Back to Journals » Drug Design, Development and Therapy » Volume 15
Factors Associated with Benzodiazepines Prolonged-Term Use in Post-Stroke Subjective Sleep Disturbance: A Single-Centre Retrospective Study from China
Authors Ma G, Sun L, Qie Z, He J, Cui F
Received 24 December 2020
Accepted for publication 25 May 2021
Published 9 June 2021 Volume 2021:15 Pages 2469—2481
DOI https://doi.org/10.2147/DDDT.S298552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Guozhong Ma,1 Liuqing Sun,2 Zhiwei Qie,3 Jinzhao He,1 Fang Cui2
1Department of Neurology, Heyuan People’s Hospital, Heyuan, Guangdong Province, 517000, People’s Republic of China; 2Department of Neurology, Hainan Hospital of Chinese People’s Liberation Army General Hospital, Sanya, Hainan Province, 572013, People’s Republic of China; 3Beijing Institute of Radiation Medicine, Beijing, 100850, People’s Republic of China
Correspondence: Guozhong Ma
Department of Neurology, Heyuan People’s Hospital, No. 733 Wenxiang Road, Yuancheng District, Heyuan, Guangdong Province, 517000, People’s Republic of China
Tel/Fax +86-0762-3185571
Email [email protected]
Fang Cui
Department of Neurology, Hainan Hospital of Chinese People’s Liberation Army General Hospital, Haitang District, Sanya, Hainan Province, 572013, People’s Republic of China
Tel/Fax +86- 0898-37330625
Email [email protected]
Purpose: To investigate the prevalence of short- and long-term benzodiazepine and z-drugs (BZD) for the treatment of post-stroke subjective sleep disturbance (SSD) and to evaluate the risk factors associated with prolonged BZD treatment in this patient body.
Patients and Methods: Between 1st January 2018 and 1st December 2018, we identified 542 inpatients suffering from acute stroke in Heyuan People’s Hospital. Of these, 290 inpatients were included in our final analysis. These patients were divided into three groups according to the treatment they received: non/occasional BZD (non-BZD), short-term BZD (short-term) and prolonged-term BZD (prolonged-term) treatment. We investigated the prevalence of each BZD treatment term and identified differences between the groups. Univariate logistic regression analysis was used to identify potential predictors for the prolonged use of BZD. Multinomial logistic regression analysis was used to assess the correlation between the prolonged use of BZD and potential predictors.
Results: The prevalence of cases receiving short and prolonged BZD treatments were 40.35% and 31.72%, respectively; none of the patients received polysomnography (PSG) screening from obstructive sleep apnoea (OSP). Treatment strategies were limited to BZD and traditional Chinese medicine; none of the patients received cognitive-behavioral treatment (CBT) or other forms of treatment. Logistic regression analysis showed that the short-term use was associated with z-drugs (odds ratio [OR]: 2.189, 95% confidence interval [CI]: 1.419– 3.378), non-communication barriers (OR =0.535, 95% CI: 0.325– 0.880) and posterior circulation infarct (POCI) (OR =2.199, 95% CI: 1.112– 4.349). The prolonged-term use was associated with z-drugs (OR =3.012, 95% CI: 1.637– 5.542), non-communication barriers (OR =0.530, 95% CI: 0.307– 0.916), partial anterior circulation infarct (PACI) (OR =0.455, 95% CI: 0.250– 0.827), and non pain after stroke (OR =0.315, 95% CI: 0.207– 0.480).
Conclusion: The status of BZD abuse for post-stroke SSD is worrying. Additional research attention and treatment options are needed for the treatment of post-stroke SSD. In particular, the potential combination of stroke and OSP appears to be underestimated and neglected. Post-stroke SSD patients should receive more comprehensive assessment and rigid follow-up to avoid the prolonged use of BZD. Additional and effective therapeutic strategies (such as positive pressure ventilation treatment or CBT) are urgently needed for cause-specific intervention.
