Back to Journals » Cancer Management and Research » Volume 11
Ezrin promotes pancreatic cancer cell proliferation and invasion through activating the Akt/mTOR pathway and inducing YAP translocation
Authors Quan C, Sun J, Lin Z, Jin T, Dong B, Meng Z, Piao J
Received 21 January 2019
Accepted for publication 29 May 2019
Published 12 July 2019 Volume 2019:11 Pages 6553—6566
DOI https://doi.org/10.2147/CMAR.S202342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Chunji Quan,1,2,* Jie Sun,1,3,* Zhenhua Lin,1,3 Tiefeng Jin,1,3 Bing Dong,1,3 Ziqi Meng,1,3 Junjie Piao1,3
1Department of Pathology and Cancer Research Center, Yanbian University Medical College, Yanji 133002, People’s Republic of China; 2Department of Pathology, Affiliated Hospital of Yanbian University, Yanji 133000, People’s Republic of China; 3Key Laboratory of the Science and Technology, Department of Jilin Province, Yanji 133002, People’s Republic of China
*These authors contributed equally to this work
Background: Ezrin and YAP are abnormally expressed in various cancers, and play pivotal roles in cancer initiation and development. However, the mechanisms of Ezrin in pancreatic cancer have not been fully elucidated. In this study, we aimed to elucidate the functions and mechanisms of Ezrin in the pathogenesis of pancreatic cancer.
Methods: Effects of Ezrin deregulation on pancreatic cancer phenotype were determined in Capan-1 and BxPC-3 cells using MTT, colony formation, transwell, wound-healing, and chick chorioallantoic membrane assays. To find out the underlying mechanism of Ezrin, multiple assays were performed to detect the effect of Ezrin on Akt pathway activation and YAP expression. Then, Ezrin and YAP expression was analyzed in pancreatic cancer and normal pancreas samples. Finally, the prognostic value of Ezrin and YAP was evaluated in pancreatic cancer patients.
Results: Ezrin promoted proliferation, invasion, epithelial–mesenchymal transition (EMT) progression, and angiogenesis of pancreatic cancers. Mechanistically, Ezrin activated Akt/mTOR pathways and induced YAP phosphorylation and nucleus translocation. The PI3K/Akt pathway inhibitor, rapamycin, and LY294002 could partially attenuate the effect of Ezrin on cell proliferation, invasion, EMT progression, and YAP phosphorylation and translocation. Moreover, both Ezrin and YAP were significantly overexpressed in pancreatic cancer tissues compared with adjacent normal pancreas, and correlated with poor prognosis in pancreatic cancer patients. Multivariate survival analysis showed that Ezrin was an independent prognostic marker for pancreatic cancer. Furthermore, the expression status of Ezrin and YAP had positive correlations in pancreatic cancer tissues.
Conclusion: Ezrin promoted pancreatic cancer proliferation, invasion, migration, and EMT progression, partially through activating the PI3K/Akt pathway, and also regulated YAP phosphorylation and translocation, partially through the PI3K/Akt pathway. Ezrin and YAP were significantly overexpressed in pancreatic cancers, and correlated with poor prognosis in pancreatic cancer patients.
Keywords: Ezrin, YAP, PI3K/Akt pathway, pancreatic cancer, survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human neoplasms, with a 5-year survival rate of only 8%.1 Until now, surgical removal and chemotherapy have been the most effective treatment. Unfortunately, only less than 20% of patients are suitable for surgical removal, owing to late detection and early metastasis. Thus, more extensive studies are needed to understand the molecular mechanism of PDAC initiation and progression.
