is needed.
Finally, as for the finding that current smoking was more common in the non-exacerbator phenotype, we speculate that this is due to a healthy smoker effect where patients with no exacerbations are not motivated to quit smoking.
Strengths and Limitations
The major strength is that this study is based on real-world data from multiple centres in both primary and secondary care. The majority of the study population was based on primary care patients where mild COPD and no exacerbations is common, which increases the generalisability of the results. We also believe that our broad definition of exacerbation, including both emergency visits and steroid courses covers all relevant exacerbations without including mild airway infections or other infections treated with antibiotics with no worsening of respiratory symptoms.
A limitation is that the response rate was 58%, potentially causing response bias. However, an attrition analysis showed that responders and non-responders did not differ by sex or age. As for the responders, there was attrition due to lack of lung function data. Excluded patients did not differ by sex. Complete lung function data were not available for all older patients, which might have skewed the results. The limitation of the period of exacerbations to six months was made to minimise the risk for recall bias. On the other hand, with a longer data collection period, some of the non-exacerbators could potentially have had exacerbations. However, as significant differences were found despite the limited time study period, we believe the results are valid. The record review of comorbidity was based on ICD codes, which may underestimate the true number of comorbid conditions. Finally, the cross-sectional nature of the analyses is a limitation, as no causal associations can be proved.
Conclusion
The non-exacerbator phenotype is common, especially in patients with no maintenance treatment or a single bronchodilator, and is characterized by preserved lung function, low symptom burden and absence of chronic bronchitis. Having no exacerbations during the previous six months was also associated with absence of underweight and obesity in women, and by absence of heart failure in men.
We suggest that awareness of this group of patients is important as they may need less resource-intense management and follow-up, but that management of comorbid conditions is important to avoid exacerbations.
Acknowledgments
We thank all participating centres, and Ulrike Spetz-Nyström and Eva Manell at the Department of Medical Sciences, Respiratory, Allergy & Sleep Research, Uppsala University, for reviewing the patient records.
Funding
The study was supported by grants from the Uppsala-Örebro Health Care region, the Swedish Heart and Lung Association, the Swedish Asthma and Allergy Association and the Bror Hjerpstedts Foundation.
Disclosure
Professor Christer Janson reports personal fees from AstraZeneca, Novartis, Boehringer Ingelheim, GlaxoSmithKline, Chiesi, Orion and Sanofi, outside the submitted work. Dr Karin Lisspers reports personal fees from Novartis, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline, outside the submitted work. Dr Hanna Sandelowsky reports personal fees from Boehringer Ingelheim, Chiesi, Novartis, AstraZeneca, GlaxoSmithKline, and TEVA, outside the submitted work. Dr Björn Ställberg reports personal fees from AstraZeneca, Novartis, Boehringer Ingelheim, GlaxoSmithKleine, Meda/Mylan, Chiesi, and Teva, outside the submitted work. Dr Josefin Sundh reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi and Novartis, outside the submitted work. The authors report no other conflicts of interest related to this study.
References
1. Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35(1):1–6. doi:10.1016/j.ccm.2013.10.010
2. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi:10.1056/NEJMoa1105482
3. Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi:10.1371/journal.pone.0037483
4. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
5. Rouzic OL, Roche N, Cortot AB, et al. Defining the “frequent exacerbator” phenotype in COPD: a hypothesis-free approach. CHEST. 2018;153(5):1106–1115. doi:10.1016/j.chest.2017.10.009
6. Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP
7. Stöber A, Lutter JI, Schwarzkopf L, et al. Impact of lung function and exacerbations on health-related quality of life in COPD patients within one year: real-world analysis based on claims data. COPD. 2021;16:2637–2651. doi:10.2147/COPD.S313711
8. Doll H, Miravitlles M. Health-related QOL in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. PharmacoEconomics. 2005;23(4):345–363. doi:10.2165/00019053-200523040-00005
9. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
10. Mantero M, Rogliani P, Di Pasquale M, et al. Acute exacerbations of COPD: risk factors for failure and relapse. COPD. 2017;12:2687–2693. doi:10.2147/COPD.S145253
11. Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi:10.1164/rccm.201911-2207OC
12. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
13. Calverley PCA, Anderson J, Celli B, et al. Salmeterol and Fluticasone Propionate and Survival in Chronic Obstructive Pulmonary Disease. N Engl J Med. ;356(8):775–789
14. Janson C, Johansson G, Ställberg B, et al. Identifying the associated risks of pneumonia in COPD patients: arctic an observational study. Respir Res. 2018;19(1):172. doi:10.1186/s12931-018-0868-y
15. Praxis-studien. Astma KOL; 2022. Available from: http://www.praxisstudien.se/.
16. de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J. 2012;40(1):28–36. doi:10.1183/09031936.00141611
17. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi:10.1164/ajrccm.160.6.9902115
18. Ferreira IM, Brooks D, White J, Goldstein R Nutritional supplementation for stable chronic obstructive pulmonary disease.Cochrane Database Syst Rev. ;12:CD000998.