Keywords: benzodiazepine and z-drugs, BZD, prolonged-use, post-stroke, subjective sleep disturbance, SSD, risk factors, obstructive sleep apnea, OSP, China
Introduction
Stroke, including both ischemic stroke and hemorrhagic stroke, is defined as an acute defect in neurological function caused by cerebrovascular disease and has become a substantial burden for global.1,2 As a result of population aging, urban expansion, unhealthy lifestyles, and an increase in cerebrovascular risk factors, the prevalence of stroke is increasing annually in China and now represents the third leading cause of death. In 2018, the prevalence of stroke in the population of China was 149.49 per 100,000.3,4
Sleep disorders refer to dissatisfaction with sleep quality, sleep timing, or the amount of sleep obtained, thus resulting in distress and impairment during the daytime. There are a number of specific sleep disorders, including insomnia disorders, sleep-related breathing disorders, central disorders of hypersomnolence, circadian rhythm sleep-wake disorders, sleep-related movement disorders, parasomnias, and other sleep disorders.5 Sleep disorders may increase the risk of stroke but may also occur as a result of stroke events and affect stroke patient recovery.5 Insomnia disorder is a sleep disorder that is reported to affect 38.6% of stroke survivors;7 far higher than in the general population of China (15%).8 The main treatments for insomnia are CBT, pharmacological intervention (predominantly BZD), and other forms of complementary therapy (eg, light therapy, acupuncture, yoga, and meditative movement).9 When used for insomnia, CBT can include sleep hygiene, relaxation training, stimulus control, sleep restriction, and cognitive therapy.9 Although CBT is recommended as the first-line treatment for chronic insomnia,9 BZD remains as the first choice treatment option in developing countries; this is because of the lack of popularization for CBT.10 For insomniacs, current clinical guidelines recommend the use of BZD only over the short term use (≤4 weeks).9 This is because psychological and physical adverse events may arise following the prolonged use of BZD.11 However, the prolonged use of BZD is common in the United States,12 England,13 France,14 Germany,15 and other areas of the world,16 and has led to a serious public health problem. This worldwide dilemma is related to a series of factors, including demographic factors,14 treatment-related factors,17 psychological factors,17 personality-related factors,18 and some other factors.19
Until now, the research community has not paid sufficient attention to the prolonged use of BZD in post-stroke insomniacs. Furthermore, we know little about the prevalence of this form of treatment or potential risk factors. Understanding of such information is essential if we are to manage these patients effectively. According to the diagnostic and statistical manual of mental disorders (DSM-5),20 the definitions of insomnia involve the one or more of the following essential aspects: (i) difficulty initiating sleep, (ii) difficulty maintaining asleep, (iii) early-morning awakening and inability to return to sleep; and clinically significant distress or impairment in daytime functioning; lasting for 3 or more days/week for 3 months, despite appropriate opportunity to sleep, and not explained by another sleep disorder, such as OSP. Notably, up to 72% of stroke Transient ischemic attack (TIA) patients may suffer from obstructive OSP,21 a specific form of sleep-related breathing disorder. OSP refers to the complete or partial obstruction of the airway, thus resulting in snoring, disturbed sleep, and daytime sleepiness.22 Due to certain objective reasons, we were unable to distinguish other sleep disorders, especially OSP, in our cohort of patients suffering from insomnia. Furthermore, we set the SSD observation length as 1 week rather than 3 months. Due to the reasons mentioned above, we used the term “subjective sleep disturbance (SSD)” as an alternative for “insomnia” in this study.
The aim of this study was to investigate the prevalence of prolonged BZD treatment for SSD, and associated factors, in adult patients who had suffered from stroke at least 3 months previously.
Patients and Methods
Data Sources
We used a medical records database based on the hospital information system (HIS) of Heyuan People’s Hospital and software provided by the Donghua Co-creation Software Co., Ltd., Beijing, China. The HIS is used in our hospital to record all inpatient and outpatient information. We used the HIS to acquire a range of inpatient information, including contact information (telephone and address), demographic data, patient history, family history, physical examination, current history (including the chief complaint, the course of disease, and accompanying symptoms), laboratory and imaging examination results, scores from different scales, medication usage, and diagnoses upon admission and discharge. We also acquired a range of information relating to outpatient visits, including visit time, diagnosis, prescription information, prescribers, and the patient’s condition at follow-up. All patients are given a unique identifier (ID) in the HIS at their first attendance. This ID is mandatory for all patients in our hospital and is an effective way of tracing patient data.
If information was missing in the HIS database, we investigated further and acquired the missing information by contacting the patients by outpatient appointments or telephone/WeChat interviews. Due to some communication barriers patients (eg, aphasia, dysarthria, audio disability, visual disability), we provided a written communication protocol (for patients with aphasia, dysarthria and audio disability) and a sound communication protocol for those with visual disabilities. When completing these investigations, disabled patients were assisted by family members and staff associated with the study. Once we had obtained informed consent from each patient, we created a standardized data collection form to retrieve all relevant information. This form was populated with a range of information from the HIS, including sociodemographic data (gender, age) and patient histories (BZD treatment, sleep-disturbing agents, traditional Chinese medicine, history of drinking tea/coffee/alcoholic beverages, history of psychotic disorders/chronic SSD/pain. We also recorded the type of stroke as either total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), lacunar infarct (LACI), and posterior circulation infarct (POCI), in accordance with the classification put forward by the Oxfordshire Community Stroke Project.23 We also used the HIS records, and interviews, to acquire data relating to stroke-related scale (discharge modified Rankin Scale [mRS] score); National Institutes of Health Stroke Scale score post-intervention, NIHSS), OSP-related factors24 (eg, body mass index [BMI], nocturnal gasping/choking, and daytime sleepiness), comorbid diseases (pain after stroke, communication barriers, post-stroke psychological diseases), and treatment-related factors (CBT, multiple prescriptions, type of BZD, and the use of traditional Chinese medicines). This research was performed between 30th July 2020 and 20th October 2020; some supplementary research was performed between 10th March 2021 and 20th March 2021.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Heyuan People’s Hospital (Reference number: HY2020K17; 29th July 2020). All participants provided written informed consent. Family members provided consent if patients were unable to do so themselves.