Ezrin, a member of the ezrin, radixin, and moesin (ERM) protein family, is essential for linking the actin cytoskeleton to the cell membrane, and is recognized as a key organizer of specialized membrane domains, such as apical microvilli, lamellipodia, filopodia, and macropinocytosis.2,3 As a cytoskeletal linker, Ezrin participates in multiple cellular processes, such as migration, morphogenesis, adhesion, apoptosis, chemoresistance, and stress responses.4 In addition, it has been reported that Ezrin is abnormally expressed in various types of tumors, and participates in cancer initiation and progression.5,6 In our previous study, we showed that Ezrin was significantly overexpressed in pancreatic cancer tissues, and correlated with tumor size, lymph-node metastasis, clinical stage, and prognosis.7
Mechanically, Ezrin could affect various signaling pathways and molecules involved in tumor progression, such as β4-integrin, hepatocyte growth factor receptor, and Src family kinases.8 In cervical cancers, Ezrin regulates the PI3K/Akt pathway, and affects the proliferation and invasion of cervical cancer cells.9 Gautreau et al reported that Ezrin mediates the PI3K/Akt pathway through binding to P85 in kidney-derived epithelial cells.10 However, Zhong et al found that knockdown of Ezrin had no effect on p-Akt expression in pancreatic cancer cells.11 Thus, we intended to determine whether the PI3K/Akt pathway is involved in the diverse functions of Ezrin in pancreatic cancer. On the other hand, we previously found that Ezrin affected skin fibroblast proliferation by regulating yes-associated protein (YAP).12 In pancreatic cancers, YAP was reported to enhance tumor cell proliferation, invasion, and metastasis.13 These results led us to hypothesize that Ezrin may regulate pancreatic cancer progression partially through YAP regulation.
To test our hypothesis, in this study we identify the function and molecular mechanism of Ezrin in pancreatic cancer, and further explore the relations between Ezrin, YAP, and Akt pathways in vitro and ex vivo.
Materials and methods
Ethics statement
This study complied with the Helsinki Declaration and was approved by the Human Ethics Committee and the Research Ethics Committee of Yanbian University Medical College, People's Republic of China. Through the surgery consent form, patients were informed that the resected specimens were kept by our hospital and might be used for scientific research, and that their privacy would be maintained. Follow-up survival data were collected retrospectively by analysis of medical records.
Tissue specimens
A total of 187 samples were collected from Yanbian University Hospital. The samples were routinely processed with 10% buffered formalin fixation and paraffin embedded. The study protocol was approved by the institutional review board of Yanbian University Medical College. Overall survival (OS) time was defined as the time from operation to cancer-related death only. Two experienced pathologists reviewed the H&E-stained slides and one appropriate paraffin block was selected for this study as described previously.7
Cell culture and transfection
Human pancreatic cancer cells (Mia PaCa-2, BxPC-3, ASPC-1, SW1990, Capan-1, and Panc-1) were supplied by the Cancer Research Center of Yanbian University and approved by the Research Ethics Committee of Yanbian University Medical College. BxPC-3 and ASPC-1 cell lines were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA) with 10% FBS (Gibco). MiAPaca-2, Panc-1, SW1990, and Capan-1 were cultured in DMEM (Gibco) supplemented with 10% FBS (Gibco). These cells were cultured at 37°C in a humidified atmosphere with 5% CO2.
Capan-1 cells in six-well plates were transfected with si-Ezrin for 6 hours by the Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) method according to the manufacturer’s protocol, and recovered in RPMI 1640 medium containing 10% FBS for 48 hours. The Ezrin-overexpressed cell line was constructed using pcDNA 3.1–Ezrin plasmid (Genechem, Shanghai, People's Republic of China). Ezrin-expressed plasmid was transfected into BxPC-3 cells using Lipofectamine 3000.
Immunohistochemistry (IHC)
IHC staining was performed using a Dako LSAB kit (Dako, Glostrup, Denmark). Tissue slides were deparaffinized, rehydrated, and incubated with H2O2. Subsequently, slides were incubated with 5% BSA, followed by the primary antibody, Ezrin (1:1,000; Cell Signaling, USA) and YAP (1:1,000; Cell Signaling), at 4°C overnight. Then, slides were treated with horseradish peroxidase-tagged second antibody, followed by DAB detection. Counterstaining was performed using hematoxylin.