19. Vestbo J, Prescott E, Almdal T, et al. Body Mass, Fat-Free Body Mass, and Prognosis in Patients with Chronic Obstructive Pulmonary Disease from a Random Population Sample - findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. ;173(1):79–83
20. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
21. GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf; 2022. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
22. Cooper BG, Stocks J, Hall GL, et al. The Global Lung Function Initiative (GLI) Network: bringing the world’s respiratory reference values together. Breathe. 2017;13(3):e56–64. doi:10.1183/20734735.012717
23. Varol Y, Ozacar R, Balci G, Usta L, Taymaz Z. Assessing the effectiveness of the COPD Assessment Test (CAT) to evaluate COPD severity and exacerbation rates. COPD. 2014;11(2):221–225. doi:10.3109/15412555.2013.836169
24. Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized Exacerbations of COPD: risk Factors and Outcomes in the ECLIPSE Cohort. Chest. 2015;147(4):999–1007. doi:10.1378/chest.14-0655
25. Donaldson GC. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
26. Putcha N, Drummond M, Wise R, Hansel N. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015;36(04):575–591. doi:10.1055/s-0035-1556063
27. Sundh J, Ställberg B, Lisspers K, Montgomery SM, Janson C. Co-morbidity, body mass index and quality of life in COPD using the clinical COPD questionnaire. COPD. 2011;8(3):173–181. doi:10.3109/15412555.2011.560130
28. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(3):228–237. doi:10.1164/rccm.201210-1843CI
29. Sundh J, Johansson G, Larsson K, et al. The phenotype of concurrent chronic bronchitis and frequent exacerbations in patients with severe COPD attending Swedish secondary care units. COPD;2015. 2327. doi:10.2147/COPD.S91362
30. Watson L, Vestbo J, Postma DS, et al. Gender differences in the management and experience of chronic obstructive pulmonary disease. Respir Med. 2004;98(12):1207–1213. doi:10.1016/j.rmed.2004.05.004
31. Åberg J, Hasselgren M, Montgomery S, et al. Sex-related differences in management of Swedish patients with a clinical diagnosis of chronic obstructive pulmonary disease. COPD. 2019;14:961–969. doi:10.2147/COPD.S193311
32. Celli B, Vestbo J, Jenkins CR, et al. Sex differences in mortality and clinical expressions of patients with chronic obstructive pulmonary disease: the TORCH Experience. Am J Respir Crit Care Med. 2011;183(3):317–322. doi:10.1164/rccm.201004-0665OC
33. Lamprecht B, Vanfleteren LE, Studnicka M, et al. Sex-related differences in respiratory symptoms: results from the BOLD Study. Eur Respir J. 2013;42(3):858–860. doi:10.1183/09031936.00047613
34. Lisspers K, Larsson K, Janson C, et al. Gender differences among Swedish COPD patients: results from the Arctic, a real-world retrospective cohort study. npj Prim Care Respir Med. 2019;29(1):45. doi:10.1038/s41533-019-0157-3
35. Putcha N, Anzueto AR, Calverley PMA, et al. Mortality and exacerbation risk by body mass index in patients with COPD in TIOSPIR and UPLIFT. Ann ATS. 2022;19(2):204–213. doi:10.1513/AnnalsATS.202006-722OC
36. Lambert AA, Putcha N, Drummond MB, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68–77. doi:10.1016/j.chest.2016.08.1432
37. Axson EL, Bottle A, Cowie MR, Quint JK. Relationship between heart failure and the risk of acute exacerbation of COPD. Thorax. 2021;76(8):807–814. doi:10.1136/thoraxjnl-2020-216390
38. Abusaid GH, Barbagelata A, Tuero E, Mahmood A, Sharma G. Diastolic dysfunction and COPD exacerbation. Postgrad Med. 2009;121(4):76–81. doi:10.3810/pgm.2009.07.2033
39. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi:10.1183/09031936.00128008
40. de Boer AR, Vaartjes I, Gohar A, et al. Heart failure with preserved, mid-range, and reduced ejection fraction across health care settings: an observational study. ESC Heart Fail. 2022;9(1):363–372. doi:10.1002/ehf2.13742
41. Lopez-Campos JL, Abad-Arranz M, Calero Acuña C, et al. Determinants for changing the treatment of COPD: a regression analysis from a clinical audit. COPD;2016. 1171. doi:10.2147/COPD.S103614
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