Patients
This retrospective study included a group of consecutive post-stroke patients with SSD who attended Heyuan People’s Hospital between 1st January 2018 and 1st December 2018. Eligible patients needed to attend regular outpatient visits to our clinic (at least once every month) and all information needed to be recorded on the HIS system.
Patients were included if they complied to the following criteria: (1) an acute stroke event had occurred 7 days prior to admission and had been confirmed by magnetic resonance imaging (MRI) or computed tomography (CT); (2) BZD had not been used regularly within the 6 months prior to stroke (regular use was defined as 3 days or more per week); (3) aged 18 to 90 years; and (4) long-term resident in Heyuan district. In addition, if a patient developed an auditory or visual disability, it was important that each disability appeared separately. Finally, all patients needed to meet the diagnostic criteria of SSD (see below).
Patients were excluded if they had a long-term history of taking sleep-disturbing agents within the 6 months prior to stroke (long-term use was defined as 3 days or more each week and for more than 3 consecutive months), including antiepileptic agents (eg, carbamazepine, valproate sodium, phenytoin, ethosuximide, phenobarbital, gabapentin, levetiracetam, and pregabalin), psychotherapeutic agents (eg, fluoxetine, paroxetine, olanzapine, risperidone, clozapine, escitalopram, sertraline, and haloperidol), hormonal agents (eg, prednisone acetate, methylprednisolone, and dexamethasone), anti-allergenic drugs (eg, loratadine, promethazine, and cetirizine), central stimulants (eg, amphetamine, cocaine, caffeine, and theophylline), and illegal drugs (eg, cannabis, ketamine, cocaine and ecstasy). When combined with clinical situations, it is likely that these medicines/illegal drugs may have interfered with sleep homeostasis;25 consequently, patients who reported the use of such drugs were excluded. We also excluded patients if they had a long-term history of taking traditional Chinese medicine within the 6 months prior to stroke (long-term use was defined as 3 days or more day per week for more than 3 consecutive months); we excluded these patients because Chinese herbal medicine may influence sleep homeostasis.26 We also excluded patients who had shown a tendency to drink tea, coffee, or alcoholic beverages, within the 6 months prior to stroke (defined as 3 days or more day per week and for more than 3 consecutive months and if the amount of tea, coffee, and alcohol, consumed was >500 mL/day, >500 mL/day and >24g/day, respectively). We excluded these patients because tea, coffee, and alcohol may interfere with sleep homeostasis.27,28 We also excluded patients who had a history of psychotic disorders prior to stroke (defined as disorders related to anxiety, depression, and schizophrenia); we excluded these patients because in clinical situations, these psychotic disorders may influence sleep homeostasis.29–31 These four categories of patients were also excluded because they could have exerted potential confounding effects. In addition, we also excluded patients with impaired cognition (defined as a post-intervention Mini-Mental State Exam [MMSE] value <24.32 These patients were excluded because the accuracy of the retrospective information could not be assured. We also excluded patients who refused to cooperate and those with a history of chronic SSD prior to stroke (defined as 6 months prior to stroke with symptoms appearing 3 days or more each week for more than 3 consecutive months). Finally, we excluded patients who had experienced persistent pain within the previous 6 months since the stroke event (persistent was defined as the appearance of symptoms on 3 or more days per week for more than 3 consecutive months).
Definition of SSD
The diagnosis of SSD required that a patient experienced at least one of the following conditions: (i) difficulty initiating sleep, (ii) difficulty maintaining asleep, (iii) early-morning awakening followed by the inability to return to sleep, and (iv) clinically significant distress or impairment in terms of daytime function, despite having an appropriate opportunity to sleep. Furthermore, the condition lasted for 3 or more days for at least 1 week. We determined the extent of sleep disturbance by examining HIS records and by outpatient appointments, or by telephone/WeChat interviews. These assessments were performed independently by two investigators; the two investigators needed to arrive at a consensus by discussion if there were any disputes. The duration of SSD was calculated from the date of the earliest SSD complaint, as recorded in the HIS records. It was important that SSD had occurred within 3 months of the stroke event. We adhered to these guidelines because there is a strong association between stroke and sleep disturbance, especially within the first 3 months of the stroke event, beyond this period, it is possible for other factors that are not related to stroke to induce insomnia.33 We also followed these guidelines because they had been used in previous studies,33 thus allowing for comparative analysis.