Immunofluorescence (IF)
IF was performed as described previously.7 Cells were grown on coverslips to 70% confluence. Then, cells were fixed with 4% paraformaldehyde for 10 minutes and permeabilized with 0.5% Triton X-100 for 10 minutes. Blocking was performed with 3% albumin bovine V (Solarbio, Beijing, People's Republic of China) for 1 hour at room temperature. After washing with PBS, cells were incubated with primary antibody at 4°C overnight, followed by incubation with fluorescence-tagged secondary antibody for 1 hour at room temperature. After washing with PBS, the cells were counterstained with DAPI (Beyotime, Shanghai, People's Republic of China), and the coverslips were mounted with Antifade Mounting Medium (Beyotime). Finally, the IF signals were visualized and recorded with a Leica SP5II confocal microscope.
Western blot
Cells were lysed and then size fractionated using SDS-PAGE. After loading, the protein samples were transferred to polyvinylidene difluoride (PVDF) membranes. Then, the membrane was blocked with 5% skim milk, followed by incubation with the primary antibody and secondary antibody. Immunocomplexes were developed using ECL prime Western blotting detection reagent (CW Biotech, Beijing, People's Republic of China).
Chick chorioallantoic membrane (CAM) assay
Fertilized chicken eggs were kept in an incubator at 37°C with 60% humidity. A total of 1×106 cells were inoculated on the 10-day-old chick CAM, in which an artificial air sac was created under aseptic conditions. Then, the window was resealed with adhesive tape and eggs were returned to the incubator. The membrane was observed under a microscope and the total number of vessel branch points directly beneath the disc was counted at 24 and 120 hours.
Wound-healing assay
Cells were seeded on six-well plates. Then, wounds were scratched using a pipette tip. Fresh medium containing 100 ng/mL of mitomycin C and 0.1% FBS was added. Cell migration ability was detected by measuring the distance of wound closure. The images were captured using a microscope at 0 and 48 hours.
Nuclear and cytosol separation
Cell cytoplasm and nucleus were separated using a Nuclear Extract Kit (CW Biotech). Supernatants were used as the cytoplasmic fraction. Pellets were resuspended with lysis buffer, followed by centrifugation. Then, supernatants were used as the nuclear fraction.
Spheroid invasion assay
A total of 3×103 cells/well were seeded in 96-well plates. Cell were then incubated for 24 hours to allow spheroid formation. Spheroids were collected and embedded in 100 μL of Matrigel, and plated in a 96-well flat-bottom plates, previously coated with 50 μL of Matrigel. After Matrigel solidification, 100 μL of invasion medium, with vascular endothelial growth factor A (VEGF-A), placental growth factor, or epidermal growth factor (EGF) (50 ng/mL), was added and plates were incubated at 37°C for up to 72 hours. Spheroids were visualized and photographed using a microscope.
Statistical analysis
SPSS software 17.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. All quantitative data are presented as mean ± SD. All results were confirmed in at least three independent experiments and data from one representative experiment are presented.
Results
Ezrin positively regulates cell proliferation of pancreatic cancer cells
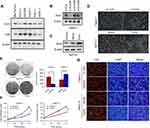
First, the endogenous expression levels of Ezrin and YAP were detected in different types of pancreatic cancer cell lines. The expression level of Ezrin was highest in Capan-1 and lowest in BxPC-3 (Figure 1A). However, there was no significant relationship between endogenous expression levels of Ezrin and YAP in pancreatic cancer cells. Subsequently, knockdown of Ezrin was performed by siRNAs in Capan-1 cells. Ezrin and empty vector plasmids were transfected into the BxPC-3 cells (Figure 1B and C). si-Ezrin#1 was selected for further study, and microscopic images of the cells before and after transfection were shown in Figure 1D.
To identify the function of Ezrin in pancreatic cancer cell proliferation, we performed the MTT assay, colony formation assay, and 5-ethynyl-2´-deoxyuridine (Edu) staining. The colony formation assay showed that Ezrin overexpression increased colony formation capacity in BxPC-3 cells, while Ezrin knockdown decreased colony formation capacity in Capan-1 cells (Figure 1E). The MTT assay also showed that Ezrin overexpression increased cell viability in BxPC-3 cells, and Ezrin knockdown decreased cell viability in Capan-1 cells. Consist with these results, Edu staining also showed that Ezrin overexpression increased DNA replication of BxPC-3 cells, and Ezrin knockdown attenuated DNA replication of Capan-1 cells (Figure 1F and G). Taken together, these results validated that Ezrin positively regulates the proliferation of pancreatic cancer cells.
Ezrin promotes migration, invasion, and epithelial–mesenchymal transition (EMT) progression in pancreatic cancer cells
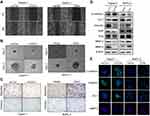
To test whether Ezrin could regulate migration and invasion in pancreatic cancer cells, wound-healing and transwell assays were performed. The results of the wound-healing assay showed that Ezrin knockdown reduced the migration of Capan-1, and Ezrin overexpression promoted the migration of BxPC-3 (Figure 2A). Then, the ability of Ezrin to regulate ECM invasion of pancreatic cancer was tested by a spheroid invasion assay. As shown in Figure 2B, Ezrin knockdown significantly inhibited Matrigel-embedded spheroids to markedly invade the surrounding matrix in Capan-1 cells, and Ezrin overexpression stimulated spheroids to invade the surrounding matrix in BxPC-3 cells. Knockdown of Ezrin was also associated with reduced invasion and migration abilities in Capan-1 cells, while Ezrin overexpression was associated with improved invasion and migration abilities in BxPC-3 cells (Figure 2C). The role of Ezrin on cell proliferation, migration, and invasion was also confirmed in Panc-1 cells (Figure S1). These results indicate that Ezrin regulates the invasion and migration of pancreatic cancer cells.
EMT, as a fundamental cell-biological process, plays key roles in embryogenesis and wound healing. The activation of EMT during cancer enhances the metastatic phenotype.14 To test whether Ezrin could regulate EMT progression, we detected the expression of EMT-related proteins by Western blot. The results showed that Ezrin knockdown significantly increased E-cadherin and Zo-1 (epithelial markers), while Vimentin, MMP-2, and MMP-9 (mesenchymal markers), and snail and slug (transcription factors) were significantly reduced. Similarly, Ezrin overexpression reduced the expression of E-cadherin and Zo-1, and increased Vimentin, MMP-2, MMP-9, snail, and slug (Figure 2D). IF staining also showed that Ezrin knockdown increased the expression of E-cadherin and Zo-1, and reduced Vimentin and MMP-2, while Ezrin overexpression reduced the expression of E-cadherin and Zo-1, and increased Vimentin and MMP-2 (Figure 2E). Taken together, these results demonstrate that Ezrin enhances EMT progression in pancreatic cancer cells.
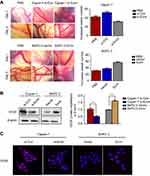
Ezrin promotes angiogenesis of pancreatic cancer
Zhao et al reported that Ezrin could regulate angiogenesis in umbilical vein endothelial cells.15 Accordingly, to identify whether Ezrin participated in the angiogenesis of pancreatic cancer, we performed in vivo CAM angiogenesis assays. As shown in Figure 3A, the number of vessels was significantly decreased in Ezrin knockdown cells, while the number of vessels was significantly increased in Ezrin-overexpressed cells. These results indicate that Ezrin was involved in the angiogenesis of pancreatic cancer. Consistent with these results, the results of Western blot and IF revealed that Ezrin knockdown decreased the expression of VEGF-A, and Ezrin overexpression increased the expression of VEGF-A (Figure 3B and C). These results demonstrate that Ezrin could regulate angiogenesis in pancreatic cancers.
Ezrin promotes proliferation and EMT progression partially through activating the PI3K/Akt pathway
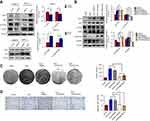
The activation of PI3K/Akt is well known as one of the hallmarks of cancer. It participates in multicellular functions, including proliferation, growth, glucose metabolism, differentiation, and EMT progression.16 Gautreau et al reported that Ezrin bound to P85, the regulatory subunit of PI3K, to mediate the PI3K/Akt pathway.10 However, it was also reported that Ezrin could not regulate the PI3K/Akt pathway, but could regulate the ERK pathway.11 Thus, Western blot was performed to test whether Ezrin could affect the Akt and ERK pathways in pancreatic cancer cells. As shown in Figure 4A, Ezrin could regulate the phosphorylation of Akt and mTOR, but not ERK1/2.