Definition of BZD
We defined BZD in accordance with the anatomical therapeutic chemical classification system codes (ATC codes) put forward by the World Health Organization (WHO).34 Patients were excluded if they received BZDs for any purpose other than insomnia. BZDs were divided into two groups: a benzodiazepine group (alprazolam, estazolam, and clonazepam) and a z-drug group (zopiclone, zolpidem).
Definitions for BZD Treatment Periods
The included patients were divided into three groups: non-BZD, short-term, and prolonged term. We defined non-BZD and short-term as < 7 days and 7–28 days within the study period, respectively. The prolonged term group was further sub-divided into medium-term (4 weeks–3 months) and long-term (>3months) within the study period. To be included in the prolonged term group, patients needed to be taking medicine at least 3 days each week; otherwise, they were assigned into the short-term use group. The duration of BZD treatment was calculated from the date of the earliest administration of BZD for the treatment of SSD (as determined by HIS records) and were traced to follow-up using the outpatients prescription records on HIS). The observation period for BZD treatment was 12 months. If the BZD prescription records were intermittent or had been terminated, we confirmed the reasons for such changes by outpatient appointments or telephone/WeChat interviews. We needed to ascertain whether these patients discontinued BZD on their own initiative or had received instruction from other sources. In these cases, we superimposed detailed information relating to the use of BZDs from records.
Observation Factors
We evaluated 15 observation factors, including gender, age, pain after stroke (this pain needed to be new-onset after stroke, and feature musculoskeletal pain, neuropathic pain, shoulder pain, stroke-related headache, and spasticity-related pain), stroke type (TACI, PACI, LACI, and POCI), discharge mRS, post-intervention NIHSS, communication barriers (including aphasia, dysarthria, auditory disability, and visual disability; defined as a score of “moderate” and above on our in-house Self-assessment Rating Scale shown in Figure 1). We also evaluated multiple prescriptions (defined as the use of ≥2 different BZD medicines during the follow-up period), post-stroke psychological disease (including anxiety disorders and/or depression disorders; anxiety disorders were defined by a score on the Self-rating Anxiety Scale ≥4535 while depression disorder was defined as a score on the Self-Rating Depression Scale ≥5036), BMI (classified into 3 categories: ≥30 kg/m2, 25–30 kg/m2 and <25 kg/m2), nocturnal gasping/choking (occurring at least 3 nights a week, as noted by self-reporting), daytime sleepiness (daytime sleepiness was defined as a score on the Epworth sleepiness scale >6).37 We also evaluate the type of treatment: CBT, type of BZD (benzodiazepines or z-drugs), and traditional Chinese medicine (including Chinese patent medicine and Chinese herbal medicine).
We selected these observation factors based on previous literature related to the prolonged use of BZD in other populations, post-stroke insomnia,15,17–19,38 and our own clinical practice.
Statistical Analysis
All statistical analysis was performed with SAS version 9.4 Windows (SAS Institute, Inc., Cary, NC, USA). Comparisons between groups were performed using the rank-sum test because of groupings. Ordered logistic regression was used to analyze factors associated with the short and prolonged use of BZD in post-stroke SSD patients. Three groups (non BZD, short-term and prolonged term) were used as dependent variables, with the “non BZD” group acting as the reference group. A total of 13 observation factors (gender, age, pain after stroke, stroke type, discharge mRS, post-intervention NIHSS, communication barriers, multiple prescriptions, post-stroke psychological diseases, BMI, nocturnal gasping/choking, daytime sleepiness, and BZD type) were used as dependent variables. Categorical variables were included in the form of dummy variables (see Table 1). If collinearity between independent variables, then these variables were selectivity removed or used in ordered logistic regression. The fitting of an ordered logistic regression model involved the Stepwise test; entry and exit, with regards to regression model probability, were set to 0.1 and 0.15, respectively (SLENTRY=0.1, SLSTAY=0.15). Model fitness was assessed by the Hosmer and Lemeshow test. Unless stated otherwise, P <0.05 was considered to be statistically significant.
 |
Table 1 Assignment of Factor-Specific Variables |
Results
Demographic and Clinical Characteristics
The protocol used to collect data during this study is shown in Figures 2 and 3. We included a total of 290 post-stroke patients with SSD in our final analysis. Demographic and clinical characteristics are given in Table 2.