Furthermore, to test the hypothesis that Ezrin promotes proliferation and EMT progression through regulating the PI3K/Akt pathway, we applied an mTOR inhibitor, rapamycin, and a PI3K inhibitor, LY294002. The results showed that loss of E-cadherin and upregulation of Vimentin by Ezrin were partially reversed when the Akt/mTOR pathway was blocked by rapamycin and LY294002 in BxPC-3 cells (Figure 4B). Concomitant with the change in EMT-related proteins, treatment with rapamycin and LY294002 significantly reversed the effect of Ezrin on BxPC-3 cell proliferation and migration (Figure 4C and D). These results indicate that Ezrin regulated proliferation and EMT progression partially through activating the Akt/mTOR pathway.
Ezrin induces YAP phosphorylation and nuclear translocation through the Akt/mTOR pathway
Previously, we reported that Ezrin regulated fibroblast size through YAP-mediated proliferation.12 Thus, we explored whether Ezrin could regulate YAP in pancreatic cancer cells. As shown in Figure 5A, Ezrin knockdown significantly increased the phosphorylation of YAP, while Ezrin overexpression significantly decreased it.
Accumulation of YAP in the nucleus induces cell growth, and its exclusion from the nucleus will induce cell cycle arrest and stop proliferation.17 By cytosolic and nuclear fractionation experiments, the translocation of YAP from the nucleus to the cytoplasm was observed in Ezrin knockdown Canpan-1 cells. The translocation of YAP from the cytoplasm to the nucleus was also observed in Ezrin-overexpressed BxPC-3 cells (Figure 5B). These results suggest that Ezrin regulats YAP translocation in pancreatic cancer cells. Ma et al reported that the PI3K/Akt pathway modulated YAP expression through regulating CD166, indicating that YAP was a downstream target of the PI3K/Akt pathway.18 Thus, we supposed that Ezrin could regulate YAP expression and translocation through the PI3K/Akt pathway. To test this hypothesis, we applied rapamycin and LY294002 again. The results showed that treatment with rapamycin and LY294002 attenuated the effect of Ezrin in regulating YAP translocation (Figure 5C). These results suggest that PI3K/Akt pathway activation is involved in Ezrin-mediated YAP translocation.
High expression of Ezrin and YAP correlates with poor prognosis
First, the mRNA expression of Ezrin and YAP in pancreatic cancer was analyzed using the Oncomine dataset.19 The results showed that mRNA expression of Ezrin and YAP was significantly higher in pancreatic cancer than in normal pancreas (Figure 6A). To identify the clinical significance of Ezrin and YAP in pancreatic cancer, we performed IHC analysis in 108 cases of pancreatic cancer tissues and 79 cases of adjacent normal pancreas. IHC staining showed that Ezrin was mainly expressed in cytoplasm, and YAP was expressed in both cytoplasm and nucleus (Figure 6E). The positive rate of Ezrin in pancreatic cancer tissues (86.1%, 93/108) was significantly higher than in adjacent normal pancreas (35.4%, 28/79) (Table 1). Similarly, the positive rate of YAP in pancreatic cancer tissues (85.2%, 92/108) was significantly higher than in adjacent normal pancreas (31.6%, 25/79) (Table 1). In addition, the expression of Ezrin was related to the pathological grading, clinical stage, lymph-node metastasis, and invasion of nerves/vessels (P<0.05), but was not related to gender, age, tumor location, or tumor size (P>0.05) (Figure 6C). The expression of YAP was closely related to pathological grade and lymph-node metastasis (P<0.01), but not related to gender, age, tumor location, tumor size, or clinical stage (P>0.05) (Figure 6D).
 |
Table 1 Expression of Ezrin and YAP in pancreatic cancer and adjacent normal tissues |
Survival data for pancreatic cancer patients with Ezrin and YAP expression were obtained from the UALCAN dataset.20 The results predicted that high expression of Ezrin and YAP mRNA levels was correlated with poor prognosis in pancreatic cancer patients (Figure 6B). Kaplan–Meier analysis showed that pancreatic cancer patients with high expression of Ezrin had shorter survival times than patients with low expression of Ezrin, while pancreatic cancer patients with high expression of YAP had shorter survival times than patients with low expression of YAP (Figure 6F). Univariate analysis showed that tumor grade, lymph-node metastasis, Ezrin, YAP, and clinical stage affected the overall survival rate of patients, and multivariate analysis showed that only the expression of Ezrin (Wald=6.297, P=0.012) and pathological grade (Wald=5.465, P=0.019) were independent risk factors (Table 2).
 |
Table 2 Survival analyses (Cox regression model) of various factors in patients with pancreatic cancer |
The mRNA expression data obtained from the Starbase dataset were analyzed to detect the relations between Ezrin and YAP.21 The results showed that the mRNA expression of Ezrin was positively correlated with YAP expression (Figure 7A) (r=0.503, P<0.05).
Consistently, statistical analysis based on IHC staining in pancreatic cancer tissues showed that the protein expression levels of Ezrin and YAP were positively related in pancreatic cancer tissues (r=0.821, P<0.05) (Figure 7B and Table 3).
 |
Table 3 Relationships between Ezrin and YAP expression in pancreatic cancer tissues |
Discussion
To date, accumulated understanding on the molecular mechanisms of pancreatic cancer has shown that many molecular events are responsible for pancreatic cancer initiation and progression. Mutations in oncogenes and tumor suppressor genes, increased expression of growth factors, and aberrations in signaling pathways contribute to pancreatic cancer development, and many therapeutic inhibitors have been designed to target K-ras, epidermal growth factor receptor (EGFR), and PI3K/Akt signaling in pancreatic cancer. Although these findings improve the survival of patients, the effects are limited because of side effects and chemoresistance. Thus, more extensive studies are required to understand the molecular mechanisms of this disease.
Ezrin, a member of the ERM protein family, is essential for linking the actin cytoskeleton to the cell membrane. Accumulating evidence suggests that Ezrin is abnormally expressed in various types of cancer. In this study, we showed that Ezrin was frequently upregulated in pancreatic cancers. The expression of Ezrin was related to pathological grading, clinical stage, lymph-node metastasis, invasion of nerves/vessels, and poor prognosis of pancreatic cancer. Studies from other laboratories have consistently demonstrated that autoantibodies against Ezrin are induced early in PDAC, and their combination with other serological markers may provide a predictive and diagnostic signature.22 We also found that Ezrin regulated the proliferation, invasion, and migration of pancreatic cancer cells in vitro, indicating that Ezrin has multiple functions in pancreatic cancer initiation and progression.
EMT is well known as a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties, and generally occurs in the initiation of metastasis in cancer progression. In the present study, we observed that Ezrin induced EMT progression in pancreatic cancer cells. Huang et al reported that knockdown of Ezrin inhibited the metastasis induced by either HGF/Met autocrine or non-autocrine signaling in syngeneic wild-type and HGF transgenic mouse hosts.23 Li et al reported that Ezrin was required for EGF-mediated lung metastasis of colorectal cancer xenografts.24 Although an increasing number of reports has identified Ezrin as participating in tumor metastasis, the mechanism is not clearly understood.
Mechanically, Ezrin could interact with EGFR, and knockdown of Ezrin causes a decrease in Y1068 and Y845 phosphorylation of EGFR.25 PI3K/Akt and ERK1/2 pathways, as downstream pathways of EGFR, are highly supposed to be activated by Ezrin. In agreement with these hypotheses, we found that Ezrin could activate the PI3K/Akt pathway, but had no effect on ERK1/2. Consistently, Krishnan et al showed that the action of Ezrin in Ewing’s sarcoma is dependent on the AKT/mTOR signal transduction cascade, but not on mitogen-activated protein kinase (MAPK).26 In addition, Ezrin was reported to bind to P85 to regulate the PI3K/Akt pathway in kidney-derived cell lines.10 Thus, a further study is required to verify whether Ezrin activates the PI3K/Akt pathway through regulating EGFR or binding to P85 in pancreatic cancer. Anyway, these results confirm that Ezrin regulates pancreatic cancer proliferation and invasion through modulating the PI3K/Akt pathway.