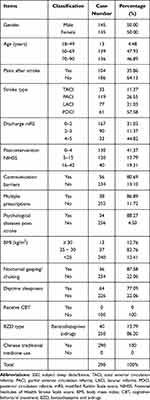 |
Table 2 Demographic and Clinical Characteristics of the Post-Stroke SSD Patients |
 |
Figure 2 Flow chart for recruiting participants. Abbreviation: BZD, benzodiazepine and z-drugs. |
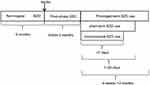 |
Figure 3 Participant flowchart. Abbreviations: SSD, subjective sleep disturbance; BZD, benzodiazepine and z-drugs. |
Treatment Durations for the Administration of BZD
We identified 81 SSD patients with non/occasional BZD use, 117 SSD patients who received BZD over the short-term, and 92 SSD patients who received BZD over prolonged periods of time. When considering the patients who received BZD over a prolonged period, 57 and 35 were classified as medium- and long-term, respectively (Table 3).
 |
Table 3 Different Treatment Terms for BZD |
Differences Between Groups
When considering the 15 observation factors, we found six factors that showed significant differences between groups: pain after stroke (P<0.0001), stroke-type (P =0.0006), post-intervention NIHSS (P=0.0050), discharge-mRS (P<0.0001), communication barriers (P=0.0044), and BZD type (P<0.0001). The specific details of these comparisons between groups are shown in Table 4.
 |
Table 4 Differences Between Groups (n, %) |
Predictors for the Short- and Prolonged-Term Use of BZD
Logistic regression indicated that short-term use was associated with z-drugs (OR: 2.189, 95% CI: 1.419–3.378), non-communication barriers (OR =0.535, 95% CI: 0.325–0.880) and posterior circulation infarct (POCI) (OR =2.199, 95% CI: 1.112–4.349). The prolonged-term use was associated with z-drugs (OR =3.012, 95% CI: 1.637–5.542), non-communication barriers (OR =0.530, 95% CI: 0.307–0.916), partial anterior circulation infarct (PACI) (OR =0.455, 95% CI: 0.250–0.827), and non pain after stroke (OR =0.315, 95% CI: 0.207–0.480). For further details, please refer to Table 5.
 |
Table 5 Predictors for BZD Short-Term and Prolong-Term Use |
Discussion
In this study, we investigated the current state of BZD use in the treatment of post-stroke patients with SSD from a single center in China. We observed the widespread prevalence of BZD use in the post-stroke SSD population; 40.34 and 31.73% of patients received BZD over short and prolonged terms, respectively. Regression analyses suggested that the use of BZD over short and prolonged terms were associated with some specific factors. In comparison with existing literature, our present analysis featured four novel aspects. First, previous studies relating to the prolonged use of BZD focused mainly on the general population14 or other specific populations, such as older age groups.18 None of the previous studies attempted to investigate the use of BDZ in the post-stroke SSD population. A high prevalence of post-stroke sleep disturbance has been reported in the literature. Because BZD is the primary form of therapy for sleep disturbance, it follows that we needed to investigate the current status of BZD use in this group of patients. When affected by stroke, this population are more vulnerable than the general population. There is a clear need to identify the risk factors that can avoid the use of BZD over long periods of time. Research has shown that there is a bidirectional relationship between stroke and post-stroke sleep disturbance. Sleep disturbance increases the risk of stroke recurrence; however, stroke can also interfere with normal sleep mechanisms.6 Therefore, the appropriate management of post-stroke sleep disturbance is of great significance for post-stroke rehabilitation. Second, most previous studies relating to the misuse of BZD involved developed countries; data relating from developing countries have yet to be published. China is the largest developing country and has the highest numbers of stroke patients in the world.2 Because our study focused on stroke patients in China, our findings provide valuable information relating to the misuse of BZD in developing countries. Third, previous studies relating to the prolonged use of BZD focused on a series of risk factors, including demographic factors, treatment-related factors, psychological factors, and personality-related factors. However, these previous studies failed to consider OSP. Given that 72% of stroke and TIA patients suffer from OSP,21 we consider that it was vital that we incorporated OSP-related factors in our present analysis. For objective reasons, we were unable to adopt the gold standard PSG for the OSP diagnosis, and only use the factor related to OSP as a substitute. Therefore, although no correlation with OSP-related factor and short-term/prolong term use was derived, in view of the importance of OSP, it is very necessary to use PSG indicator to analyze the correlation between OSP and prolong term use more accurately in the future.Finally, previous studies tended to simply divide their patient body into long-term and non-long-term groups; in the present study, we grouped the durations of BZD treatments into non/occasional, short-term, and prolonged term. Previous research showed that the use of BDZ over the short-term was related to poor outcomes after stroke.39 Our new grouping strategy is more reasonable and is likely to provide more information relating to the prevention and control of stroke.