YAP was also identified as being partially involved in the oncogenic role of Ezrin in pancreatic cancer. YAP is the major downstream effector of the Hippo pathway, which regulates tissue homeostasis, organ size, regeneration, and tumorigenesis.27 Emerging evidence suggests that hyperactivation of YAP is widespread in cancers.28 Consistent with this, we found that YAP was frequently overexpressed in pancreatic cancer tissues, and correlated with pathological grading, lymph-node metastasis, and survival in pancreatic cancer. In addition, a statistically significant positive correlation between Ezrin and YAP was observed in pancreatic cancer tissues. Our in vitro studies also showed that upregulation of Ezrin inhibited phosphorylation of YAP and induced nucleus translocation.
It is widely recognized that nuclear YAP functions as an oncogene in tumorigenesis and tumor progression in multiple types of cancer. In the absence of phosphorylation, YAP localizes to the nucleus, where it binds and activates the TEA-domain DNA-binding transcription factors (TEAD 1–4), thereby stimulating the expression of a variety of genes, including connective tissue growth factor (CTGF), BIRC5, cysteine-rich angiogenic inducer 61 (CYR61), amphiregulin (AREG), and endothelin 2 (EDN2).29 In the present study, we found that the expression of nuclear YAP was increased with Ezrin overexpression in pancreatic cancer cells. These results suggest that Ezrin promotes pancreatic cancer tumorigenesis partially through regulating YAP nuclear translocation. Liang et al reported that mTOR activation led to YAP accumulation.30 We found that PI3K and mTOR inhibitor could partially reverse the effect of Ezrin on YAP accumulation in pancreatic cancer cells, suggesting that activation of the PI3K/Akt pathway is required for the effect of Ezrin in regulating YAP.
However, it remains to be seen whether Ezrin protein can directly interact with YAP. Since YAP and ERM protein families are implicated in cancer progression, the characterization of these putative interactions could contribute toward the better understanding of not only the role of Ezrin proteins in the regulation of tumorigenesis with YAP, but also the molecular events leading to cancer and metastasis.
Conclusion
Our results showed that Ezrin and YAP was significantly overexpressed in pancreatic cancers, and correlated with poor prognosis in cancer patients. In vitro, Ezrin promoted pancreatic cancer proliferation, invasion, migration, and EMT progression, partially through activating the PI3K/Akt pathway, and Ezrin also could regulate YAP phosphorylation and translocation, partially through the PI3K/Akt pathway (Figure S2).
Acknowledgments
This study was supported by grants from the Jilin Province Science and Technology Development Program (20170520015JH) and the National Natural Science Foundation of China (no. 315603120).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
2. Baumgartner M, Sillman AL, Blackwood EM, et al. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci USA. 2006;103(36):13391–13396. doi:10.1073/pnas.0605950103
3. Chiasson-MacKenzie C, Morris ZS, Liu CH, Bradford WB, Koorman T, McClatchey AI. Merlin/ERM proteins regulate growth factor-induced macropinocytosis and receptor recycling by organizing the plasma membrane: cytoskeletoninterface. Genes Dev. 2018;32(17–18):1201–1214. doi:10.1101/gad.317354.118
4. Celik H, Bulut G, Han J, et al. Ezrin inhibition up-regulates stress response gene expression. J Biol Chem. 2016;291(25):13257–13270. doi:10.1074/jbc.M116.718189
5. Bartova M, Hlavaty J, Tan Y, et al. Expression of ezrin and moesin in primary breast carcinoma and matched lymph node metastases. Clin Exp Metastasis. 2017;34(5):333–344. doi:10.1007/s10585-017-9853-y
6. Kobel M, Gradhand E, Zeng K, et al. Ezrin promotes ovarian carcinoma cell invasion and its retained expression predicts poor prognosis in ovarian carcinoma. Int J Gynecol Pathol. 2006;25(2):121–130. doi:10.1097/01.pgp.0000185410.39050.ac
7. Piao J, Liu S, Xu Y, et al. Ezrin protein overexpression predicts the poor prognosis of pancreatic ductal adenocarcinomas. Exp Mol Pathol. 2015;98(1):1–6. doi:10.1016/j.yexmp.2014.11.003
8. Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adh Migr. 2011;5(2):199–206. doi:10.4161/cam.5.2.15081
9. Kong J, Di C, Piao J, et al. Ezrin contributes to cervical cancer progression through induction of epithelial-mesenchymal transition. Oncotarget. 2016;7(15):19631–19642.
10. Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96(13):7300–7305. doi:10.1073/pnas.96.13.7300
11. Zhong ZQ, Song MM, He Y, Cheng S, Yuan HS. Knockdown of Ezrin by RNA interference reverses malignant behavior of human pancreatic cancer cells in vitro. Asian Pac J Cancer Prev. 2012;13(8):3781–3789.
12. Quan C, Yan Y, Qin Z, Lin Z, Quan T. Ezrin regulates skin fibroblast size/mechanical properties and YAP-dependent proliferation. J Cell Commun Signal. 2018;12(3):549–560. doi:10.1007/s12079-017-0406-6
13. Jiang Z, Zhou C, Cheng L, et al. Inhibiting YAP expression suppresses pancreatic cancer progression by disrupting tumor-stromal interactions. J Exp Clin Cancer Res. 2018;37(1):69. doi:10.1186/s13046-018-0740-4
14. Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35(4):645–654. doi:10.1007/s10555-016-9648-7
15. Zhao LP, Huang L, Tian X, et al. Knockdown of ezrin suppresses the migration and angiogenesis of human umbilical vein endothelial cells in vitro. J Huazhong Univ Sci Technol Med Sci. 2016;36(2):243–248. doi:10.1007/s11596-016-1574-y
16. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. doi:10.1080/19336918.2015.1016686
17. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94(4):1287–1312. doi:10.1152/physrev.00005.2014
18. Ma L, Wang J, Lin J, Pan Q, Yu Y, Sun F. Cluster of differentiation 166 (CD166) regulated by phosphatidylinositide 3-Kinase (PI3K)/AKT signaling to exert its anti-apoptotic role via yes-associated protein (YAP) in liver cancer. J Biol Chem. 2014;289(10):6921–6933. doi:10.1074/jbc.M113.524819
19. Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepato-Gastroenterology. 2008;55(88):2016–2027.
20. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002
21. Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–97. doi:10.1093/nar/gkt1248
22. Capello M, Cappello P, Linty FC, et al. Autoantibodies to Ezrin are an early sign of pancreatic cancer in humans and in genetically engineered mouse models. J Hematol Oncol. 2013;6:67. doi:10.1186/1756-8722-6-67
23. Huang L, Qin Y, Zuo Q, et al. Ezrin mediates both HGF/Met autocrine and non-autocrine signaling-induced metastasis in melanoma. Int J Cancer. 2018;142(8):1652–1663. doi:10.1002/ijc.31196
24. Li Y, Lin Z, Chen B, et al. Ezrin/NF-kB activation regulates epithelial- mesenchymal transition induced by EGF and promotes metastasis of colorectal cancer. Biomed Pharmacother. 2017;92:140–148. doi:10.1016/j.biopha.2017.05.058
25. Krieg J, Hunter T. Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem. 1992;267(27):19258–19265.
26. Krishnan K, Bruce B, Hewitt S, Thomas D, Khanna C, Helman LJ. Ezrin mediates growth and survival in Ewing’s sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clin Exp Metastasis. 2006;23(3–4):227–236. doi:10.1007/s10585-006-9033-y
27. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nature Rev Cancer. 2015;15(2):73–79. doi:10.1038/nrc3876
28. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature Rev Cancer. 2013;13(4):246–257. doi:10.1038/nrc3458
29. Eibl G, Rozengurt E. KRAS, YAP, and obesity in pancreatic cancer: a signaling network with multiple loops. Semin Cancer Bio. 2019;54:50–62. doi:10.1016/j.semcancer.2017.10.007
30. Liang N, Zhang C, Dill P, et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211(11):2249–2263. doi:10.1084/jem.20140341
Supplementary materials
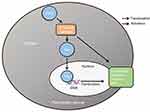 |
Figure S2 Scheme of proposed molecular mechanism of Ezrin-induced pancreatic cancer cell proliferation, invasion, and EMT progression. Abbreviation: EMT, epithelial–mesenchymal transition. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.