There are some similarities and differences worth noting between our findings and previous studies. Consistent with previous studies relating to the long-term use of BZD in the general population,17,40 we found that pains after stroke was significantly associated with the prolonged use of BZD in post-stroke SSD; The reason may be that pain causes the patient to be in an uncomfortable state, unable to maintain a calm state, and then interferes with sleep; and the specific mechanism underlying this association is not entirely clear and requires further research. Unlike previous studies based on the prolonged use of BZD in the general population,14,18 some putative factors (eg, gender, post-intervention NIHSS and multiple prescriptions) were not significantly associated with the prolonged use of BZD in post-stroke SSD patients. These differences may be attributable to differential pathogenesis and a range of other factors, including sample size, confounding factors, and grouping criteria. Further research is now needed to determine the specific mechanism involved. NIHSS and mRS are the two most common assessment tools for stroke and are considered to be strongly associated with the prognosis of patients suffering from stroke.41,42 In the present study, we showed that communication barriers rather than NIHSS/mRS was associated with the prolonged use of BZD post-stroke SSD patients. Patient with communication barriers find it difficult to express themselves or receive external information; this may lead to adverse events such as psychological diseases and sleep disturbance. This finding highlights the need for clinicians to pay more attention to communication barriers after stroke. On stroke type, PACI rather than LACI was the prevented factors associate with prolonged use of BZD in post-stroke SSD patients.The reason may be that most PACI patients had relatively mild symptoms and better prognosis. But for LACI, the area of that is small but may locate in the key parts such as medulla oblongata, pons and posterior limb of internal capsule, thus affecting critical neurological function.
Our study also identified some other aspects that should be addressed further; these factors are widespread but often overlooked. In particular, post-stroke patients experiencing sleep disturbance were not assessed in a way that could secondary factors, such as OSP. Despite the severity and high prevalence of OSP comorbidity in stroke patients,21 both the American Heart Association and the Chinese Medical Association recommend screening patients with OSP for stroke prevention;43,44 our present data showed that OSP was severely underestimated. None of our patients received PSG screening for OSP and only a very small number received 24-hour ECG primary screening for OSP. The ‘Report on Stroke Prevention and Treatment in China (2019),2 representing the latest and largest national survey of stroke in China, characterized a host of important risk factors, including smoking, obesity, and alcohol intake. However, this survey failed to mention OSP status but placed OSP on the list of risk factors. This unsatisfactory situation suggests that lack of attention to OSP screening represents a serious national issue in China.
Second, the strategy for post-stroke SSD patients was over simplistic and did not consider individualized therapies. There are several aspects to this problem. Firstly, the missed diagnosis of OSP leads to a large number of potential OSP patients losing the opportunity of receiving treatment involving positive pressure ventilation. Secondly, none of the SSD patients were given CBT; CBT is difficult to provide for the general population of China due to inefficiencies in the health-care system, medical staff shortages, and cultural differences.45 This problem could be exacerbated in post-stroke patients with insomnia due to impaired communication. Thirdly, post-stroke psychological diseases and pain are strongly associated with sleep disturbance,40,46 but they were considered or processed appropriately.
Third, our study showed that z-drugs were prescribed on a much larger scale than traditional benzodiazepine. These z-drugs were initially perceived to be much safer than traditional benzodiazepine but associated with the same risks for dependence and withdrawal.47 In the present study, we also found that z-drugs are a risk factor for both short-term and prolonged use. The consequences associated with the prolonged use of z-drugs on such a large scale should be investigated more explicitly, including the identification of specific adverse outcomes. Finally, we found that both patients and physicians placed high value on traditional Chinese medicine; this form of medicine was widely used. However, we failed to identify any significant differences between treatment groups. This preference could be linked to the unique oriental culture; value-based decisions are not always correct. Further rigorous evidence is now needed to confirm the effects of traditional Chinese medicine for the treatment of patients with SSD.
This study has some limitations that need to be considered. First, there was only a small number of patients that were treated with BZD for a prolonged period. A larger sample size is vital if we are to validate our findings in future. Second, we failed to acquire some vital information, particularly PSG results. Consequently, we used the term “SSD” in the present study rather than “insomnia”. The definitions of SSD are overly general and lack precise definitions. It is important to identify cases of OSA from the population of stroke patients and precisely define “insomnia”; this would make analysis more robust in future. Third, because the diagnosis of post-stroke SSD was based on medical records, we need to consider that some patients may not have reported their insomnia symptoms to a physician, thus leading to misdiagnosis. Such events could have affected our results. In addition, we used non-diagnostic tools (clinical symptoms) instead of clinical diagnostic tools (eg, the Pittsburgh Sleep Quality Index or the Athens Insomnia Scale) to define post-stroke SSD. Existing literature shows that the use of different assessment tools can lead to differences in the identification of sleep disturbance.33 This may have affected our results. Finally, our findings were based on a single-center and a retrospective study. Future work should involve a prospective design involving several different centers.
Conclusion
In this study, we investigated the current state of BZD treatment in a Chinese population of post-stroke patients with SSD and evaluated the factors that are related to the prolonged use of this form of treatment. Our findings will help clinicians to identify groups that will require the prolonged use of BZD earlier. We found that the prolonged use of BZD is common in post-stroke patients with SSD. This was a worrying discovery and requires further research attention. Furthermore, there is a clear need to perform further studies to clarify the role of OSP in the prolonged use of BZD in post-stroke patients.
Acknowledgments
This study was supported by the Heyuan City Government under Science and Technology Plan Project of Heyuan City (Reference: 200707171500645) and the Innovation Research Team Project of the Natural Science Foundation of Hainan (Reference: 2018CXTD348).
Disclosure
None of the authors have any conflicts of interest to declare.
References
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211. doi:10.1055/s-0038-1649503
2. Wang Longde CJZZ. Report on stroke prevention and treatment in China (2019). Version 1 ed. Beijing: People’s Health Publishing House; 2020:189.
3. Wang YJ, Li ZX, Gu HQ, et al. China stroke statistics 2019: a report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc Neurol. 2020;5(3):211–239. doi:10.1136/svn-2020-000457
4. Longde W. Brief report on stroke prevention and treatment in China 2019. Chin J Cerebrovas Dis. 2020;17(5):272–281. doi:10.3969/j.issn.1672-5921.2020.05.008
5. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
6. Mims KN, Kirsch D. Sleep and Stroke. Sleep Med Clin. 2016;11(1):39–51. doi:10.1016/j.jsmc.2015.10.009
7. Leppavuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T. Insomnia in ischemic stroke patients. Cerebrovasc Dis. 2002;14(2):90–97. doi:10.1159/000064737
8. Cao XL, Wang SB, Zhong BL, et al. The prevalence of insomnia in the general population in China: a meta-analysis. PLoS One. 2017;12(2):e170772. doi:10.1371/journal.pone.0170772
9. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi:10.1111/jsr.12594
10. Kuo C. 5 - Cognitive behavioral therapy around the globe. In: Stein DJ, Bass JK, Hofmann SG, editors. Global Mental Health and Psychotherapy. Academic Press; 2019:87–126.
11. Gudex C. Adverse effects of benzodiazepines. Soc Sci Med. 1991;33(5):587–596. doi:10.1016/0277-9536(91)90216-y
12. Votaw VR, Geyer R, Rieselbach MM, McHugh RK. The epidemiology of benzodiazepine misuse: a systematic review. Drug Alcohol Depend. 2019;200:95–114. doi:10.1016/j.drugalcdep.2019.02.033
13. Davies J, Rae TC, Montagu L. Long-term benzodiazepine and Z-drugs use in England: a survey of general practice [corrected]. Br J Gen Pract. 2017;67(662):e609–13. doi:10.3399/bjgp17X691865
14. Airagnes G, Lemogne C, Renuy A, et al. Prevalence of prescribed benzodiazepine long-term use in the French general population according to sociodemographic and clinical factors: findings from the CONSTANCES cohort. BMC Public Health. 2019;19(1):566. doi:10.1186/s12889-019-6933-8
15. Janhsen K, Roser P, Hoffmann K. The problems of long-term treatment with benzodiazepines and related substances. Dtsch Arztebl Int. 2015;112(1–2):1–7. doi:10.3238/arztebl.2015.0001
16. Casati A, Sedefov R, Pfeiffer-Gerschel T. Misuse of medicines in the European Union: a systematic review of the literature. Eur Addict Res. 2012;18(5):228–245. doi:10.1159/000337028
17. Takano A, Ono S, Yamana H, et al. Factors associated with long-term prescription of benzodiazepine: a retrospective cohort study using a health insurance database in Japan. BMJ Open. 2019;9(7):e29641. doi:10.1136/bmjopen-2019-029641
18. Mokhar A, Tillenburg N, Dirmaier J, Kuhn S, Harter M, Verthein U. Potentially inappropriate use of benzodiazepines and z-drugs in the older population-analysis of associations between long-term use and patient-related factors. PEERJ. 2018;6:e4614. doi:10.7717/peerj.4614
19. Kurko TA, Saastamoinen LK, Tahkapaa S, et al. Long-term use of benzodiazepines: definitions, prevalence and usage patterns - a systematic review of register-based studies. Eur Psychiatry. 2015;30(8):1037–1047. doi:10.1016/j.eurpsy.2015.09.003
20. American Psychiatric Association. Diagnostic and statistical manual of mental disorders fifth editon (DSM-V). Arlington, VA: American Psychiatric Publishing; 2013:362–365.
21. Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137. doi:10.5664/jcsm.27760
22. Maspero C, Giannini L, Galbiati G, Rosso G, Farronato G. Obstructive sleep apnea syndrome: a literature review. Minerva Stomatol. 2015;64(2):97–109.
23. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi:10.1016/0140-6736(91)93206-o
24. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea? The rational clinical examination systematic review. JAMA. 2013;310(7):731–741. doi:10.1001/jama.2013.276185
25. Foral P, Knezevich J, Dewan N, Malesker M. Medication-induced sleep disturbances. Consult Pharm. 2011;26(6):414–425. doi:10.4140/TCP.n.2011.414
26. Ni X, Shergis JL, Guo X, et al. Updated clinical evidence of Chinese herbal medicine for insomnia: a systematic review and meta-analysis of randomized controlled trials. Sleep Med. 2015;16(12):1462–1481. doi:10.1016/j.sleep.2015.08.012
27. Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The safety of ingested caffeine: a comprehensive review. Front Psychiatry. 2017;8(80). doi:10.3389/fpsyt.2017.00080
28. Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539–549. doi:10.1111/acer.12006
29. Chouinard S, Poulin J, Stip E, Godbout R. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957–967. doi:10.1093/oxfordjournals.schbul.a007145
30. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254–1269. doi:10.4088/jcp.v66n1008
31. Cutler AJ. The role of insomnia in depression and anxiety: its impact on functioning, treatment, and outcomes. J Clin Psychiatry. 2016;77(8):e1010. doi:10.4088/JCP.14076tx3c
32. Pangman VC, Sloan J, Guse L. An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. APPL NURS RES. 2000;13(4):209–213. doi:10.1053/apnr.2000.9231
33. Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101222. doi:10.1016/j.smrv.2019.101222
34. Health NIOP. WHO collaborating centre for drug statistics methodology: ATC/DDD index. 2021.
35. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi:10.1016/S0033-3182(71)71479-0
36. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi:10.1001/archpsyc.1965.01720310065008
37. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
38. Suh M, Choi-Kwon S, Kim JS. Sleep disturbances after cerebral infarction: role of depression and fatigue. J Stroke Cerebrovasc Dis. 2014;23(7):1949–1955. doi:10.1016/j.jstrokecerebrovasdis.2014.01.029
39. Colin O, Labreuche J, Deguil J, et al. Preadmission use of benzodiazepines and stroke outcomes: the Biostroke prospective cohort study. BMJ Open. 2019;9(1):e22720. doi:10.1136/bmjopen-2018-022720
40. Delpont B, Blanc C, Osseby GV, Hervieu-Bègue M, Giroud M, Béjot Y. Pain after stroke: a review. Rev Neurol-France. 2018;174(10):671–674. doi:10.1016/j.neurol.2017.11.011
41. Kwah LK, Diong J. National institutes of health stroke scale (NIHSS). J Physiother. 2014;60(1):61. doi:10.1016/j.jphys.2013.12.012
42. Runde D. Calculated decisions: modified rankin scale (mRS) for neurologic disability. Emerg Med Pract. 2019;21(Suppl 6):D4–5.
43. Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. STROKE. 2014;45(12):3754–3832. doi:10.1161/STR.0000000000000046
44. Zhang P, Li YP, Wu HJ, et al. Guidelines for the diagnosis and treatment of insomnia in Chinese adults (2017 Edition). Chin J Neurol. 2018;51(05):324–335. doi:10.3760/cma.j.issn.1006-7876.2018.05.002
45. Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res. 2012;36(5):427–440. doi:10.1007/s10608-012-9476-1
46. Glidewell RN, Moorcroft WH, Lee-Chiong T. Comorbid insomnia: reciprocal relationships and medication management. Sleep Med Clin. 2010;5(4):627–646. doi:10.1016/j.jsmc.2010.08.012
47. Schifano F, Chiappini S, Corkery JM, Guirguis A. An insight into Z-drug abuse and dependence: an examination of reports to the European medicines agency database of suspected adverse drug reactions. Int J Neuropsychopharmacol. 2019;22(4):270–277. doi:10.1093/ijnp/pyz007
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.